Abstract
Inheritance of apical membrane is proposed to maintain vertebrate neural stem cell proliferation. However, evidence for this is contradictory. Using direct clonal analysis and live imaging in chick neural tube, we show that divisions that separate apical and basal components generate an apical daughter, which becomes a neuron, and a basal daughter, which rapidly re-establishes apico-basal polarity and divides again. Using a recently described real-time reporter of Notch activity, we confirm progenitor status and demonstrate that division orientation can influence Notch signalling. In addition, we reveal loss of apical complex proteins on neuronal differentiation onset, suggesting that removal of this inherited complex is part of the neuronal differentiation mechanism. These findings reconcile contradictory data, link asymmetric division to Notch signalling dynamics and identify apical complex loss as a new step towards neuronal differentiation.
Keywords: vertebrate neurogenesis, asymmetric cell fate, mitotic spindle, Notch signalling, chick
Introduction
The role of mitotic spindle orientation with respect to the apical/luminal surface of the neuroepithelium and the decision to adopt neuronal or progenitor cell fate remains controversial. In the cortex, data suggest that apical membrane promotes self-renewing neural stem cell divisions [1–6], but also indicate that inheritance of both apical and basal cell compartments is required to maintain neural progenitor status [7, 8]. The spinal cord is a simpler tissue and is amenable to real-time imaging of individual cells [9, 10]. In early chick neural tube, divisions that give rise to a neuron and a progenitor have cleavage planes non-perpendicular to the luminal surface, whereas divisions that generate progenitors divide mainly with a perpendicular cleavage plane [9]. One explanation for the more varied orientation of the latter is a lack of proneural gene expression at early stages that confers potential to adopt a neuronal fate [9, 11]. These observations suggest that as proneural gene expression commences mitotic spindle orientation comes to influence cell fate choice. However, randomization of mitotic spindle position in chick neural tube analysed at a cell population level is reported to disrupt this tissue, but not to alter cell fates [12]. These findings contrast with real-time imaging data in zebrafish neural tube, which shows that not only do non-perpendicular cleavage plane divisions generate daughters with different fates, but also the apical daughter cell rather than remaining a progenitor, becomes a neuron [10]. Direct monitoring of single cells following manipulation of mitotic spindle in the neural tube of other vertebrates is now required to resolve these contradictions. High-level proneural gene expression promotes neuronal differentiation and is inhibited by Notch signalling, which maintains progenitors in the neuroepithelium [13, 14]. It is therefore also important to determine the relationship between mitotic spindle orientation and Notch activity; neighbouring cells that need not be siblings can provide Notch signals, but such signalling between sibling cells might prevail during asymmetric cell fate assignment.
Here we test the function of mitotic spindle orientation in cell fate choice in the chick neural tube, using mis-expression of the protein Inscuteable (Insc) as a tool to generate divisions with near perpendicular spindle orientations, known as apico-basal (A/B) divisions. In vertebrates, as in Drosophila [15], Insc links the apical Par protein complex (Par3, Par6 and aPKC) via Partner of Insc (Pins) homologues (LGN/Gpsm2 and AGS3/Gpsm1) with Gαi proteins and associated microtubule and dynein-binding proteins that contact the mitotic spindle [16]. Mis-expression of Insc in the vertebrate neuroepithelium causes the mitotic spindle to associate with the apical cortex and so generate divisions that separate apical and basal cellular compartments [6, 7, 17]. Here we use a range of approaches including direct clonal analysis and live imaging to determine cell fates generated by A/B divisions in the chick neural tube. We deploy a novel reporter to monitor Notch activity following A/B divisions and characterize the dynamics of A/B polarity during the establishment of daughter cell fates.
Results
Apico-basal divisions increase neuron production
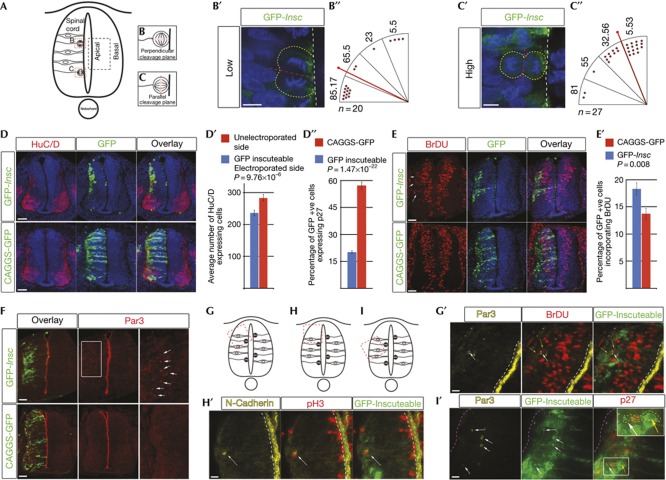
To investigate the role of mitotic spindle orientation in cell fate choice, a green fluorescent protein (GFP)-inscuteable (GFP-Insc) expression vector was transfected into the stage HH10-12 chick neural tube by in ovo electroporation (which expresses low-level Insc; supplementary Fig S1 online). A minimal plasmid concentration that systematically generated apico-basally orientated divisions was determined (Fig 1A–C) and the consequences of such divisions were assessed after 48 h. Many GFP-expressing cells were found in the mantle zone with neuronal morphology (Fig 1D). In contrast, in control pCAGGS-GFP-transfected embryos, most GFP-expressing cells retained contact with the apical surface (Fig 1D, bottom panels). Increase in neuron numbers was then confirmed with the neuronal marker HuC/D (Fig 1D′) and post-mitotic marker p27 (Fig 1D″; supplementary Fig S2A online), and decreased cell proliferation was indicated by reduced numbers of BrdU-incorporating cells (Fig 1E). These data demonstrate increased neuronal differentiation and depletion of the neural progenitor pool on induction of A/B divisions.
Figure 1.
Insc mis-expression rotates the mitotic spindle, increases neuron numbers and generates ectopic cycling cells. (A) Transverse section of chick spinal cord showing mitotic cells with perpendicular (B) or parallel (C) cleavage planes. (B′) Most cells transfected with low levels of GFP-Insc divide with perpendicular cleavage planes. (C′) Cells transfected with higher levels of GFP-Insc mainly divide with parallel cleavage planes. (B″,C″) Quantification of cleavage planes relative to apical surface. Each red dot represents a cell in anaphase. Numbers above each quadrant represent average cleavage plane angle relative to apical surface. Median angle of division=bold red line. Scale bars (B′,C′), 5 μm (D) GFP-Insc mis-expression increases the number of HuC/D-expressing cells (quantified in D′, five sections each from five embryos, paired t-test, error bars represent s.e.m.). (D″) Quantification of GFP-Insc mis-expressing cells that also express p27 (five sections each from five embryos, unpaired t-test, error bars represent s.e.m. Total GFP-Insc cells=1,336, CAGGS-GFP cells=6,786). (E) GFP-Insc mis-expression reduces percentage of GFP-expressing cells incorporating BrDU (quantified in E′, five sections each from five embryos, unpaired t-test, error bars represent s.e.m. Total GFP-Insc cells=1,635, total CAGGS-GFP cells=4,017) and mislocalised BrDU-incorporating cells in the mantle zone (white arrows) Scale bars (D,E,F), 40 μm. (F) Ectopic Par3 in the mantle zone of GFP-Insc-transfected embryos. G,H,I mark regions shown in G′,H′,I′. (G′,H′) Cycling cells in mantle zone associate with Par3 and N-cadherin (white arrows). (I′) Ectopic Par3 is localized in ends of GFP-Insc-expressing cells (white arrows). These cells do not co-express p27 (cell outlined by yellow dashed line, yellow arrow indicates nucleus). White dashed line=apical surface, magenta dashed line=basal surface in I′. Scale bars (G′,H′,I′), 10 μm. GFP, green fluorescent protein; Insc, Inscuteable.
Apico-basal divisions generate ectopic neural progenitors
Insc mis-expression also led to abnormal distribution of BrDU-positive cells in the mantle zone (12/14 embryos; Fig 1E), which also expressed the neural progenitor marker Sox2 (5/5 embryos; supplementary Fig S2B online). Previous work found that such ectopic proliferative cells lack A/B polarity [12] and such change is associated with oncogenic transformation of tissue [18]. However, in embryos transfected with GFP-Insc, apical Par-complex proteins Par3, aPKC and the sub-apical adhesion protein N-cadherin were additionally ectopically localized in the mantle zone (Par3, 10/12 embryos; aPKC, 9/10 embryos; N-cadherin 5/5 embryos; Fig 1F; supplementary Fig S2C,D online far right panels). The presence of ectopic apical protein in this progenitor cell population was then confirmed by localization of Par3 in BrdU-incorporating cells (Fig 1G′); co-expression of N-cadherin and the G2/M phase marker pH3 (Fig 1H′); and non-overlapping expression of Par3 and the post-mitotic marker p27 (26/26 cells; Fig 1I′). Closer analysis revealed that Par3 was localized to one end of each ectopic progenitor cell (Fig 1I′, white arrows) further supporting the retention of A/B polarity. In addition, experiments using a truncated version of the G-protein regulator LGN (Ct-cLGN) that interferes dominantly with endogenous LGN was used to compare the effects of Insc-induced A/B divisions and the randomization of the mitotic spindle (after [12]). These experiments showed that although less effective, randomization also altered cell fates and lead to ectopic Par-complex protein expression in the mantle layer (supplementary Fig S3A,A′,B online). Overall, induction of A/B divisions in the spinal cord increases neuron numbers and creates an ectopic population of neural progenitor cells that exhibit A/B polarity.
Apico-basal divisions generate a neuron and a progenitor
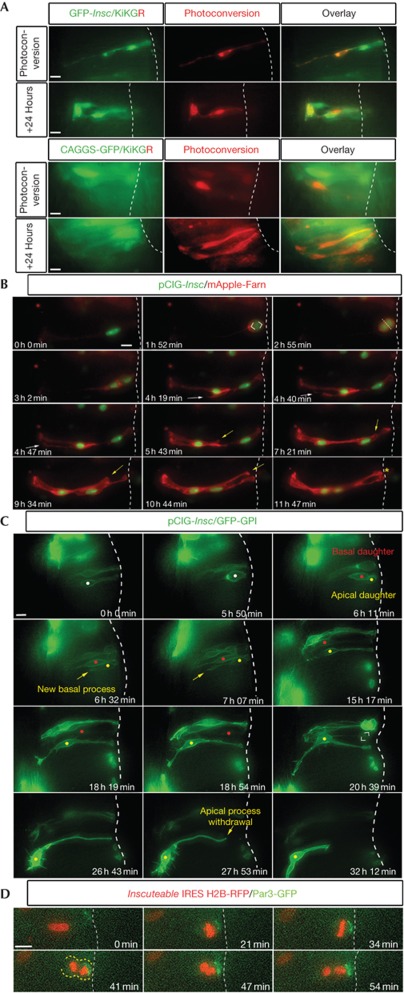
To determine the fates of daughter cells generated by A/B divisions, single cells mis-expressing GFP-Insc or control plasmid pCAGGS-GFP and KiKGR, a photo-convertible fluorescent protein (see Methods) were marked by UV-laser-mediated photo-conversion and slices incubated for 24 h (at least one cell cycle) and then imaged (Fig 2A). The majority of cells (7/9) co-expressing GFP-Insc and KiKGR gave rise to one cell that spanned the neural tube width (in the manner of a progenitor), whereas the other adopted a neuronal morphology (lacking apical process attachment and projecting an axon along the neural tube perimeter). In contrast, all cells expressing control pCAGGS-GFP and KiKGR (8/8) produced progeny with cell shape profiles that extended across the neural tube width and lacked neuronal morphology. This direct clonal analysis demonstrates that A/B divisions produce cells with asymmetric fates, a neuron and a progenitor.
Figure 2.
Insc mis-expression induces stem cell mode divisions. (A) Clonal analysis reveals induction of a neuron and a progenitor by GFP-Insc mis-expression (see text for details). (B) Following an A/B division (1 h 52 min–2 h 55 min), the apical daughter cell extends a new basal process (4 h 19 min, white arrows) and the basal daughter extends a new apical process (5 h 43 min, yellow arrows), which re-establishes apical surface (white dotted line) contact (11 h 47 min, yellow asterisk). (C) Cell indicated with white dot undergoes A/B division, generating a basal daughter (red dot) that remains as a progenitor and divides again (20 h 39 min) and an apical daughter (yellow dot) that extends a new basal process (6 h 32 min) and undergoes neuronal differentiation (27 h 53 min). (D) Following an A/B division (starting at 21 min), Par3 GFP is restricted to an apical crescent that is inherited by the apical daughter (41–54 min). Scale bars, 10 μm. GFP, green fluorescent protein; Insc, Inscuteable.
Apical cell differentiation and basal cell re-polarization
Long-term time lapse was used to determine cell behaviour following Insc-induced division. Cells transfected with pCAGGS-inscuteable-IRES-nucGFP (pCIG-Insc) and mApple-Farn (membrane marker) divided apico-basally, with the basal daughter retaining the original basal process while its apical sibling produced a new one (n=7/7; Fig 2B; supplementary Movie S1 online). In all cases, the basal cell also extended a new apical process; even when these cells became located laterally within the neural tube this new process strikingly re-attached the cell to the luminal surface (Fig 2B; supplementary Movie S1 online). To extend these observations, time-lapses of cells expressing pCIG-Insc and GFP-GPI (monitored through a single filter set, giving increased cell viability) were performed. In divisions where the fate of the apical daughter was clear, the majority of cells became neurons (13/15). Conversely, most basal daughter cells divided again (17/19; Fig 2C; supplementary Movie S2 online) with a small subset (4/19) dividing ectopically in the mantle zone (supplementary Fig S4A online; supplementary Movie S3 online). This determines the identity of the ectopic BrDU-positive progenitor cell population (see Fig 1E) as mis-placed basal daughter cells and as such mis-localized cells express apical markers this further supports re-establishment of A/B polarity in basal daughters.
To address directly whether basal daughter cells re-acquire apical polarity or inherit a small portion of apical membrane, Par3 localization was analysed in cells co-transfected with pCAGGS-Inscuteable-IRES-H2B RFP and low-level (25 ng/μl) pCAGGS-Par3-GFP (Par3-GFP). In the resulting A/B divisions, all detectable Par3-GFP was inherited by the apical daughter (7/7 divisions; Fig 2D; supplementary Movie S4 online). To confirm this, inheritance of endogenous Par3 protein was also analysed using immunocytochemistry and the apical cell was again found to inherit most of this protein (36/36 A/B divisions; supplementary Fig S4B online). These data suggest that the rapid re-polarization of the basal daughter involves de-novo synthesis of apical complex proteins.
Asymmetric inheritance during normal neurogenesis
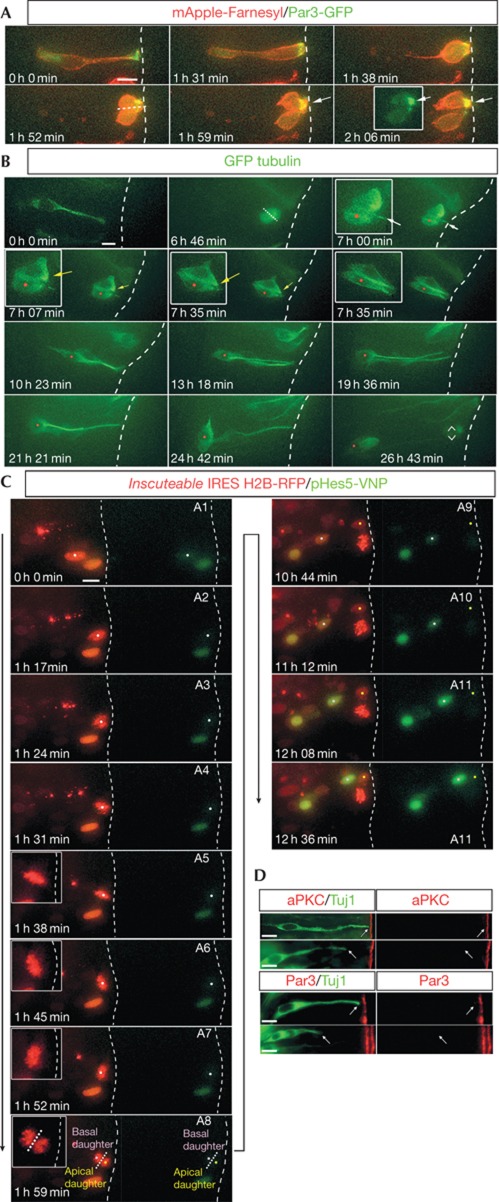
It has been suggested that small changes in spindle orientation are sufficient to partition A/B components in such divisions [1, 2, 19]. To investigate this in the chick neural tube, we monitored inheritance of Par3 at mitosis in cells transfected with either H2B-RFP or mApple-Farn together with low levels of Par3-GFP to visualize normal Par3 localization. This revealed divisions with moderately non-perpendicular cleavage planes in which Par3-GFP was inherited largely by only one daughter cell (n=7/7; Fig 3A; supplementary Movie S5 online). Furthermore, in divisions in which cellular processes were visible and subsequent cell fates of both daughter cells could be established, neuronal cell fate correlated with retention of an apical process, whereas the basal sibling grew a new apical process and divided again (n=6; Fig 3B; supplementary Movie S6 online). These observations suggest that small changes in mitotic spindle orientation during normal neurogenesis can lead to asymmetric segregation of apical membrane and that re-growth of an apical process by basal cells is characteristic of normal neural stem cell mode divisions in the chick spinal cord.
Figure 3.
Asymmetric inheritance of apical components and differential activation of notch signalling. (A) Cell division during normal neurogenesis with a cleavage plane perpendicular to the apical surface (white dotted line, 1 h 52 min), where almost all of the Par3-GFP is inherited by one of the daughter cells (1 h 59 min–2 h 08 min, white arrows). (B) A short apical process (white arrow) is retained by the apical cell (red dot, 7 h 0 min) that goes on to differentiate into a neuron, whereas its sibling basal cell forms a new apical process (yellow arrow) and divides again (26 h 43 min). This is a re-analysis of a movie (supplementary Movie S2 online) previously presented in [9] to assess apical process inheritance. (C) Differential activation of Notch signalling in the basal daughter following an A/B division. The cell marked by a white dot enters mitosis (A3, 1 h 24 min) following which the metaphase chromosomes undergo a characteristic rocking motion (A4–A6, 1 h 38 min–1 h 52 min). The plane of division is fixed at 1 h 52 min (A6) following which the cell enters anaphase (1 h 59 min, A7) with an A/B orientation. The notch active daughter returns towards the apical surface (A9–12). (D) Newly differentiated neurons expressing Tuj1 (green) lack Par3 and aPKC (both red) expression at the tips of the apical process. Scale bars, 10 μm. GFP, green fluorescent protein.
Notch signalling is elevated in basal daughter cells
Using a novel live reporter for Notch signalling pHes5-VNP, based on the chicken Hes5-1 promoter-driving expression of a destabilized nuclear Venus fluorescent protein coupled with the Hes5-1 3′UTR to confer instability [20] (supplementary Methods online), we and our collaborators have shown that most Notch-active cells undergoing mitosis in the chick neural tube at HH10-12 generate two Notch-active daughters, consistent with most cells remaining progenitors at this stage [20]. However, a subset of cells generated only one Notch-active daughter; this could reflect the influence of non-cell autonomous signals and/or asymmetric cell division. Here we investigated Notch signalling following induction of A/B divisions by co-transfection of pCAGGS-Insc-IRES-H2B-RFP and the pHes5-VNP reporter. This revealed that all basal daughters increased Notch signalling, whereas the apical daughter had low or no activity (a threefold increase above that in the apical daughter was detected between 7 and 10 h, n=6; the precise time of activation is influenced by level of transfected reporter plasmid; Fig 3C; supplementary Figs S4 and S5 online; supplementary Movie S7 online). This differential activation indicates that Notch signalling can be influenced by mitotic spindle orientation.
Loss of apical protein complex by apical daughter cells
Work in the mouse cortex suggests that Par3 promotes progenitor status [21] via Notch signalling [22]. Par3 mis-expression in the chick neural tube also generates a phenotype consistent with this [23]. These observations appear to contradict the asymmetric inheritance of the apical complex by the apical daughter as it then differentiates into a neuron. However, we show above that A/B polarity is rapidly re-established in basal cells that behave as progenitors and so the Par-complex is also characteristic of progenitor cells. One possibility is that apical daughters, although inheriting the majority of the Par-complex, quickly lose this as they differentiate. Tuj-1 marks cells commencing neuronal differentiation [24]. We found that such Tuj-1 cells lack both Par3 (n=76) and aPKC (n=54) at the tip of the apical process, which is withdrawn as differentiation commences [9] (Fig 3D). This suggests that rapid loss of the apical protein complex is part of the neuronal differentiation mechanism.
Discussion
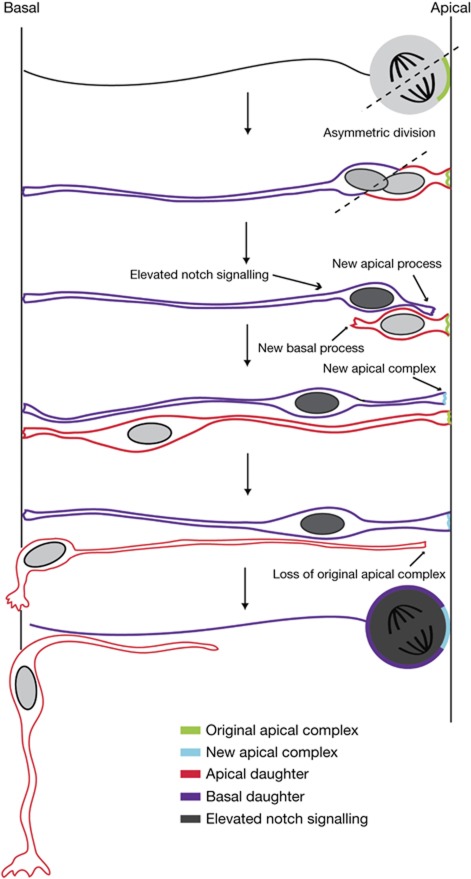
We show here that induction of A/B divisions generates an increase in neuron numbers, and by carrying out direct clonal analysis and real-time monitoring of individual cells we demonstrate that A/B divisions generate a neuron and a progenitor. We conclude that mitotic spindle orientation can influence cell fate choice in the chick neural tube (summarized in Fig 4). This contrasts with a previous study in this tissue, in which mitotic spindle position was randomised and no change in cell fates was detected [12]. We reconcile this by demonstrating that randomization of mitotic spindle position can elicit a slight increase in neurons, consistent with the differing incidence of A/B divisions generated by these two approaches. These different findings might also be explained by use of a different technical approach, retrospective clonal analysis dependent on a co-transfection strategy [12].
Figure 4.
Cell behaviour during neurogenesis in the chick neural tube. Schematic of key steps underlying neural stem cell behaviour in the chick neural tube. Asymmetric divisions generate daughter cells inheriting apical (red) or basal (purple) cell poles. On division, the apical daughter inherits the original apical complex (green) and the basal daughter inherits the basal process. The basal daughter then makes new apical complex proteins (light blue), extends a new apical process and elevates Notch signalling (dark grey). The apical daughter generates a new basal process, but downregulates apical proteins, evident as it commences apical process withdrawal during neuronal differentiation. The notch-active basal daughter cell, which retains apico-basal polarity, now divides again.
Randomizing mitotic spindle orientation or inducing A/B divisions also generates ectopic neural progenitor cells ([7, 12] and this study) and we find that these cells possess A/B polarity. By monitoring the behaviour of individual Insc-expressing cells in real-time, we further identify the origin of these ectopic progenitors as displaced basal daughters that fail to re-contact the apical surface (supplementary Fig S4A online). The finding that on division Par3 largely localizes in the apical daughter further suggests that re-polarization of the basal cell may involve synthesis of new apical complex proteins as well as re-localization of any inherited proteins. Our observations of normal neurogenesis reveal that moderately non-perpendicular cleavage planes can generate daughter cells with asymmetric inheritance of the Par-complex and that basal daughter cells re-grow an apical process. This strongly suggests that re-establishment of apical polarity is a normal neurogenesis step (Fig 4), in keeping with observations in the zebrafish [10].
In the vertebrate cortex, the prevailing hypothesis identifies apical properties as critical determinants of the self-renewing progenitor cell state, reviewed in Gotz and Huttner [19]. However, there is now evidence that possession of both apical and basal components correlates with progenitor status in the cortex [7, 8] and in the neural tube ([10], this study). Furthermore, cells that inherit only apical components tend to exit the cell cycle in the cortex [7] and become neurons in fish [10] and here in the chick neural tube. In the cortex, the incidence of differential apical process inheritance is reported to be insufficient to account for cortical neurogenesis [7]. However, given the rapid regulation of A/B polarity in basal daughter cells observed by live imaging, here and in the zebrafish [10], the incidence of this event may have been under-estimated.
Our data and that of Alexandre et al [10] and Konno et al [7] point to inheritance of the original basal process as the identifier of the progenitor daughter. Although there is no clear correlation between bisection of the basal process and adoption of symmetrical cell fates in cortex or spinal cord [14, 25, 26], the basal process of radial glial cells, which appear at later stages in the cortex, is clearly inherited by the daughter cell that continues as a progenitor [27, 28]. This is consistent with observations in the neural tube and supports a requirement for basal process inheritance for maintenance of this established neural stem cell.
It is clear that mitotic spindle orientation is just one influence on cell fate choice and that signalling via Notch and other pathways also directs this process. This study shows, for the first time, that Notch signalling is differentially activated following induction of an A/B division in the live neuroepithelium, suggesting that mitotic spindle orientation influences Notch activity during normal neurogenesis. The systematic detection of elevated Notch activity in the basal daughter also confirmed the progenitor status of this cell and strongly suggests that asymmetric division results in a cell intrinsic difference in the ability of sibling cells to respond to Notch signalling; although it is also formally possible that asymmetric partition of cellular components leads to changes that affect the ability of neighbouring cells to deliver Notch signalling.
These data are consistent with the finding that the ability of Par3 to promote the neural progenitor cell state depends on Notch signalling [22]. These authors and others [21, 23] conclude that inheritance of Par3 promotes progenitor cell fate, but it is likely that the cellular context in which Par3 is expressed determines its action. We show that while the basal daughter expresses Par3 and exhibits Notch activity, the apical daughter cell inherits Par3 but lacks Notch signalling. However, we also find that the apical daughter rapidly downregulates Par3 as neuronal differentiation commences. Retraction of the apical end-foot by differentiating neurons occurs in spinal cord and cortex [9, 29] and must involve detachment from abutting apical end-feet of neighbouring cells. Indeed, this loss of the apical protein complex may facilitate release from the luminal surface; a critical physical requirement for normal neuronal differentiation. In the future, it will be important to investigate the mechanisms underlying re-establishment of A/B polarity in neural progenitors and the loss of Par-complex proteins as cells embark on neuronal differentiation.
Methods
In ovo electroporation and plasmids. Spinal cords were transfected using standard conditions. GFP-Insc (pCAGGS-GFP-Insc) was a kind gift of Domingos Henrique. The Ct-cLGN-IRES-GFP-GPI construct was kindly provided by Xavier Morin. See supplementary Methods online for further detail.
Immunofluorescence. Immuncytochemistry was performed on a 20-μm thick cryosections following standard procedures (supplementary Methods online).
Embryo slice culture and time-lapse imaging. Slices were prepared, cultured and imaged as previously described [9, 30] (supplementary Methods online).
Clonal analysis. Single cells were labelled and their lineages traced using the photo-convertible fluorescent protein KiKGR (MBL International; supplementary Methods online).
Angle measurements. Cleavage plane orientations were measured in anaphase and telophase cells (after [9]; supplementary Methods online).
Statistical analysis. All cell counts involving percentages of GFP-positive cells expressing markers were compared using unpaired t-tests; electroporated versus unelectroporated sides were assessed using paired t-tests.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Acknowledgments
We thank Jason R. Swedlow for technical advice and D. Henrique, F. Vilas-Boas and R. Fior for the Notch reporter construct, critical discussion and comments on the manuscript. This study was supported by a Wellcome Trust programme grant no. 083611/Z/07/Z to K.G.S. and Prof. Swedlow.
Author contributions: K.G.S. conceived the project. K.G.S. and R.M.D. designed experiments. R.M.D. carried out all the experiments. K.G.S. and R.M.D. analysed the data and wrote the manuscript.
Footnotes
The authors declare that they have no conflict of interest.
References
- Kosodo Y, Roper K, Haubensak W, Marzesco AM, Corbeil D, Huttner WB (2004) Asymmetric distribution of the apical plasma membrane during neurogenic divisions of mammalian neuroepithelial cells. EMBO J 23: 2314–2324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marthiens V, ffrench-Constant C (2009) Adherens junction domains are split by asymmetric division of embryonic neural stem cells. EMBO Rep 10: 515–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Tsai J-W, Imai JH, Lian W-N, Vallee RB, Shi S-H (2009) Asymmetric centrosome inheritance maintains neural progenitors in the neocortex. Nature 461: 947–955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappello S et al. (2006) The Rho-GTPase cdc42 regulates neural progenitor fate at the apical surface. Nat Neurosci 9: 1099–1107 [DOI] [PubMed] [Google Scholar]
- Chenn A, McConnell SK (1995) Cleavage orientation and the asymmetric inheritance of notchl immunoreactivity in mammalian neurogenesis. Cell 82: 631–641 [DOI] [PubMed] [Google Scholar]
- Postiglione MP, Jüschke C, Xie Y, Haas GA, Charalambous C, Knoblich JA (2011) Mouse inscuteable induces apical-basal spindle orientation to facilitate intermediate progenitor generation in the developing neocortex. Neuron 72: 269–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konno D, Shioi G, Shitamukai A, Mori A, Kiyonari H, Miyata T, Matsuzaki F (2008) Neuroepithelial progenitors undergo LGN-dependent planar divisions to maintain self-renewability during mammalian neurogenesis. Nat Cell Biol 10: 93–101 [DOI] [PubMed] [Google Scholar]
- Shioi G, Konno D, Shitamukai A, Matsuzaki F (2009) Structural basis for self-renewal of neural progenitors in cortical neurogenesis. Cerebral Cortex 19: i55–i61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcock AC, Swedlow JR, Storey KG (2007) Mitotic spindle orientation distinguishes stem cell and terminal modes of neuron production in the early spinal cord. Development 134: 1943–1954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandre P, Reugels AM, Barker D, Blanc E, Clarke JDW (2010) Neurons derive from the more apical daughter in asymmetric divisions in the zebrafish neural tube. Nat Neurosci 13: 673–679 [DOI] [PubMed] [Google Scholar]
- Bertrand N, Castro DS, Guillemot F (2002) Proneural genes and the specification of neural cell types. Nat Rev Neurosci 3: 517–530 [DOI] [PubMed] [Google Scholar]
- Morin X, Jaouen F, Durbec P (2007) Control of planar divisions by the G-protein regulator LGN maintains progenitors in the chick neuroepithelium. Nat Neurosci 10: 1440–1448 [DOI] [PubMed] [Google Scholar]
- Pierfelice T, Alberi L, Gaiano N (2011) Notch in the vertebrate nervous system: an old dog with new tricks. Neuron 69: 840–855 [DOI] [PubMed] [Google Scholar]
- Yoon K, Gaiano N (2005) Notch signaling in the mammalian central nervous system: insights from mouse mutants. Nat Neurosci 8: 709–715 [DOI] [PubMed] [Google Scholar]
- Kraut R, Chia W, Jan LY, Jan YN, Knoblich JA (1996) Role of inscuteable in orienting asymmetric cell divisions in Drosophila. Nature 383: 50–55 [DOI] [PubMed] [Google Scholar]
- Zhong W, Chia W (2008) Neurogenesis and asymmetric cell division. Curr Opin Neurobiol 18: 4–11 [DOI] [PubMed] [Google Scholar]
- Zigman M et al. (2005) Mammalian inscuteable regulates spindle orientation and cell fate in the developing retina. Neuron 48: 539–545 [DOI] [PubMed] [Google Scholar]
- Martin-Belmonte F, Perez-Moreno M (2012) Epithelial cell polarity, stem cells and cancer. Nat Rev Cancer 12: 23–38 [DOI] [PubMed] [Google Scholar]
- Gotz M, Huttner WB (2005) The cell biology of neurogenesis. Nat Rev Mol Cell Biol 6: 777–788 [DOI] [PubMed] [Google Scholar]
- Vilas-Boas F, Fior R, Swedlow J, Storey K, Henrique D (2011) A novel reporter of notch signalling indicates regulated and random notch activation during vertebrate neurogenesis. BMC Biol 9: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa MR, Wen G, Lepier A, Schroeder T, Götz M (2008) Par-complex proteins promote proliferative progenitor divisions in the developing mouse cerebral cortex. Development 135: 11–22 [DOI] [PubMed] [Google Scholar]
- Bultje RS, Castaneda-Castellanos DR, Jan LY, Jan Y-N, Kriegstein AR, Shi S-H (2009) Mammalian Par3 regulates progenitor cell asymmetric division via notch signaling in the developing neocortex. Neuron 63: 189–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afonso C, Henrique D (2006) PAR3 acts as a molecular organizer to define the apical domain of chick neuroepithelial cells. J Cell Sci 119: 4293–4304 [DOI] [PubMed] [Google Scholar]
- Menezes J, Luskin M (1994) Expression of neuron-specific tubulin defines a novel population in the proliferative layers of the developing telencephalon. J Neurosci 14: 5399–5416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosodo Y et al. (2008) Cytokinesis of neuroepithelial cells can divide their basal process before anaphase. EMBO J 27: 3151–3163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shitamukai A, Konno D, Matsuzaki F (2011) Oblique radial glial divisions in the developing mouse neocortex induce self-renewing progenitors outside the germinal zone that resemble primate outer subventricular zone progenitors. J Neurosci 31: 3683–3695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishell G, Kriegstein AR (2003) Neurons from radial glia: the consequences of asymmetric inheritance. Curr Opin Neurobiol 13: 34–41 [DOI] [PubMed] [Google Scholar]
- Miyata T, Kawaguchi A, Okano H, Ogawa M (2001) Asymmetric inheritance of radial glial fibers by cortical neurons. Neuron 31: 727–741 [DOI] [PubMed] [Google Scholar]
- Ochiai W, Minobe S, Ogawa M, Miyata T (2007) Transformation of pin-like ventricular zone cells into cortical neurons. Neurosci Res 57: 326–329 [DOI] [PubMed] [Google Scholar]
- Das RM, Wilcock AC, Swedlow J, Storey KG (2012) High-resolution live imaging of cell behaviour in the developing neuroepithelium. J Vis Exp (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.