Abstract
Objective
To describe the incidence of post-injury multiple organ failure (MOF) and its relationship to nosocomial infection and mortality in trauma centers employing evidence-based standard operating procedures (SOPs).
Design
Prospective cohort study wherein SOPs were developed and implemented to optimize post-injury care.
Setting
Seven U.S. Level I trauma centers.
Patients
Severely injured patients (> 16 years old) with a blunt mechanism, systolic hypotension (< 90 mmHg) and/or base deficit (> 6 meq/L), need for blood transfusion within the first 12 hrs, and an abbreviated injury score (AIS) ≥ two excluding brain injury were eligible for inclusion.
Measurements and Main Results
1,002 patients were enrolled and 916 met inclusion criteria. Daily markers of organ dysfunction were prospectively recorded for all patients while receiving intensive care. Overall, 29% of patients developed MOF. Development of MOF was early (median time of two days), short - lived, and predicted an increased incidence of NI, whereas, persistence of MOF predicted mortality. However, surprisingly, NI did not increase subsequent MOF and there was no evidence of a “second-hit” induced late onset MOF.
Conclusions
MOF remains common after severe injury. Contrary to current paradigms, the onset is only early, and not bimodal, nor is it associated with a “second-hit” induced late onset. MOF is associated with subsequent NI and increased mortality. SOP-driven interventions may be associated with a decrease in late MOF and morbidity.
Keywords: trauma, injury, standards of care, infection, multiple organ failure, mortality
INTRODUCTION
Multiple organ failure (MOF) was first described as a sequela to shock and infection over 30 years ago by Baue with the name first coined by Eiseman (1, 2). Modern resuscitation techniques developed in the 1960’s led to improved immediate survival. However, late death increased as a result of the emergence of organ failure (3). MOF onset had a recognized bimodal temporal pattern frequently linked to a “second-hit” inciting event, such as nosocomial infection (NI). New treatment paradigms were directed at rapidly reversing physiologic abnormalities and reducing possible secondary insults. Among these were low tidal volume ventilation protocols to minimize the consequences of acute respiratory distress syndrome (ARDS), appropriate resuscitation guidelines to avoid overly aggressive crystalloid resuscitation, higher trigger (lower hemoglobin levels) prior to autologous red blood cell transfusion, as well as the use of glycemic control regimens to decrease post-operative infection rates (4-10). Although these standard operating procedures (SOPs) are associated with an improvement in mortality, the impact of these SOPs on MOF after severe injury is currently unknown (11).
The ’Inflammation and Host Response to Injury’ Large Scale Collaborative Project is a National Institute of General Medical Sciences (NIGMS)-funded collaborative study aimed at identifying the genomic and proteomic responses to injury associated with different clinical outcomes after trauma. As part of this multi-center clinical investigation, participating institutions developed and adopted standard operating procedures (SOP’s) of best practices for the management of severely injured patients to optimize outcome and minimize differences in care between centers. This paper describes the incidence of MOF after severe blunt trauma and its relationship to nosocomial infection and mortality in trauma centers employing evidence-based standard operating procedures.
MATERIALS AND METHODS
This study is a primary analysis of data derived from an ongoing multi-center prospective cohort study. Standard operating procedures were developed and implemented across all seven Level I Trauma centers to minimize variation in post-injury care, including early goal-directed resuscitation, glycemic control, venous thrombo-embolism prophylaxis, appropriate low tidal volume ventilation, ventilator-associated pneumonia management, and restrictive transfusion guidelines (4-10, 12). Patients admitted to one of the seven institutions over a four year period (November, 2003 through September, 2007) were included in the analysis. Cohort inclusion criteria included blunt mechanism of injury, presence of pre-hospital or emergency department systolic hypotension (< 90 mmHg) or an elevated base deficit (> 6 meq/L) within 30 minutes of arrival, a blood transfusion requirement within the first 12 hrs after injury, and any body region exclusive of the brain with an abbreviated injury score (AIS) ≥ 2, allowing exclusion of patients with isolated traumatic brain injury. Patients less than 16 or greater than 90 years of age, and those with cervical spinal cord injury, were also excluded. The study was approved by the Institutional Review Board (IRB) of each institution. In addition to local institutional oversight, the program was reviewed and approved by the IRB at Massachusetts General Hospital.
Clinical data were de-identified, entered and stored in a web-based data collection platform (TrialDB) by trained research nurses. Integrity of the data was maintained through ongoing curation and external data review by an independent chart abstractor. Patients were admitted to the intensive care units and daily markers of organ dysfunction were prospectively recorded. These included PaO2/FiO2 ratios for patients requiring ventilator support, serum creatinine levels (mg/dl), total bilirubin levels (mg/dl), pressure-adjusted heart rate, level of cardiac inotrope support, platelet count (platelets/ml 10−3) and Glasgow Coma Score (GCS). These parameters allowed computation of the Marshall multiple organ dysfunction scores (MODS) for respiratory, renal, hepatic, cardiovascular, hematologic, and neurological systems to be determined up to 28 days while in the ICU (13). Using the Marshall scoring system, the diagnosis of MOF was defined as patients who had two or more consecutive days with a MODS score greater than or equal to six at least 48 hrs out from the time of injury. The score was calculated excluding the neurological component (as measured by GCS). The initial day of the two consecutive days with a score greater than or equal to six was considered as the onset day of MOF. Resolution was defined as two consecutive days with a score less than six. The initial day of the two consecutive days with a score less than six was considered the resolution day. Carrying forward the last observed value filled in intermittent missing data. On all days following the last observed MOF value, patients were assumed to be MOF-free and were assigned a score of zero. Patients could experience repeated episodes of MOF based upon the above definition.
The diagnosis of nosocomial infection required specific clinical criteria along with positive cultures. All time variables to the respective outcome event were determined from the day of initial injury, while the time to the first infection event was used in those patients with multiple infections. Diagnosis of ventilator-associated pneumonia (VAP) followed CDC criteria but also required a quantitative culture threshold of equal or greater than 104 CFU/ml for bronchoalveolar lavage specimens. Diagnosis of catheter-related blood stream infections required positive peripheral cultures with the identical organism obtained from either a positive semi quantitative culture (>15 CFU/segment), or positive quantitative culture (>103 CFU/segment) from a catheter segment specimen. Urinary tract infections required > 105 organisms/ml of urine. The day of first nosocomial infection was defined as the first day of any surgical site infection, pneumonia, urinary tract infection, blood stream infection, or catheter related blood stream infection occurring after study day two.
Patients who developed MOF were compared with those who did not develop MOF in a univariate fashion. Stepwise logistic regression modeling was then employed to determine independent risk factors for the development of MOF. Model covariates considered in the backward elimination process included selected patient demographics, early resuscitation requirements, injury characteristics, shock parameters, comorbidities and early operative and ICU interventions. For this analysis, missing covariate values were mean imputed.
Cox proportional odds regression was used to model the effect of MOF on mortality and nosocomial infection. Mortality and nosocomial infection were treated as two distinct outcomes and were modeled separately. All patients who remained alive in the hospital on days three through 28 were considered to be at risk for death or nosocomial infection. The first two days were excluded to avoid acute resuscitation-induced organ dysfunction. Two summary measures of MOF were used as independent variables in the Cox model. First, the sum of the MOF scores on days two and three were used as a measure of baseline MOF intensity. Second, the cumulative sum of all MOF scores from day two through each study day, inclusive, were used as a measure of long term MOF exposure. On each of study days two to 28, these two scores were used to predict death or nosocomial infection on the following day. Baseline covariates included age, sex, ISS, APACHE, head AIS > three, 0-24 hour volume of PRBCs, 0-24 hour volume of crystalloids and surgical exposure were included as potential baseline confounders in multivariate models even though not statistically different because of their expected contribution to MOF development. Surgical exposure was treated as a time varying covariate that could increase the risk of nosocomial infection (particularly surgical site infections). The surgery score was initially assigned a value of zero. A value of one was assigned starting on the first day the patient had any procedure that did not violate the bowel or did not require damage control. A value of two was assigned on the first day the patient had a damage control procedure or a procedure that violated the bowel.
SAS 9·2 (SAS Institute Inc., Cary, NC) was used for all statistical calculations. All data were summarized as mean ± standard deviation, median [inter-quartile range], or percentage (%). Student-t or Mann-Whitney statistical tests were used to compare continuous variables, while Chi-Square or Fischer’s Exact test were used for categorical variables.
RESULTS
Over the nearly four-year study period, 1,002 severely injured patients were enrolled. Eighty six patients died before post injury day two, leaving 916 patients who met the inclusion criteria. Table 1 provides demographics. The majority of patients were young, non-Hispanic, white males with a low incidence of comorbid disease processes. Median ISS was 29, median APACHE II was 30 and at least a third of the patients sustained an AIS > three grade injury in a major body compartment with an early mortality of 8·6% confirming severity of initial trauma (Table 2). Patients received a median of 10·5 L of crystalloid, approximately five units of packed cells and approximately three units of fresh frozen plasma (ratio of 1·7:1) (Table 3). Over 50% of the patients required mechanical ventilation on arrival to the emergency department, and nearly a quarter of patients were supported with vasopressor agents during the first 24 hrs.
Table 1.
Cohort Demographics (N=1,002)
| Age; median (IQR) | 40 (26, 54) |
| Sex (% male) | 65 |
| Race (% of total) | |
| White | 88.2 |
| African-American | 7.4 |
| Asian | 2.6 |
| Other | 1.8 |
| Ethnicity (% of total) | |
| Non-Hispanic | 84.6 |
| Hispanic | 12.2 |
| Unknown | 3.2 |
| Pre-Existing Comorbidity (% of total) | |
| Hypertension | 15 |
| Alcohol use | 13.4 |
| Diabetes | 7 |
| Liver disease | 4.4 |
| COPD | 3.5 |
IQR: Interquartile range; COPD: Chronic obstructive pulmonary disease
Table 2.
Injury Characteristics (N=1,002)
| Motor Vehicle Collision | 86.1 |
| Occupant | 55.2 |
| Motorcyclist | 14.3 |
| Pedestrian | 13.9 |
| Cyclist | 2.7 |
| Fall | 8.4 |
| Machinery | 1.8 |
| Assault | 0.9 |
| Other | 2.9 |
| Injury Severity | |
| AIS > 3 (% of total) | |
| Head | 34.3 |
| Abdomen | 43.2 |
| Thorax | 62.5 |
| Extremity | 61.8 |
| ISS; median (IQR) | 29 (22, 41) |
| Admission Motor GCS; median (IQR) | 3 (1, 6) |
| Admission APACHE II; median (IQR) | 30 (25, 34) |
| Initial ED SBP; mean (+ SD) | 111 (32) |
| Worst ED SBP; mean (+ SD) | 84 (25) |
| Time: injury to arrival (hours); median (IQR) | 1.3 (0.8, 1.6) |
AIS: abbreviated injury scale; ISS: injury severity score; GCS: Glasgow coma scale; APACHE: acute physiology and chronic health evaluation; ED: emergency department; SBP: systolic blood pressure; SD: standard deviation; IQR: interquartile range
Table 3.
Treatment Characteristics [N=1,002, all data: median (IQR) unless noted]
| Resuscitation Parameters | |
| Crystalloid (liters) | |
| 0 – 12 hours | 10.5 (7.6, 15.0) |
| >12 - 24 hours | 2.4 (1.6, 4.5) |
| PRBC (liters) | |
| 0 – 12 hours | 1.8 (1.0, 3.5) |
| FFP (milliliters) | |
| 0 – 12 hours | 700 (0, 1750) |
| Worst Base Deficit | |
| 0 – 12 hours (n=988) | 10 (7, 14) |
| >12 – 24 hours (n=825) | 2 (0, 6) |
| Worst Lactate | |
| 0 – 12 hours (n=877) | 5.1 (3.6, 7.3) |
| >12 – 24 hours (n=616) | 3.0 (2.0, 4.7) |
| ED Mechanical Ventilation (% of total) | 56 |
| Pressor use 0 – 24 hours (% of total) | 24 |
| Operative Procedures: 0 – 24 hours (% of total) | |
| Ortho | 19 |
| Abdominal | 43 |
| Thoracic | 8 |
| Outcome, [N (% of 916 that survived at least 48 hours)] | |
| Nosocomial Infection | 452 (49.3) |
| Multiple Organ Failure | 269 (29.4) |
| Mortality | 104 (11.3) |
IQR: interquartile range; PRBC: packed red blood cells; FFP: fresh frozen plasma; ED: emergency department
Twenty nine percent of patients developed MOF. Patients who met MOF criteria were older, male, more severely injured, and had a higher proportion of comorbidities – particularly liver disease, than those who did not meet MOF criteria (Table 4). Patients diagnosed with MOF received greater resuscitation volumes of crystalloid, packed cells and fresh frozen plasma than those who did not meet criteria. Importantly, in this severely injured population, the physiologic parameters of shock, including base deficit and lactate, revealed that those with MOF had sustained greater depths of shock that lasted for a more prolonged period than those who did not have MOF. Of the 269 patients who developed MOF, 224 patients (83%) had one episode of MOF, 39 (15%) had two episodes and six (2%) had three episodes.
Table 4.
Comparison of patients who developed MOF compared to those who never developed MOF
| MOF | No MOF | P | |
|---|---|---|---|
| Demographics | |||
| Patients N (% of total) | 269 (29.4) | 647 (70.6) | |
| Age: median (IQR) | 46 (28, 59) | 38 (25, 50) | <0.0001 |
| Sex (% male) | 77 | 59 | <0.0001 |
| Comorbidity (% of total) | |||
| Hypertension | 21.2 | 13.1 | 0.0022 |
| Diabetes | 11.5 | 5.6 | 0.0016 |
| Liver disease | 7.8 | 2.9 | 0.001 |
| Alcohol use | 15.2 | 13.9 | 0.60 |
| COPD | 5.2 | 2.9 | 0.09 |
| Injury Characteristics | |||
| AIS ≥ 3 (% of total) | |||
| Head | 36.8 | 33.1 | 0.28 |
| Abdomen | 45.7 | 40.8 | 0.17 |
| Thorax | 71.0 | 57.5 | 0.0001 |
| Extremity | 69.1 | 60.9 | 0.018 |
| ISS: median (IQR) | 41 (29, 50) | 35 (27, 43) | <0.0001 |
| Resuscitation Parameters | |||
| Crystalloid 1st 12 hours, L | 12.52 (8.76, 17.21) | 9.73 (7.22, 13.20) | <0.0001 |
| Crystalloid 12-24 hours, L | 3.34 (1.80, 6.25) | 2.20 (1.60, 4.00) | <0.0001 |
| PRBC 1st 12 hours, L | 2.80 (1.40, 4.95) | 1.40 (0.70, 2.45) | <0.0001 |
| FFP 1st 12 hours, L | 1.2 (0.40, 2.54) | 0.40 (0, 1.20) | <0.0001 |
| Vasopressor use (% of total) | 39.2 | 12.8 | <0.0001 |
| ED mechanical ventilation | 59.9 | 50.9 | 0.013 |
| Physiologic Parameters | |||
| Worst BD 1st 12 hours | 11 (15, 8.1) | 9.1 (12, 7) | <0.0001 |
| Worst BD 12-24 hours | 3.7 (6.9, 1) | 2.0 (5, 0) | <0.0001 |
| Worst lactate 1st 12 hours | 6.1 (4.4, 8.4) | 4.6 (3.3, 6.1) | <0.0001 |
| Worst lactate 12-24 hours | 3.8 (2.4, 5.7) | 2.6 (1.8, 3.9) | <0.0001 |
IQR-Interquartile range, AIS–Abbreviated Injury Score, ISS–Injury Severity Score, PRBC–packed red blood cells, FFP–fresh frozen plasma, BD–base deficit, L–liters. Unless noted, all Resuscitation and Physiologic Parameter data expressed as median (IQR)
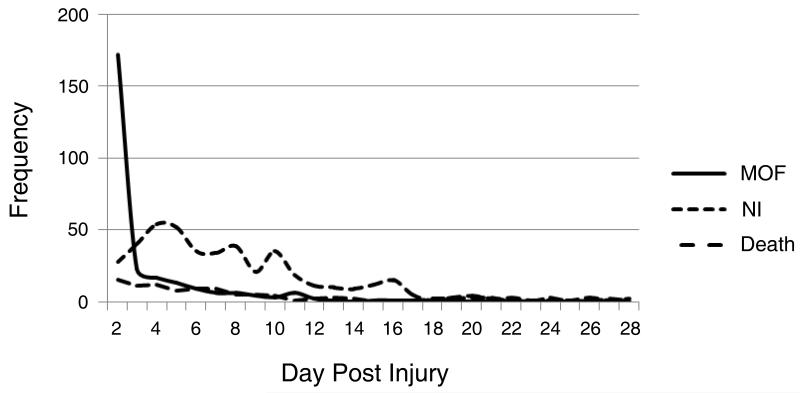
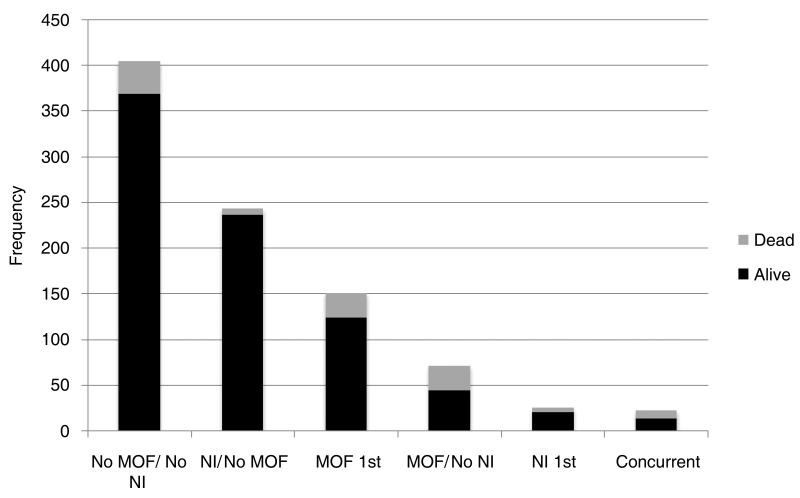
Multiple organ failure occurred early after injury [median day of first MOF diagnosis - [day two (Interquartile range (IQR): 2, 4)] and the number of new cases declined rapidly with increasing days post injury (Figure 1). Contrary to current paradigms, there was no late bimodal peak of organ failure identified. In addition, the first episode of MOF lasted a median of only four days (IQR: 2, 15). Interestingly, the median day of onset of nosocomial infection was seven (IQR: 5, 10), much later than the onset of MOF (Figure 1). While patients who developed MOF more commonly developed a subsequent nosocomial infection (75% vs. 39%, P<0·001 (Figure 2), in contrast to current belief, patients diagnosed with a nosocomial infection rarely developed subsequent MOF (Figure 2). As demonstrated in Figure 2, these findings are in contrast to the classical MOF paradigm implicating a secondary insult, such as nosocomial infection, as a contributor to MOF development.
Figure 1.
Day of onset and frequency of multiple organ failure, nosocomial infection and death. MOF – multiple organ failure; NI – nosocomial infection.
Figure 2.
Temporal relationship of nosocomial infection (NI), multiple organ dysfunction (MOF) and mortality. No MOF/No NI – patients who never experience either condition; NI/No MOF – patients who had nosocomial infection, never had MOF; MOF 1st – patients who had MOF before a diagnosis of nosocomial infection; MOF/No NI – patients who had MOF, never had nosocomial infection; NI 1st – patients who had nosocomial infection before a diagnosis of MOF; Concurrent – patients who had MOF and nosocomial infection diagnosed on the same day.
Table 5 shows that predictors of MOF included male gender, pre-existing liver disease, greater injury severity, the use of early vasopressors, higher volumes of FFP and crystalloid resuscitation, as well as more severe depth of shock (lactate values in first 12 hrs) and duration of shock (worse base deficit values in hours 12 – 24 post injury). Red cell transfusion alone was not an independent predictor of MOF; however, transfusion was an entry requirement. Center contribution was also not evaluated as an independent predictor due to the standardization in care from the implementation of the SOPs. In fact, the rate of MOF, nosocomial infection, and MOF were similar between sites. Important predictors of nosocomial infection are shown in Table 6. Early severe and prolonged MOF was associated with the subsequent development of nosocomial infection. Independent predictors of mortality (Table 7) include the presence of increased age, higher severity of injury (APACHE II and ISS scores), and blood transfusion. The presence of MOF at baseline did not predict subsequent mortality, but persistence (or non-resolution) and higher cumulative exposure of MOF was a predictive of subsequent mortality.
Table 5.
Multivariate model of predictors of multiple organ failure
| Hazard Ratio | 95% C.I. | P | |
|---|---|---|---|
| Demographics | |||
| Male gender | 2.19 | 1.51 – 3.18 | <0.0001 |
| Comorbidity | |||
| Hypertension | 2.10 | 1.34 – 3.29 | 0.0011 |
| Liver disease | 2.29 | 1.08 – 4.845 | 0.03 |
| Injury Characteristics | |||
| AIS ≥3 | |||
| Thorax | 1.67 | 1.13 – 2.46 | 0.01 |
| Extremity | 1.72 | 1.19 – 2.48 | 0.004 |
| APACHE II (per point) | 1.06 | 1.03 – 1.09 | 0.0002 |
| ISS | 1.01 | 0.99 – 1.03 | 0.075 |
| Resuscitation Parameters | |||
| Crystalloid 12-24 hours, L (per liter) | 1.09 | 1.03 – 1.15 | 0.003 |
| FFP 1st 12 hours, L (per liter) | 1.31 | 1.15 – 1.51 | <0.0001 |
| Vasopressor use | 2.09 | 1.51 – 3.18 | 0.0004 |
| Physiologic Parameters | |||
| Worst BD 12-24 hours | 0.95 | 0.91 – 0.99 | 0.02 |
| Worst lactate 1st 12 hours | 1.07 | 1.01 – 1.14 | 0.04 |
AIS: Abbreviated injury score; FFP: Fresh frozen plasma; BD: base deficit; CI: confidence interval; APACHE: Acute physiology and chronic health evaluation
Table 6.
Multivariate model of predictors of nosocomial infection after injury
| Hazard Ratio | 95% C.I. | P | |
|---|---|---|---|
| Demographics | |||
| Age (per year) | 1.00 | 0.99 – 1.01 | 0.86 |
| Gender; Female | 0.89 | 0.72 – 1.09 | 0.26 |
| Injury Characteristics | |||
| AIS ≥3 | |||
| Head | 0.82 | 0.66 – 1.01 | 0.07 |
| APACHE II (per point) | 1.01 | 0.99 – 1.03 | 0.32 |
| ISS (per point) | 1.01 | 1.00 – 1.02 | 0.003 |
| Surgery score | 0.95 | 0.79 – 1.15 | 0.60 |
| Resuscitation Parameters | |||
| RBC 1st 24 hours (per liter) | 1.035 | 0.997 - 1.075 | 0.0748 |
| Crystalloid 1st 24 hours (per liter) | 0.999 | 0.985 - 1.104 | 0.9359 |
| Outcome | |||
| Baseline MOF (per point) | 1.05 | 1.01 – 1.08 | 0.006 |
| Cumulative MOF (per point) | 1.01 | 1.00 – 1.02 | <0.001 |
AIS: Abbreviated injury scale score; APACHE: Acute physiology and chronic health evaluation score; ISS: Injury severity scale score; RBC: Red blood cell transfusion; MOF: Multiple organ failure score; CI: confidence interval
Table 7.
Multivariate model of predictors of mortality after injury
| Hazard Ratio | 95% C.I. | P | |
|---|---|---|---|
| Demographics | |||
| Age (per year) | 1.02 | 1.00 – 1.03 | 0.014 |
| Gender; Female | 1.35 | 0.83 – 2.19 | 0.233 |
| Injury Characteristics | |||
| AIS ≥3 | |||
| Head | 1.48 | 0.94 – 2.35 | 0.092 |
| APACHE II (per point) | 1.09 | 1.04 – 1.15 | <0.001 |
| ISS (per point) | 1.03 | 1.01 – 1.05 | 0.002 |
| Surgery score | 0.60 | 0.42 – 0.86 | 0.006 |
| Resuscitation Parameters | |||
| RBC 1st 24 hours (per liter) | 1.09 | 1.02 – 1.16 | 0.008 |
| Crystalloid 1st 24 hours (per liter) | 0.989 | 0.966 - 1.1012 | 0.3544 |
| Outcome | |||
| Baseline MOF (per point) | 1.03 | 0.97 – 1.10 | 0.32 |
| Cumulative MOF (per point) | 1.03 | 1.02 – 1.04 | <0.0001 |
AIS: Abbreviated injury scale score; APACHE: Acute physiology and chronic health evaluation score; ISS: Injury severity scale score; RBC: Red blood cell transfusion; MOF: Multiple organ failure score; CI: confidence interval
DISCUSSION
The results of this study in severely injured blunt trauma patients identify several unique new trends in outcome. The development of MOF remains common, and is associated with higher rates of subsequent nosocomial infection, while persistence of MOF predicted increased mortality. Unlike the currently accepted paradigm, there was a single decreasing early peak in MOF incidence without evidence of the traditional secondary late-onset bimodal peak. Additionally, while MOF predicted the development of nosocomial infection, the development of infection, as a potential “second-hit” inciting event, surprisingly, was not temporarily associated with subsequent development of MOF. This may be partly explained by a stricter definitions in VAP based on BAL quantitative cultures compared to previous reports (2,14-15). However, the strict definitions used in this study was essential given the high incidence of SIRS and underlying chest injury which would have severely increased the number of false positive pneumonias detected (7).
Similar to previous studies, increasing age and male gender are associated with an increased incidence of MOF (14-16). It is unclear if the effect of age is the result of limited physiologic reserve, comorbid conditions that alter the response to injury, a modified response to injury associated with pre-injury medications or other unknown factors (17-19). In addition, being male was associated with greater than a two-fold increased risk of developing MOF. Sperry and colleagues have shown similar results, and also an association with higher IL-6 production in males post-injury as a marker of a plausible excessive pro-inflammatory mechanism in males after injury (20).
Expectedly, injury severity characteristics have been shown to be associated with the subsequent development of MOF (16, 21). Using univariate analysis, increasing injury severity was associated with higher rates of MOF. Interestingly, however, in this severely injured cohort, physiologic markers of depth and duration of shock revealed that severe base deficit and higher lactates were even more significant predictors of MOF. Likewise, early elevated lactate levels and continued evidence of shock measured by elevated base deficit for 12 to 24 hours after injury were also independent predictors of MOF. As a potential partial explanation, these patients required more aggressive resuscitation as measured by crystalloid, blood and plasma transfusion volumes, which also correlated with MOF development, similar to prior studies (19, 22). However, since transfusion was an entry criterion for recruitment, only prolonged crystalloid resuscitation and FFP transfusion remained independent predictors of subsequent MOF.
Ciesla and colleagues previously showed that early organ dysfunction occurring within 48 hours of injury was infrequently sustained and resolved with completion of resuscitation (23). Similarly, there was an early peak in onset of MOF; with the majority of cases present on day two after injury but, unexpectedly, there followed a rapid deteriorating smooth slope of new onset cases with no late bimodal peak of new cases of MOF at six to eight days after injury, as historically described. Moore and colleagues described this second peak of MOF and attributed the second peak to deleterious pro-inflammatory consequences such as late infection; the commonly accepted “second-hit” phenomenon (24). The absence of a late peak of MOF in the current study may be due to the implementation of the SOP’s, and resolution of the underlying pathologic processes, which, while not preventing, enabled “tolerance” of subsequent infection without a secondary impact or increased mortality. As an example, increasing utilization of lung protective ventilation has been demonstrated to decrease activation of the immune system and incidence of MOF and is associated with decreased plasma inflammatory cytokine responses, and improved outcome in patients with acute lung injury or ARDS (23, 25, 26). Further support of this hypothesis is found by analyzing the relationship of organ failure and infection in this study. The incidence of nosocomial infection in this severely injured cohort approached 50%. However, while MOF was an identifiable risk factor for the development of nosocomial infection, nosocomial infection was not temporally associated with the development of MOF. It is entirely plausible that the late peak of MOF seen by Moore, while temporally associated with infection, was not caused by infection, but caused by management strategies that at the time were standard of care, but have since been shown to be injurious.
In contrast, persistence of sustained MOF is poorly tolerated and associated with a significantly increased mortality. The Canadian Critical Care Trials Group found that daily MODS scores provided additional prognostic value over the baseline score. Cabre and associates showed that a prolonged burden of MOF was associated with higher mortality, while Barie and associates found cumulative MODS scoring to be predictive of survival in an ICU setting (27-29). We accounted for two different measures of MOF; early severity of MOF was measured by the sum of the scores on days two and three after injury and the prolonged burden of MOF was measured as the cumulative (sum of) daily scores until the event in question. The two events analyzed were infection and death (after 48 hours post injury). Only cumulative MOF scores were associated with mortality in this study; the baseline MOF score was not an independent predictor of mortality as this score appeared related to physiologic factors associated with the initial injury severity.
While the results of this study are intriguing, there are limitations. First, this was an observational study and thus, only associations can be described and no cause and effect can be concluded. We implemented evidence-based SOPs in an effort to deliver and standardized best practice trauma care across centers. During the study period, SOP compliance was approximately 70% throughout the program and, compared to other benchmarks, was associated with an improvement in overall mortality.(11) However, this observational study was not designed to test prospectively whether this intervention was responsible for the outcomes. Although there appears to be a need for prospective studies to determine the effect of SOPs on present day outcomes after severe blunt trauma, based on these and previous findings, prospective validation would be unethical.
CONCLUSION
MOF after severe blunt trauma remains a major complication. In this observational study, MOF occurred early after injury, without a secondary peak post injury as has been commonly defined. The development of MOF was associated with the subsequent development of nosocomial infection and persistence of MOF predicted death; however, the development of infection, though common, was not associated with subsequent development MOF. The data document the changes in MOF pattern and implications for modern day critical care management in the form of best-practice SOP’s and requires further investigation.
Classifications: organ dysfunction, nosocomial infection, inflammation, mortality, shock, trauma, intensivist, evidence-based medicine
ACKNOWLEDGEMENTS
The participants of the Inflammation and Hospital Response to Injury Large Scale Collaborative Research Program: Lily Altstein, Ph.D., Henry V. Baker, Ph.D., Ulysses G.J. Balis, M.D., Paul E. Bankey, M.D., Ph.D., Timothy R. Billiar, M.D., Bernard H. Brownstein, Ph.D., Steven E. Calvano, Ph.D., David G. Camp II, Ph.D., J. Perren Cobb, M.D., Ronald W. Davis, Ph.D., Asit K. De, Ph.D., Celeste C. Finnerty, Ph.D., Richard L. Gamelli, M.D., Nicole S. Gibran, M.D., Laura Hennessy, R.N., David N. Herndon, M.D., Shari E. Honari, R.N., Marc G. Jeschke, M.D., Ph.D., Jeffrey L. Johnson, M.D., Matthew B. Klein, M.D., Stephen F. Lowry, M.D., Philip H. Mason, Ph.D., Grace P. McDonald-Smith, M.Ed., Bruce A. McKinley, Ph.D., Carol L. Miller-Graziano, Ph.D., Michael N. Mindrinos, Ph.D., Ernest E. Moore, M.D., Frederick A. Moore, M.D., Avery B. Nathens, M.D., Ph.D., M.P.H., Laurence G. Rahme, Ph.D., Daniel G. Remick, M.D., David A. Schoenfeld, Ph.D., Michael B. Shapiro, M.D., Richard D. Smith, Ph.D., John D. Storey, Ph.D., Robert Tibshirani, Ph.D., Mehmet Toner, Ph.D., H. Shaw Warren, M.D., Bram Wispelwey, M.S., Wenzhong Xiao, Ph.D., Wing H. Wong, Ph.D.
Source of Funding: This study was supported by the U.S. National Institutes of Health, National Institute of General Medical Sciences, Large Scale Collaborative Project, U54GM062119. The funding source had no involvement in the study design, data collection and analysis, and manuscript preparation.
Footnotes
The authors have not disclosed any potential conflicts of interest.
For information regarding this article, rtompkins@partners.org
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Joseph P. Minei, University of Texas Southwestern Medical Center and Parkland Health and Hospital System, Dallas, TX.
Joseph Cuschieri, University of Washington School of Medicine and Harborview Medical Center, Seattle, WA.
Jason Sperry, University of Pittsburgh Medical Center and Presbyterian University Hospital, Pittsburgh, PA.
Ernest E. Moore, University of Colorado Health Sciences Center and Denver Health Medical Center, Denver, CO.
Michael A. West, University of California San Francisco and San Francisco General Hospital, San Francisco, CA.
Brian G. Harbrecht, University of Louisville School of Medicine, Louisville, KY.
Grant E. O’Keefe, University of Washington School of Medicine and Harborview Medical Center, Seattle, WA.
Mitchell J. Cohen, University of California San Francisco and San Francisco General Hospital, San Francisco, CA.
Lyle L. Moldawer, University of Florida College of Medicine and Shands Hospital, Gainesville, FL.
Ronald G. Tompkins, Harvard Medical School and Massachusetts General Hospital, Boston, MA.
Ronald V. Maier, University of Washington School of Medicine and Harborview Medical Center, Seattle, WA.
REFERENCES
- 1.Baue AE. Multiple, progressive, or sequential systems failure. A syndrome of the 1970s. Arch Surg. 1975;110(7):779–781. doi: 10.1001/archsurg.1975.01360130011001. [DOI] [PubMed] [Google Scholar]
- 2.Eiseman B, Beart R, Norton L. Multiple organ failure. Surg Gynecol Obstet. 1977;144(3):323–326. [PubMed] [Google Scholar]
- 3.Acosta JA, Yang JC, Winchell RJ, et al. Lethal injuries and time to death in a level I trauma center. J Am Coll Surg. 1998;186(5):528–533. doi: 10.1016/s1072-7515(98)00082-9. [DOI] [PubMed] [Google Scholar]
- 4.Cuschieri J, Freeman B, O’Keefe G, et al. Inflammation and the host response to injury a large-scale collaborative project: patient-oriented research core standard operating procedure for clinical care X. Guidelines for venous thromboembolism prophylaxis in the trauma patient. J Trauma. 2008;65(4):944–950. doi: 10.1097/TA.0b013e3181826df7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Evans HL, Cuschieri J, Moore EE, et al. Inflammation and the host response to injury, a Large-Scale Collaborative Project: patient-oriented research core standard operating procedures for clinical care IX. Definitions for complications of clinical care of critically injured patients. J Trauma. 2009;67(2):384–388. doi: 10.1097/TA.0b013e3181ad66a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harbrecht BG, Minei JP, Shapiro MB, et al. Inflammation and the host response to injury, a large-scale collaborative project: patient-oriented research core-standard operating procedures for clinical care: VI. Blood glucose control in the critically ill trauma patient. J Trauma. 2007;63(3):703–708. doi: 10.1097/TA.0b013e31811eadea. [DOI] [PubMed] [Google Scholar]
- 7.Minei JP, Nathens AB, West M, et al. Inflammation and the Host Response to Injury, a Large-Scale Collaborative Project: patient-oriented research core--standard operating procedures for clinical care. II. Guidelines for prevention, diagnosis and treatment of ventilator-associated pneumonia (VAP) in the trauma patient. J Trauma. 2006;60(5):1106–1113. doi: 10.1097/01.ta.0000220424.34835.f1. discussion 1113. [DOI] [PubMed] [Google Scholar]
- 8.Nathens AB, Johnson JL, Minei JP, et al. Inflammation and the Host Response to Injury, a large-scale collaborative project: Patient-Oriented Research Core--standard operating procedures for clinical care. I. Guidelines for mechanical ventilation of the trauma patient. J Trauma. 2005;59(3):764–769. [PubMed] [Google Scholar]
- 9.West MA, Moore EE, Shapiro MB, et al. Inflammation and the host response to injury, a large-scale collaborative project: patient-oriented research core--standard operating procedures for clinical care VII--Guidelines for antibiotic administration in severely injured patients. J Trauma. 2008;65(6):1511–1519. doi: 10.1097/TA.0b013e318184ee35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.West MA, Shapiro MB, Nathens AB, et al. Inflammation and the host response to injury, a large-scale collaborative project: Patient-oriented research core-standard operating procedures for clinical care. IV. Guidelines for transfusion in the trauma patient. J Trauma. 2006;61(2):436–439. doi: 10.1097/01.ta.0000232517.83039.c4. [DOI] [PubMed] [Google Scholar]
- 11.Cuschieri J, Johnson J, Sperry J, et al. Benchmarking Outcomes in the Critically Injured Trauma Patient and the Effect of Implementing Standard Operating Procedures. Annals of Surgery. doi: 10.1097/SLA.0b013e31824f1ebc. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moore FA, McKinley BA, Moore EE, et al. Inflammation and the Host Response to Injury, a large-scale collaborative project: patient-oriented research core--standard operating procedures for clinical care. III. Guidelines for shock resuscitation. J Trauma. 2006;61(1):82–89. doi: 10.1097/01.ta.0000225933.08478.65. [DOI] [PubMed] [Google Scholar]
- 13.Marshall JC, Cook DJ, Christou NV, et al. Multiple organ dysfunction score: a reliable descriptor of a complex clinical outcome. Crit Care Med. 1995;23(10):1638–1652. doi: 10.1097/00003246-199510000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Cuschieri J, Bulger E, Schaeffer V, et al. Early elevation in random plasma IL-6 after severe injury is associated with development of organ failure. Shock. 34(4):346–351. doi: 10.1097/SHK.0b013e3181d8e687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sauaia A, Moore EE, Johnson JL, et al. Validation of postinjury multiple organ failure scores. Shock. 2009;31(5):438–447. doi: 10.1097/SHK.0b013e31818ba4c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sauaia A, Moore FA, Moore EE, et al. Early predictors of postinjury multiple organ failure. Arch Surg. 1994;129(1):39–45. doi: 10.1001/archsurg.1994.01420250051006. [DOI] [PubMed] [Google Scholar]
- 17.Fang JF, Chen RJ, Lin BC, et al. Liver cirrhosis: an unfavorable factor for nonoperative management of blunt splenic injury. J Trauma. 2003;54(6):1131–1136. doi: 10.1097/01.TA.0000066123.32997.BB. discussion 1136. [DOI] [PubMed] [Google Scholar]
- 18.Sakr Y, Vincent JL, Ruokonen E, et al. Sepsis and organ system failure are major determinants of post-intensive care unit mortality. J Crit Care. 2008;23(4):475–483. doi: 10.1016/j.jcrc.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 19.Tran DD, Cuesta MA, van Leeuwen PA, et al. Risk factors for multiple organ system failure and death in critically injured patients. Surgery. 1993;114(1):21–30. [PubMed] [Google Scholar]
- 20.Sperry JL, Friese RS, Frankel HL, et al. Male gender is associated with excessive IL-6 expression following severe injury. J Trauma. 2008;64(3):572–578. doi: 10.1097/TA.0b013e3181650fdf. discussion 578-579. [DOI] [PubMed] [Google Scholar]
- 21.Sauaia A, Moore FA, Moore EE, et al. Multiple organ failure can be predicted as early as 12 hours after injury. J Trauma. 1998;45(2):291–301. doi: 10.1097/00005373-199808000-00014. discussion 301-293. [DOI] [PubMed] [Google Scholar]
- 22.Watson GA, Sperry JL, Rosengart MR, et al. Fresh frozen plasma is independently associated with a higher risk of multiple organ failure and acute respiratory distress syndrome. J Trauma. 2009;67(2):221–227. doi: 10.1097/TA.0b013e3181ad5957. discussion 228-230. [DOI] [PubMed] [Google Scholar]
- 23.Ciesla DJ, Moore EE, Johnson JL, et al. The role of the lung in postinjury multiple organ failure. Surgery. 2005;138(4):749–757. doi: 10.1016/j.surg.2005.07.020. discussion 757-748. [DOI] [PubMed] [Google Scholar]
- 24.Moore FA, Sauaia A, Moore EE, et al. Postinjury multiple organ failure: a bimodal phenomenon. J Trauma. 1996;40(4):501–510. doi: 10.1097/00005373-199604000-00001. discussion 510-502. [DOI] [PubMed] [Google Scholar]
- 25.Parsons PE, Eisner MD, Thompson BT, et al. Lower tidal volume ventilation and plasma cytokine markers of inflammation in patients with acute lung injury. Crit Care Med. 2005;33(1):1–6. doi: 10.1097/01.ccm.0000149854.61192.dc. discussion 230-232. [DOI] [PubMed] [Google Scholar]
- 26.Sakr Y, Vincent JL, Reinhart K, et al. High tidal volume and positive fluid balance are associated with worse outcome in acute lung injury. Chest. 2005;128(5):3098–3108. doi: 10.1378/chest.128.5.3098. [DOI] [PubMed] [Google Scholar]
- 27.Barie PS, Hydo LJ, Fischer E. A prospective comparison of two multiple organ dysfunction/failure scoring systems for prediction of mortality in critical surgical illness. J Trauma. 1994;37(4):660–666. doi: 10.1097/00005373-199410000-00022. [DOI] [PubMed] [Google Scholar]
- 28.Cabre L, Mancebo J, Solsona JF, et al. Multicenter study of the multiple organ dysfunction syndrome in intensive care units: the usefulness of Sequential Organ Failure Assessment scores in decision making. Intensive Care Med. 2005;31(7):927–933. doi: 10.1007/s00134-005-2640-2. [DOI] [PubMed] [Google Scholar]
- 29.Cook R, Cook D, Tilley J, et al. Multiple organ dysfunction: baseline and serial component scores. Crit Care Med. 2001;29(11):2046–2050. doi: 10.1097/00003246-200111000-00002. [DOI] [PubMed] [Google Scholar]