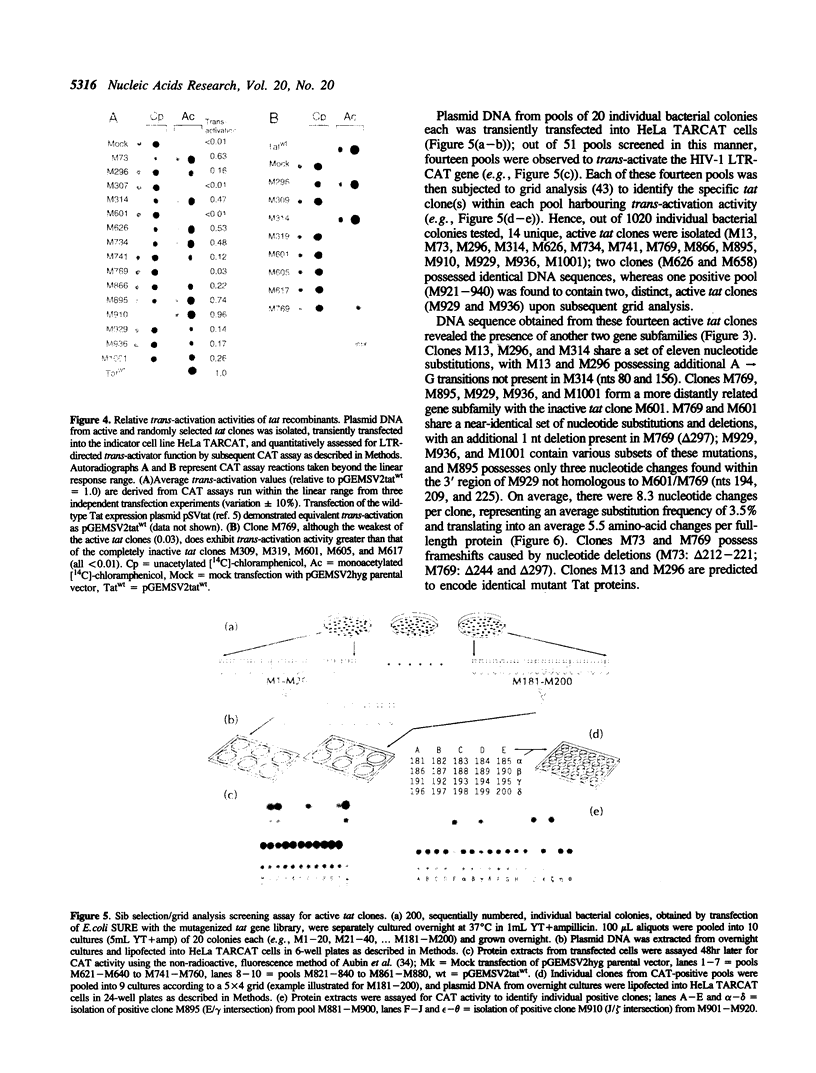
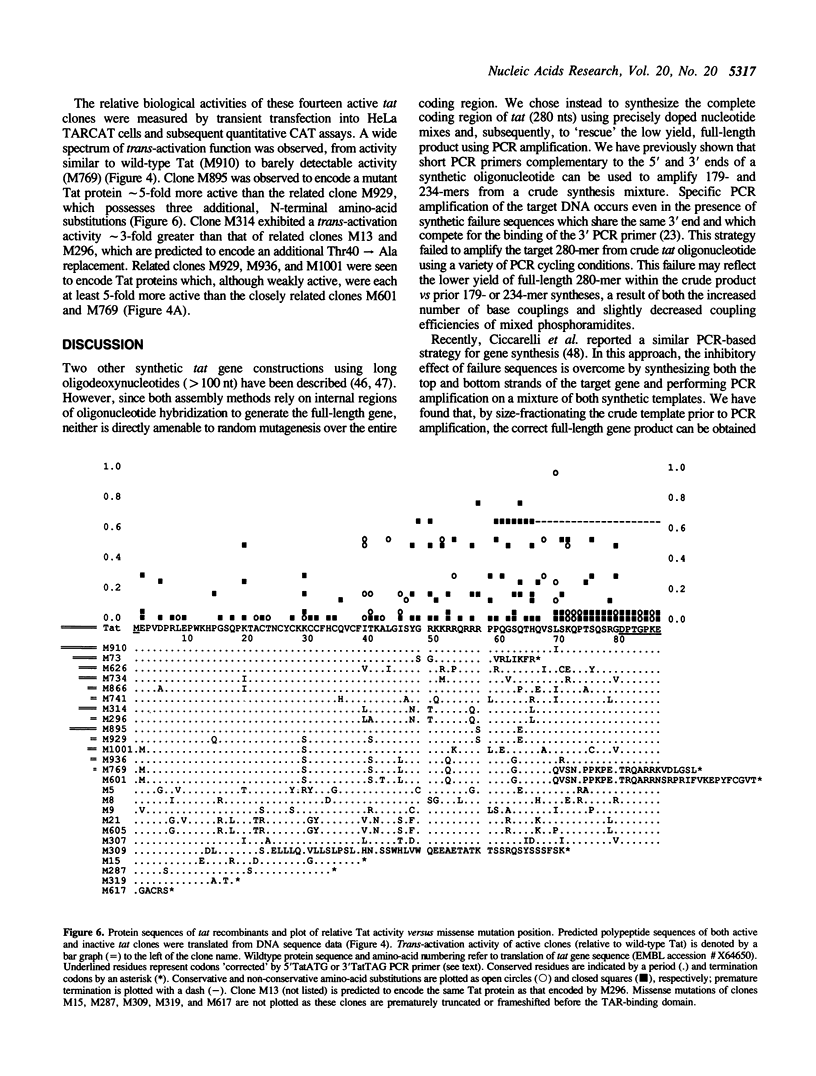
Abstract
A new method is described for the direct construction of randomly mutagenized genes by applying the polymerase chain reaction (PCR) to an oligonucleotide synthesized using doped nucleotide reservoirs. We have demonstrated the utility of this method by generating a library of mutant HIV-1 tat genes. Several arbitrarily selected, inactive tat clones were sequenced to evaluate the extent of the mutagenesis. Moreover, fourteen recombinants encoding varying levels of transcriptional trans-activator activity were isolated by transient transfection of sub-library pools into a HeLa cell line bearing an HIV-LTR-chloramphenicol acetyltransferase (CAT) reporter gene. Sequence data revealed a spectrum of alterations including nucleotide substitutions, insertions, and deletions, suggesting that mutations arose from both the doped DNA synthesis and the subsequent PCR 'rescue' of full-length product. Sequence comparison between inactive and active Tat clones revealed a selection pressure against amino-acid substitutions within the N-terminal domains of Tat, indicating the importance of this region to trans-activation competence. In addition, single and double missense mutations within the basic-rich, TAR RNA-binding domain were seen to be tolerated within active Tat clones.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams S. E., Johnson I. D., Braddock M., Kingsman A. J., Kingsman S. M., Edwards R. M. Synthesis of a gene for the HIV transactivator protein TAT by a novel single stranded approach involving in vivo gap repair. Nucleic Acids Res. 1988 May 25;16(10):4287–4298. doi: 10.1093/nar/16.10.4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubin R., Fourney R., Weinfeld M., Paterson M. C. Rapid radiolabel-sparing thin-layer chromatography method for the visual assessment of chloramphenicol acetyltransferase gene expression. Nucleic Acids Res. 1987 Dec 10;15(23):10069–10069. doi: 10.1093/nar/15.23.10069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett R. W., Erfle H. Rapid generation of DNA fragments by PCR amplification of crude, synthetic oligonucleotides. Nucleic Acids Res. 1990 May 25;18(10):3094–3094. doi: 10.1093/nar/18.10.3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Caruthers M. H., Barone A. D., Beaucage S. L., Dodds D. R., Fisher E. F., McBride L. J., Matteucci M., Stabinsky Z., Tang J. Y. Chemical synthesis of deoxyoligonucleotides by the phosphoramidite method. Methods Enzymol. 1987;154:287–313. doi: 10.1016/0076-6879(87)54081-2. [DOI] [PubMed] [Google Scholar]
- Ciccarelli R. B., Gunyuzlu P., Huang J., Scott C., Oakes F. T. Construction of synthetic genes using PCR after automated DNA synthesis of their entire top and bottom strands. Nucleic Acids Res. 1991 Nov 11;19(21):6007–6013. doi: 10.1093/nar/19.21.6007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccarelli R. B., Loomis L. A., McCoon P. E., Holzschu D. L. Insertional gene synthesis, a novel method of assembling consecutive DNA sequences within specific sites in plasmids. Construction of the HIV-1 tat gene. Nucleic Acids Res. 1990 Mar 11;18(5):1243–1248. doi: 10.1093/nar/18.5.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen B. R. Trans-activation of human immunodeficiency virus occurs via a bimodal mechanism. Cell. 1986 Sep 26;46(7):973–982. doi: 10.1016/0092-8674(86)90696-3. [DOI] [PubMed] [Google Scholar]
- Dayton A. I., Sodroski J. G., Rosen C. A., Goh W. C., Haseltine W. A. The trans-activator gene of the human T cell lymphotropic virus type III is required for replication. Cell. 1986 Mar 28;44(6):941–947. doi: 10.1016/0092-8674(86)90017-6. [DOI] [PubMed] [Google Scholar]
- Delling U., Roy S., Sumner-Smith M., Barnett R., Reid L., Rosen C. A., Sonenberg N. The number of positively charged amino acids in the basic domain of Tat is critical for trans-activation and complex formation with TAR RNA. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6234–6238. doi: 10.1073/pnas.88.14.6234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derse D., Carvalho M., Carroll R., Peterlin B. M. A minimal lentivirus Tat. J Virol. 1991 Dec;65(12):7012–7015. doi: 10.1128/jvi.65.12.7012-7015.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz J. J., Rhoads D. D., Roufa D. J. PCR-mediated chemical mutagenesis of cloned duplex DNAs. Biotechniques. 1991 Aug;11(2):204-6, 208, 210-1. [PubMed] [Google Scholar]
- Dorn P., DaSilva L., Martarano L., Derse D. Equine infectious anemia virus tat: insights into the structure, function, and evolution of lentivirus trans-activator proteins. J Virol. 1990 Apr;64(4):1616–1624. doi: 10.1128/jvi.64.4.1616-1624.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eadie J. S., Davidson D. S. Guanine modification during chemical DNA synthesis. Nucleic Acids Res. 1987 Oct 26;15(20):8333–8349. doi: 10.1093/nar/15.20.8333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert K. A., Kunkel T. A. DNA polymerase fidelity and the polymerase chain reaction. PCR Methods Appl. 1991 Aug;1(1):17–24. doi: 10.1101/gr.1.1.17. [DOI] [PubMed] [Google Scholar]
- Felgner P. L., Gadek T. R., Holm M., Roman R., Chan H. W., Wenz M., Northrop J. P., Ringold G. M., Danielsen M. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher A. G., Feinberg M. B., Josephs S. F., Harper M. E., Marselle L. M., Reyes G., Gonda M. A., Aldovini A., Debouk C., Gallo R. C. The trans-activator gene of HTLV-III is essential for virus replication. 1986 Mar 27-Apr 2Nature. 320(6060):367–371. doi: 10.1038/320367a0. [DOI] [PubMed] [Google Scholar]
- Garcia J. A., Harrich D., Pearson L., Mitsuyasu R., Gaynor R. B. Functional domains required for tat-induced transcriptional activation of the HIV-1 long terminal repeat. EMBO J. 1988 Oct;7(10):3143–3147. doi: 10.1002/j.1460-2075.1988.tb03181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz B. H., DiMaio D. Saturation mutagenesis using mixed oligonucleotides and M13 templates containing uracil. Methods Enzymol. 1990;185:599–611. doi: 10.1016/0076-6879(90)85047-r. [DOI] [PubMed] [Google Scholar]
- Hutchison C. A., 3rd, Swanstrom R., Loeb D. D. Complete mutagenesis of protein coding domains. Methods Enzymol. 1991;202:356–390. doi: 10.1016/0076-6879(91)02019-6. [DOI] [PubMed] [Google Scholar]
- Kamine J., Loewenstein P., Green M. Mapping of HIV-1 Tat protein sequences required for binding to Tar RNA. Virology. 1991 Jun;182(2):570–577. doi: 10.1016/0042-6822(91)90598-6. [DOI] [PubMed] [Google Scholar]
- Kozak M. An analysis of 5'-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987 Oct 26;15(20):8125–8148. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuppuswamy M., Subramanian T., Srinivasan A., Chinnadurai G. Multiple functional domains of Tat, the trans-activator of HIV-1, defined by mutational analysis. Nucleic Acids Res. 1989 May 11;17(9):3551–3561. doi: 10.1093/nar/17.9.3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick M. Sib selection. Methods Enzymol. 1987;151:445–449. doi: 10.1016/s0076-6879(87)51036-9. [DOI] [PubMed] [Google Scholar]
- Meyerhans A., Cheynier R., Albert J., Seth M., Kwok S., Sninsky J., Morfeldt-Månson L., Asjö B., Wain-Hobson S. Temporal fluctuations in HIV quasispecies in vivo are not reflected by sequential HIV isolations. Cell. 1989 Sep 8;58(5):901–910. doi: 10.1016/0092-8674(89)90942-2. [DOI] [PubMed] [Google Scholar]
- Meyerhans A., Vartanian J. P., Wain-Hobson S. DNA recombination during PCR. Nucleic Acids Res. 1990 Apr 11;18(7):1687–1691. doi: 10.1093/nar/18.7.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P. J., Tjian R. Transcriptional regulation in mammalian cells by sequence-specific DNA binding proteins. Science. 1989 Jul 28;245(4916):371–378. doi: 10.1126/science.2667136. [DOI] [PubMed] [Google Scholar]
- Muesing M. A., Smith D. H., Capon D. J. Regulation of mRNA accumulation by a human immunodeficiency virus trans-activator protein. Cell. 1987 Feb 27;48(4):691–701. doi: 10.1016/0092-8674(87)90247-9. [DOI] [PubMed] [Google Scholar]
- Rappaport J., Lee S. J., Khalili K., Wong-Staal F. The acidic amino-terminal region of the HIV-1 Tat protein constitutes an essential activating domain. New Biol. 1989 Oct;1(1):101–110. [PubMed] [Google Scholar]
- Rice A. P., Carlotti F. Mutational analysis of the conserved cysteine-rich region of the human immunodeficiency virus type 1 Tat protein. J Virol. 1990 Apr;64(4):1864–1868. doi: 10.1128/jvi.64.4.1864-1868.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice A. P., Carlotti F. Structural analysis of wild-type and mutant human immunodeficiency virus type 1 Tat proteins. J Virol. 1990 Dec;64(12):6018–6026. doi: 10.1128/jvi.64.12.6018-6026.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen C. A. Regulation of HIV gene expression by RNA-protein interactions. Trends Genet. 1991 Jan;7(1):9–14. doi: 10.1016/0168-9525(91)90015-i. [DOI] [PubMed] [Google Scholar]
- Rosen C. A., Sodroski J. G., Haseltine W. A. The location of cis-acting regulatory sequences in the human T cell lymphotropic virus type III (HTLV-III/LAV) long terminal repeat. Cell. 1985 Jul;41(3):813–823. doi: 10.1016/s0092-8674(85)80062-3. [DOI] [PubMed] [Google Scholar]
- Ruben S., Perkins A., Purcell R., Joung K., Sia R., Burghoff R., Haseltine W. A., Rosen C. A. Structural and functional characterization of human immunodeficiency virus tat protein. J Virol. 1989 Jan;63(1):1–8. doi: 10.1128/jvi.63.1.1-8.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadaie M. R., Rappaport J., Benter T., Josephs S. F., Willis R., Wong-Staal F. Missense mutations in an infectious human immunodeficiency viral genome: functional mapping of tat and identification of the rev splice acceptor. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9224–9228. doi: 10.1073/pnas.85.23.9224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Smith M. In vitro mutagenesis. Annu Rev Genet. 1985;19:423–462. doi: 10.1146/annurev.ge.19.120185.002231. [DOI] [PubMed] [Google Scholar]
- Sodroski J., Patarca R., Rosen C., Wong-Staal F., Haseltine W. Location of the trans-activating region on the genome of human T-cell lymphotropic virus type III. Science. 1985 Jul 5;229(4708):74–77. doi: 10.1126/science.2990041. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Southgate C., Zapp M. L., Green M. R. Activation of transcription by HIV-1 Tat protein tethered to nascent RNA through another protein. Nature. 1990 Jun 14;345(6276):640–642. doi: 10.1038/345640a0. [DOI] [PubMed] [Google Scholar]
- Sumner-Smith M., Roy S., Barnett R., Reid L. S., Kuperman R., Delling U., Sonenberg N. Critical chemical features in trans-acting-responsive RNA are required for interaction with human immunodeficiency virus type 1 Tat protein. J Virol. 1991 Oct;65(10):5196–5202. doi: 10.1128/jvi.65.10.5196-5202.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiley L. S., Brown P. H., Cullen B. R. Does the human immunodeficiency virus Tat trans-activator contain a discrete activation domain? Virology. 1990 Oct;178(2):560–567. doi: 10.1016/0042-6822(90)90354-t. [DOI] [PubMed] [Google Scholar]
- Tymms M. J., McInnes B., Alin P., Linnane A. W., Cheetham B. F. Structure-function studies of interferon-alpha based on random mutagenesis and expression in vitro. Genet Anal Tech Appl. 1990 May;7(3):53–63. doi: 10.1016/0735-0651(90)90041-d. [DOI] [PubMed] [Google Scholar]
- Wosnick M. A., Barnett R. W., Carlson J. E. Total chemical synthesis and expression in Escherichia coli of a maize glutathione-transferase (GST) gene. Gene. 1989 Mar 15;76(1):153–160. doi: 10.1016/0378-1119(89)90017-6. [DOI] [PubMed] [Google Scholar]
- Wosnick M. A., Barnett R. W., Vicentini A. M., Erfle H., Elliott R., Sumner-Smith M., Mantei N., Davies R. W. Rapid construction of large synthetic genes: total chemical synthesis of two different versions of the bovine prochymosin gene. Gene. 1987;60(1):115–127. doi: 10.1016/0378-1119(87)90219-8. [DOI] [PubMed] [Google Scholar]
- Zalacain M., González A., Guerrero M. C., Mattaliano R. J., Malpartida F., Jiménez A. Nucleotide sequence of the hygromycin B phosphotransferase gene from Streptomyces hygroscopicus. Nucleic Acids Res. 1986 Feb 25;14(4):1565–1581. doi: 10.1093/nar/14.4.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]






