Abstract
Iron sucrose originator (ISORIG) has been used to treat iron deficiency and iron deficiency anemia for decades. Iron sucrose similars (ISSs) have recently entered the market. In this non-clinical study of non-anemic rats, five doses (40 mg iron/kg body weight) of six ISSs marketed in Asian countries, ISORIG or saline solution (control) were administered intravenously over four weeks to compare their toxicologic effects. Vasodilatory effects, impaired renal function and hepatic damage were only observed in the ISS groups. Significantly elevated serum iron and transferrin saturation levels were observed in the ISS groups suggesting a higher release of iron resulting in higher amounts of non-transferrin bound (free) iron compared to ISORIG. This might explain the elevated oxidative stress and increased levels of inflammatory markers and antioxidant enzymes in the liver, heart and kidneys of ISS-treated animals. Physico-chemical analyses showed that the molecular structure of most of the ISSs differed greatly from that of the ISORIG. These differences may be responsible for the organ damage and oxidative stress observed in the ISS groups. Significant differences were also found between different lots of a single ISS product. In contrast, polarographic analyses of three different ISORIG lots were identical, indicating that the molecular structure and thus the manufacturing process for ISORIG is highly consistent. Data from this study suggest that ISSs and ISORIG differ significantly. Therefore, before widespread use of these products it would be prudent to evaluate additional non-clinical and/or clinical data proving the safety, therapeutic equivalence and interchangeability of ISSs with ISORIG.
Keywords: Anemia, inflammation, iron deficiency, iron sucrose, iron sucrose similar, oxidative stress.
INTRODUCTION
Iron deficiency (ID) is one of the world’s most prevalent nutrient deficiencies [1]. It has various causes, such as increased iron demands due to blood loss, growth, pregnancy, inadequate dietary intake due to poor nutrition, and inadequate gastrointestinal absorption due to malabsorption or interference with drugs and food components. Because iron-containing enzymes are essential for all major metabolic processes, ID can lead to inadequate synthesis of these essential enzymes and thus to deleterious effects on cells and tissues. Untreated ID can lead to iron deficiency anemia (IDA), a condition in which the number of red blood cells (RBCs), the hemoglobin (Hb) level, and the volume of packed RBCs in the blood are below the normal values [2, 3]. IDA is an additional burden in numerous disease states including chronic heart failure (CHF) , inflammatory bowel disease and chronic kidney disease (CKD) [4-9].
Iron supplementation is often necessary to manage ID with or without anemia. The key goal is to replenish body iron stores and, if present, correct the Hb deficit [2]. Although oral iron administration is the least expensive form of iron therapy [5], the intravenous (i.v.) route of iron administration has become more favored in recent years in many clinical settings due to the gastrointestinal intolerance, low iron delivery rates, limited absorption and prolonged iron store repletion times associated with oral iron supplements [10]. The iron preparations for i.v. administration that have been available already for several years include iron sucrose, sodium ferric gluconate, and low- and high-molecular weight iron dextran. Recently, preparations that allow rapid administration of high doses of iron, such as ferric carboxymaltose (available in various countries) and ferumoxytol (available in the USA), have been marketed worldwide. All iron complexes for i.v. administration consist of a polynuclear iron(III)-oxyhydroxide core shielded by a carbohydrate shell [11, 12]. However, they vary widely in their physico-chemical properties, pharmacological activity, and side effects [11, 13, 14].
Despite the rapid recovery of the ID status in response to i.v. iron administration, there are potential risks associated with i.v. iron. Due to the risk of life-threatening/serious anaphylactic reactions associated with i.v. iron dextran, in particular high-molecular weight iron dextran, this form of iron therapy is not generally recommended [15-17]. Moreover, the more labile, low molecular weight compounds, such as ferric gluconate, release larger amounts of iron into the circulation saturating transferrin and generating non-transferrin bound iron (NTBI) [14, 18]. NTBI is taken up unspecifically by the liver, endocrine tissue and heart where it may catalyze a number of reactions that lead to oxidative stress and tissue damage [19, 20]. The extent of iron release into the circulation determines the maximum allowed single dose of an i.v. iron preparation to minimize the amount of NTBI [21]. More stable iron complexes can be administered in higher doses.
Iron sucrose is an intravenous iron preparation that contains no dextran or its derivatives, and the associated incidence of allergic side effects has been shown to be rare [11, 22]. The iron sucrose originator (ISORIG, iron sucrose preparation, Venofer®, Vifor International, Switzerland) complex has been in clinical use for decades for the treatment of ID and IDA in a variety of clinical conditions [2]. In recent years, a number of new iron sucrose similar (ISS) preparations have entered the market. Their structures differ from that of the ISORIG complex due to different manufacturing processes [23]. These subtle structural differences may present a significant risk, raising potential concerns about the safety and efficacy of ISS preparations in clinical use. Non-clinical studies have indicated hemodynamic and functional differences between the ISORIG and ISS preparations and, in particular, a higher potential of ISSs to induce oxidative stress in kidneys, liver and heart [24, 25]. Additionally, in a recently published observational clinical study, switching the i.v. iron treatment of hemodialysis patients from ISORIG to an ISS led to a significant decrease in Hb levels and iron indices suggesting that the studied ISS preparation may not be therapeutically equivalent to ISORIG [26].
We previously established a non-clinical model that allows distinguishing between the potential of ISS preparations to induce oxidative stress in the liver, heart and kidneys [24, 25]. In the present study, we used this same model to compare the properties of six ISS preparations in the Asian market with those of the ISORIG complex.
METHODS
Physico-Chemical Analysis
Physico-chemical analyses were performed by the Quality Control Laboratory of Vifor (International) Ltd. (St. Gallen, Switzerland) on the samples of the ISS preparations. Molecular weight distribution was measured by gel permeation chromatography as described previously [11, 27]. The Fe(III)/Fe(II) reduction potentials were measured by polarography as described previously [11]. The turbidity point, pH and titratable alkalinity were assessed by methods described previously [27, 28].
Animals and Treatments
All experiments were approved by the Hospital Alemán Animal Care and the Teaching and Research Committee, and performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals. Forty male and forty female 2-month-old non-anemic Sprague-Dawley rats (Laboratory of Experimental Medicine, Hospital Alemán, Buenos Aires, Argentina) weighing 200-220 g were randomized into eight groups (n = 10/group) with an equal male-female distribution. The control group received isotonic saline solution and the ISORIG group received iron sucrose [Venofer®, LOT 517100, Vifor (International), Switzerland]. The ISS groups received either ISSFERP (Ferplex® SS, LOT H3797, Himont Pharmaceuticals, Lahore, Pakistan), ISSFERI (Ferijet, LOT 024-Y, Akson Pharmaceuticals, Azad Kashmir, Pakistan), ISSFERO (Ferosoft® S, LOT 6016, Hilton Pharma Ltd., Karachi, Pakistan), ISSENCI (Encifer®, Emcure Pharmaceuticals Ltd., Pune, India), ISSBACK (Fe-Back, Nang-Kuang Pharmaceutical Co. Ltd., Tainan Hsien, Taiwan) or ISSLIB (Fe-Lib, LOT A5015, Advanced International Pharmaceutical Nanotech Inc., Taiwan).
Rats were housed in metabolic cages in a temperature-controlled room (22 ± 2 °C) subject to 12 h light/dark cycles (07.00-19.00). All animals had free access to tap water and were fed standard rat chow (16-18% protein, Cooperación, Argentina) ad libitum throughout the study. Rats received a single i.v. dose by tail vein injection of the corresponding iron compound (40 mg/kg body weight) or control solution (equivalent volume) at the same time every 7 days for 4 weeks (a total of five administrations on days 0, 7, 14, 21 and 28). Treatment doses were adjusted each week according to the body weight of each animal.
Blood samples were obtained for biochemical assessment of Hb, serum iron and transferrin saturation (TSAT) 24 h after an i.v. iron treatment on days 1, 8, 15, 22 and 29. Urine was collected in each group for 24 h after each i.v. injection with methods described previously [29]. The rats were sacrificed on day 29 by subtotal exsanguination under anesthesia (sodium thiopental, 40 mg/kg body weight, intraperitoneal) according to institutional guidelines for animal care and use. Previously, blood samples were obtained for biochemistry determination. The liver, heart and kidneys of each rat were perfused with ice cold saline solution through the abdominal aorta until they were free of blood and then removed for oxidative stress evaluation, microscopy and immunohisto-chemical study.
Blood Pressure Measurement
Systolic blood pressure (SBP) was measured by tail-cuff plethysmography at baseline (day 0) and 24 h after each i.v. iron administration (days 1, 8, 15, 22, and 29). Cuff pressure was determined by a Pneumatic Pulse Transducer using a Programmed electro-sphygmomanometer PE-300 (Narco Bio-Systems, Austin, Texas, USA); rats were restrained in a plastic chamber without anesthesia. Pulses were recorded on a Physiograph MK-IIIS (Narco Bio-Systems, Austin, Texas, USA) and a minimum of three measurements were taken at each session. The SBP was calculated as an average of the three readings [29, 30].
Biochemical Procedures
Blood samples were collected from the tail vein in capillary tubes for biochemistry determination after 14 h of fasting. Hb concentration was determined by SYSMEX XT 1800i (Roche Diagnostic GmbH, Mannheim, Germany). Serum iron was determined by radial immunodiffusion (Diffu-Plate, Biocientifica S.A., Buenos Aires, Argentina) and TSAT was obtained using traditional chemical methods. Liver enzymes, including aspartate aminotransferase (AST), alanine aminotransferase (ALT) and alkaline phosphatase (ALP), were assessed in the blood samples by colorimetric and ultraviolet (UV) methods using an Autoanalyzer Modular P800 with corresponding reagents (Roche Diagnostic GmbH, Mannheim, Germany). Aliquots of urine were assessed for creatinine with the enzymatic UV method (Randox Laboratories Ltd., Crumlin, Northern Ireland). Creatinine clearance (CrCl) was determined by the standard formula, and proteinuria was determined using the sulfosalicylic acid method.
Evaluation of Oxidative Stress Parameters in Liver, Heart and Kidney
Samples of the whole liver, heart and kidney were homogenized (1:3, w:v) in ice cold 0.25 M sucrose solution. Glutathione (GSH) levels were determined in the 10,000 × g supernatant by methods described previously [31, 32]. Further samples of the corresponding perfused tissues were homogenized (1:10, w:v) in 0.05 M sodium phosphate buffer (pH 7.4) and used for the determination of malondialdehyde to evaluate lipid peroxidation by thiobarbituric acid reactive species (TBARS). The remaining homogenate was centrifuged at 4 °C for 15 min at 9,500 × g and the supernatant was used to measure catalase activity. The remaining tissue samples were homogenized (1:3, w/v) in ice cold sucrose solution (0.25 M). The supernatant obtained after centrifugation at 105,000 × g for 90 min was used to measure Cu, Zn superoxide dismutase (Cu, Zn-SOD) and glutathione peroxidase (GPx) activity. Enzyme units (U) were defined previously [14]. Specific activity was expressed as U/mg protein.
Light Microscopy and Immunohistochemical Study
Decapsulated kidney, liver and heart samples were cut longitudinally, fixed in phosphate-buffered 10% formaldehyde (pH 7.2) and embedded in paraffin. Three-micron sections were cut and stained. All observations were made with a light microscope Nikon E400 (Nikon Instrument Group, Melville, New York. USA) [24, 33, 34].
Immunolabeling of specimens was carried out using a modified avidin-biotin-peroxidase technique (Vectastain ABC kit, Universal Elite, Vector Laboratories, CA, USA) as described previously [14]. Tissue ferritin was quantified with antiferritin monoclonal antibody (Biogen, San Román, California, USA). Pro-inflammatory markers were quantified with monoclonal antibodies against rat tumor necrosis factor-alpha (TNF-α) (R&D Systems, Minneapolis, MN, USA) and interleukin-6 (IL6) (Santa Cruz Biotechnology, Santa Cruz, CA, USA) at dilutions of 1:50 and 1:100, respectively (PBS diluting agent) [29, 35].
Morphometric Analysis
Histological sections were studied in each animal with an image analyzer (Image-Pro Plus version 4 for Windows, Media Cybernetics LP, Silver Spring, MD, USA) as described previously [14].
Statistical Methods
Values are expressed as mean ± SD. All statistical analyses were performed using absolute values and processed through GraphPad Prism version 5.01 for Windows (GraphPad Software, Inc. San Diego, CA, USA). For parameters with a Gaussian distribution, comparisons among groups were performed using analysis of variance (ANOVA) and for parameters with a non-Gaussian distribution using Kruskal-Wallis test (non-parametric ANOVA) and Dunn’s multiple comparison test. A value of p<0.05 was considered significant.
RESULTS
Physico-Chemical Analyses
The physico-chemical analyses of the studied ISS preparations showed clearly that, except for ISSFERP, these products do not comply with the specifications of ISORIG [27]. All ISSs, except for ISSFERP, had lower titratable alkalinity and out of range turbidity point (Table 1). In addition, ISSFERI, ISSBACK, and ISSLIB had a very high molecular weight. ISSFERI and ISSFERO also had a lower pH than ISORIG. Interestingly, great variations in some of the physico-chemical parameters were also observed between the different lots of the two ISSs (ISSENCI and ISSBACK) that were analyzed.
Table 1.
Characteristics, pH, Titratable Alkalinity, Turbidity Point, and Molecular Weight of the Six Asian ISS Preparations Compared with the Originator Iron Sucrose (ISORIG) and the Pharmacopeia (USP). (Studies Performed by the Quality Control Laboratory of Vifor (International), St. Gallen, Switzerland)
| Parameter | USPa | ISORIG | ISSFERP | ISSFERI | ISSFERO | ISSENCI | ISSENCI | ISSBACK | ISSBACK | ISSLIB |
|---|---|---|---|---|---|---|---|---|---|---|
| Lot | - | 517100 | H3797 | 024-T | 6016 | LHA04003 | LHA05005 | F5097 | D5064 | A5015 |
| Characteristics | - | Dark brown, opaque aqueous solution | Complies | Complies | Complies | Complies | Complies | Complies | Complies | Complies |
| pH | 10.5-11.1 | 10.9 | 10.5 | 10.4 | 9.8 | 10.6 | 10.8 | 10.8 | 10.5 | 10.6 |
| Titratable alkalinity (ml) | 0.5-0.8 | 0.8 | 0.55 | 0.4 | 0.18 | 0.33 | 0.4 | 0.46 | 0.38 | 0.37 |
| Turbidity point | 4.4-5.3 | 4.9 | 5.1 | 9.7 | 4.2 | 5.8 | 6.4 | N/Ab | N/Ab | 5.33 |
| Mw (Da) | 34,000-60,000 | 45,700 | 50,600 | 215,000 | 39,200 | 50,500 | 46,400 | 293,000 | 162,000 | 246,000 |
| Mn (Da) | ≥24,000 | 33,900 | 36,100 | 85,200 | 31,300 | 35,100 | 34,200 | 175,000 | 93,300 | 151,000 |
| P | ≤1.7 | 1.3 | 1.4 | 2.52 | 1.25 | 1.44 | 1.36 | 1.67 | 1.74 | 1.63 |
[27]
Starting solution turbid
N/A, not available; Mw (Da), weight average molecular weight in Dalton; Mn (Da), number average molecular weight in Dalton; P = ratio Mw/Mn
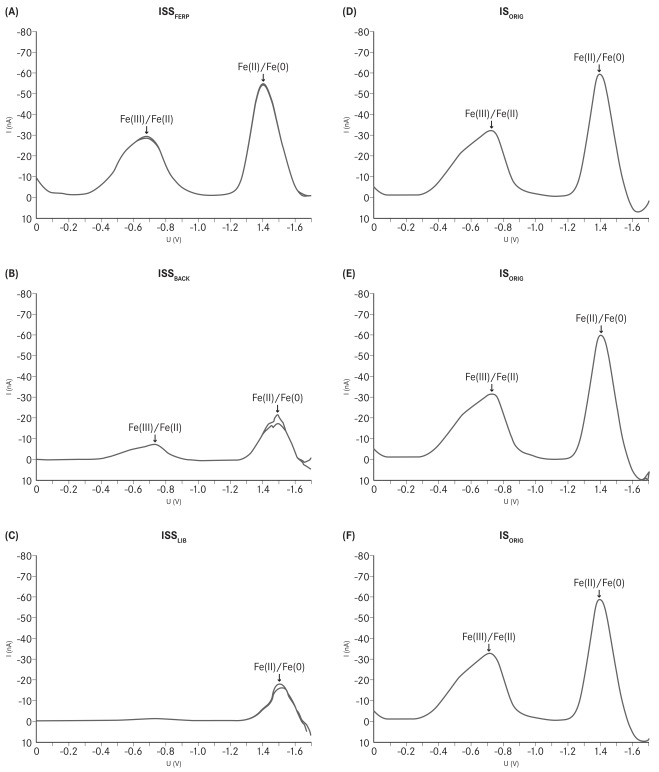
The reduction potentials were determined by polarography and are measured vs. Ag/AgCl 3M KCl, if not otherwise specified. The iron(III)/iron(II) reduction potentials varied considerably among the different investigated ISSs. The reduction potential of ISSFERP was the only one of the studied ISSs that complied with the reference value for iron sucrose and also the shape of its polarogram was found to be identical to that of ISORIG (Fig. 1). The reduction potentials of ISSBACK and ISSLIB were not determinable due to low solubility of these compounds. In addition, the shapes of the polarograms for these ISSs were different from that of ISORIG, confirming the dissimilarities between the structures of these ISSs and that of the ISORIG complex (Fig. 1). The reduction potentials of ISSFERI, ISSFERO and ISSENCI did not match the specifications for iron sucrose (-750 ± 50 mV) [27]. The polarogram of ISSFERI revealed two distinct Fe(III)/Fe(II) transitions at -495 and -765 mV. ISSFERO had a reduction potential of -566 mV, whereas ISSENCI had different values depending on the analyzed lot (-565 and -534 mV for lot LHA04003 and LHA05005, respectively). In contrast, polarograms of three randomly chosen lots of ISORIG had an identical shape and complied with the reference specification, reflecting the high consistency of the manufacturing process and thus the structure of the ISORIG complex (Fig. 1).
Fig. (1).
Polarograms of (A) ISSFERP, (B) ISSBACK (Lot 5064), (C) ISSLIB, and (D-F) three randomly chosen lots of ISORIG. (Studies performed by the Quality Control Laboratory of Vifor (International) Ltd., St. Gallen, Switzerland.)
Non-Clinical Study
Throughout the non-clinical study, no significant differences were observed in Hb concentrations between the i.v. iron-treated and control groups, as expected for non-anemic rats (Table 2). Biochemical analysis revealed that serum iron concentration and TSAT levels were significantly elevated (p<0.01) in animals from the ISS groups and the ISORIG group compared to control group on days 1, 8 and 29 (Table 2). The ISORIG group showed significantly lower values (p<0.01) in both serum iron concentration and percentage TSAT compared to the ISS groups on days 1, 8 and 29.
Table 2.
Hemoglobin (Hb), Serum Iron and Transferrin Saturation (TSAT)
| Mean ± SD | ISSFERP (n = 10) | ISSFERI (n = 10) | ISSFERO (n = 10) | ISSENCI (n = 10) | ISSBACK (n = 10) | ISSLIB (n = 10) | ISORIG (n = 10) | Control (n = 10) |
|---|---|---|---|---|---|---|---|---|
| Day 1 | ||||||||
| Hb (g/dL) | 15.8 ± 0.2 | 15.8 ± 0.1 | 16.0 ± 0.1 | 16.1 ± 0.1 | 15.9 ± 0.2 | 15.8 ± 0.2 | 15.9 ± 0.2 | 15.8 ± 0.5 |
| Serum iron (µg/dL) | 559.1 ± 60.2 | 563.2 ± 55.3 | 539.0 ± 59.1 | 542.2 ± 50.0 | 549.9 ± 47.1 | 555.1 ± 60.0 | 360.0 ± 42.0# | 309.9 ± 15.0* |
| TSAT (%) | 86.9 ± 4.3 | 90.0 ± 4.1 | 87.7 ± 5.0 | 86.8 ± 5.9 | 86.9 ± 6.0 | 89.2 ± 3.9 | 73.2 ± 6.2# | 45.1 ± 3.8* |
| Day 8 | ||||||||
| Hb (g/dL) | 16.2 ± 0.1 | 16.4 ± 0.2 | 16.3 ± 0.2 | 16.1 ± 0.1 | 16.4 ± 0.1 | 16.4 ± 0.2 | 16.4 ± 0.2 | 15.9 ± 0.4 |
| Serum iron (µg/dL) | 489.0 ± 52.0 | 500.0 ± 66.0 | 499.0 ± 58.0 | 488.3 ± 45.0 | 500.1 ± 32.0 | 479.9 ± 52.0 | 398.0 ± 33.0# | 318.0 ± 18.0* |
| TSAT (%) | 85.9 ± 3.0 | 87.0 ± 5.2 | 89.9 ± 6.1 | 89.4 ± 6.0 | 88.3 ± 4.9 | 88.2 ± 5.2 | 69.9 ± 5.5# | 48.3 ± 5.9* |
| Day 29 | ||||||||
| Hb (g/dL) | 16.4 ± 0.2 | 16.5 ± 0.2 | 16.5 ± 0.1 | 16.5 ± 0.2 | 16.5 ± 0.2 | 16.5 ± 0.1 | 16.5 ± 0.2 | 16.0 ± 0.5 |
| Serum iron (µg/dL) | 478.1 ± 45.2 | 458.9 ± 60.0 | 486.3 ± 43.0 | 453.7 ± 41.1 | 469.5 ± 56.2 | 487.9 ± 39.9 | 395.7 ± 21.0# | 301.0 ± 10.8* |
| TSAT (%) | 84.4 ± 4.2 | 89.9 ± 6.1 | 88.9 ± 5.3 | 89.0 ± 5.7 | 84.8 ± 5.1 | 86.6 ± 4.4 | 70.1 ± 4.5# | 47.2 ± 4.6* |
p < 0.01 versus all groups
p < 0.01 versus ISSFERP, ISSFERI, ISSFERO, ISSENCI, ISSBACK, ISSLIB
All ISS groups (ISSFERP, ISSFERI, ISSFERO, ISSENCI, ISSBACK, ISSLIB) presented a significant decrease in SBP throughout the study compared to the control and ISORIG groups (p<0.01) (Table 3); there were no significant differences between the ISS groups themselves.
Table 3.
Systolic Blood Pressure (mean ± SD) After Weekly i.v. Administration (40 mg Iron/kg Body Weight or Equivalent Volume) in ISS, ISORIG and Control Groups Over a 4-Week Period
| Day | ISSFERP (n = 10) | ISSFERI (n = 10) | ISSFERO (n = 10) | ISSENCI (n = 10) | ISSBACK (n = 10) | ISSLIB (n = 10) | ISORIG (n = 10) | Control (n = 10) |
|---|---|---|---|---|---|---|---|---|
| 0 | 118.1 ± 2.4 | 119.0 ± 2.9 | 118.1 ± 2.4 | 119.0 ± 2.9 | 118.1 ± 2.3 | 119.1 ± 2.9 | 118.9 ± 2.2 | 118.9 ± 1.9 |
| 1 | 112.3 ± 1.9 | 111.0 ± 0.1 | 112.3 ± 1.9 | 114.1 ± 1.9 | 112.3 ± 1.9 | 112.0 ± 1.9 | 116.2 ± 2.0# | 120.2 ± 0.5* |
| 8 | 111.4 ± 1.7 | 112.0 ± 3.1 | 114.7 ± 1.7 | 113.6 ± 3.1 | 114.7 ± 1.7 | 110.1 ± 3.1 | 117.8 ± 2.8# | 119.8 ± 2.1# |
| 15 | 112.6 ± 1.8 | 111.1 ± 2.4 | 114.9 ± 1.8 | 114.1 ± 2.4 | 116.8 ± 1.8 | 114.7 ± 2.4 | 118.0 ± 2.2# | 120.3 ± 2.1# |
| 22 | 113.7 ± 2.0 | 114.2 ± 2.5 | 114.5 ± 2.0 | 112.1 ± 2.5 | 114.5 ± 2.0 | 113.9 ± 2.5 | 119.4 ± 1.9# | 120.3 ± 2.0# |
| 29 | 115.1 ± 1.8 | 114.8 ± 3.0 | 113.6 ± 2.5 | 114.7 ± 2.6 | 113.7 ± 2.0 | 113.8 ± 3.0 | 121.2 ± 2.6# | 122.1 ± 2.4# |
p< 0.01 versus all groups
p< 0.01 versus ISSFERP, ISSFERI, ISSFERO, ISSENCI, ISSBACK, ISSLIB
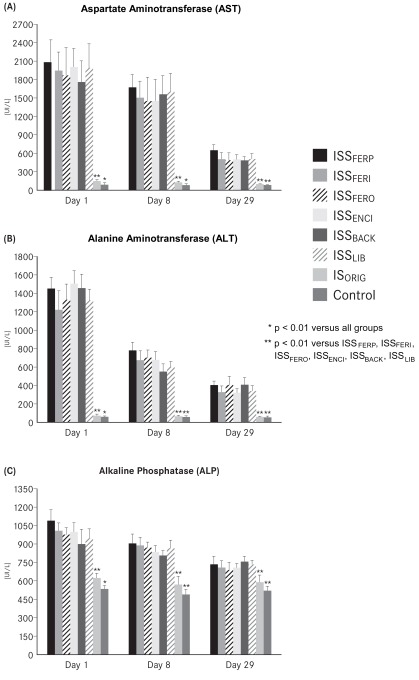
CrCl was significantly reduced in rats from all of the ISS groups on days 1, 8 and 29 compared with the ISORIG and control groups (p<0.01) (Table 4), whereas the values between the ISORIG and control group did not differ significantly. All ISS groups showed significant proteinuria on days 1, 8 and 29 (p<0.01) compared with the ISORIG and control groups, which showed no differences throughout the study (Table 4). Assessment of liver function showed that AST, ALT and ALP levels were significantly increased (p<0.01) in all ISS groups on days 1, 8 and 29 compared with ISORIG and control groups (Fig. 2A-C). Significant differences, although modest (p<0.05), were observed between the ISORIG group and the control group on days 1 and 8. However, there was no marked difference between these groups on day 29.
Table 4.
Creatinine Clearance and Proteinuria After Weekly i.v. Administration (40 mg Iron/kg Body Weight or Equivalent Volume) in the ISS, ISORIG and Control Groups Over a 4-Week Period
| Day | ISSFERP (n = 10) | ISSFERI (n = 10) | ISSFERO (n = 10) | ISSENCI (n = 10) | ISSBACK (n = 10) | ISSLIB (n = 10) | ISORIG (n = 10) | Control (n = 10) |
|---|---|---|---|---|---|---|---|---|
| CrCl | ||||||||
| 1 | 2.4 ± 0.1 | 2.3 ± 0.1 | 2.4 ± 0.2 | 2.1 ± 0.1 | 2.2 ± 0.1 | 2.3 ± 0.2 | 2.7 ± 0.1# | 2.8 ± 0.1* |
| 8 | 3.3 ± 0.1 | 2.2 ± 0.1 | 2.3 ± 0.1 | 2.2 ± 0.2 | 2.3 ± 0.2 | 2.3 ± 0.1 | 2.8 ± 0.1# | 2.9 ± 0.1# |
| 29 | 2.1 ± 0.1 | 2.2 ± 0.1 | 2.2 ± 0.1 | 2.3 ± 0.2 | 2.1 ± 0.2 | 2.4 ± 0.2 | 2.7 ± 0.1# | 2.9 ± 0.1# |
| Proteinuria | ||||||||
| 1 | 19.2 ± 5.5 | 22.1 ± 6.2 | 16.9 ± 7.1 | 18.5 ± 7.9 | 17.4 ± 5.8 | 19.9 ± 5.8 | 2.0 ± 1.3# | 1.2 ± 2.0# |
| 8 | 21.9 ± 6.6 | 26.3 ± 7.7 | 27.3 ± 9.9 | 33.2 ± 5.8 | 22.9 ± 4.9 | 25.1 ± 4.1 | 5.7 ± 2.4# | 4.1 ± 2.1# |
| 29 | 35.4 ± 7.0 | 31.8 ± 8.2 | 30.7 ± 5.9 | 39.4 ± 8.2 | 35.1 ± 5.8 | 29.8 ± 7.9 | 6.3 ± 3.0# | 3.8 ± 3.0# |
p< 0.01 versus all groups
p< 0.01 versus ISSFERP, ISSFERI, ISSFERO, ISSENCI, ISSBACK, ISSLIB
Fig. (2).
(A) Aspartate aminotransferase (AST), (B) alanine aminotransferase (ALT) and (C) alkaline phosphatase (ALP) after weekly i.v. administration (40 mg iron /kg body weight or equivalent volume) in the ISORIG, ISS and control groups over a 4- week period.
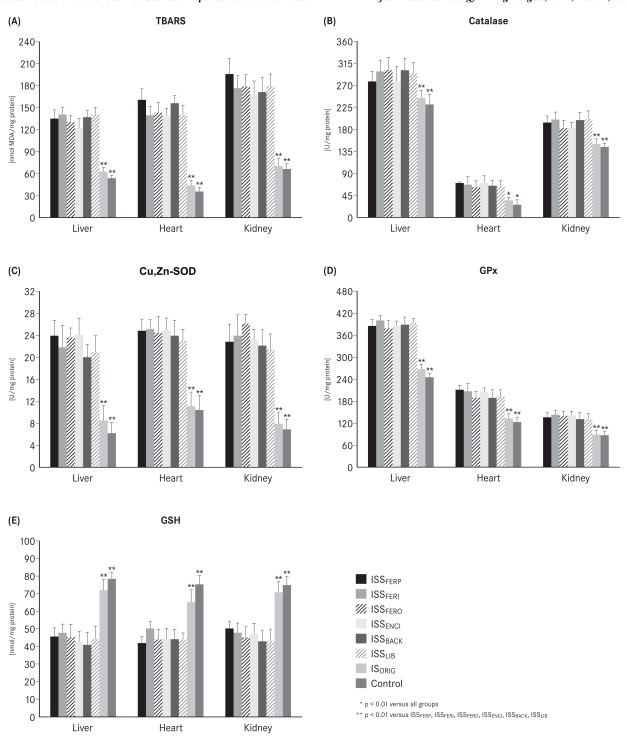
The liver, heart and kidney tissues of all the ISS groups showed a significant increase (p<0.01) in malondialdehyde (TBARS), catalase, GPx, and Cu, Zn-SOD levels and a significant decrease (p<0.01) in GSH level when compared with the ISORIG and control groups on day 29 (Fig. 3A-E). The ISORIG group showed only modest non-significant increases in the levels of malondialdehyde, catalase, GPx, and Cu, Zn-SOD in the liver and a modest non-significant decrease in the GSH levels in the liver, heart and kidneys compared to the control group on day 29. Other oxidative stress parameters did not differ between the ISORIG group and the control group in heart or kidneys at the end of the study.
Fig. (3).
(A) Thiobarbituric acid reactive species (TBARS), (B) catalase, (C) Cu,Zn-superoxide dismutase (Cu,Zn-SOD), (D) glutathione peroxidase (GPx), (E) glutathione (GSH) in liver, heart and kidney homogenates after i.v. administration (40 mg iron /kg body weight or equivalent volume) in the ISORIG, ISS and control groups on day 29.
On day 29, microscopy studies of the liver showed significantly more (p<0.01) positive staining for iron (Prussian blue) in the Kupffer cells, sinusoidal epithelial cells and hepatocytes in all ISS groups compared to the ISORIG group. The ISORIG group had iron deposits only in the Kupffer cells and displayed a significantly (p<0.01) greater area for ferritin staining in the liver compared to the ISS and control groups (Table 5).
Table 5.
Prussian Blue Staining and Ferritin Immunostaining in the Liver, Heart and Kidney Samples of the Six Asian ISS Groups, the ISORIG Group, and the Control Group on Day 29
| Mean ± SD | ISSFERP (n = 10) | ISSFERI (n = 10) | ISSFERO (n = 10) | ISSENCI (n = 10) | ISSBACK (n = 10) | ISSLIB (n = 10) | ISORIG (n = 10) | Control (n = 10) |
|---|---|---|---|---|---|---|---|---|
| Prussian blue (% positive staining/area) | ||||||||
| Liver | 6.8 ± 2.0 | 15.1 ± 3.2 | 14.6 ± 2.2 | 14.2 ± 2.6 | 14.7 ± 2.8 | 14.9 ± 1.8 | 7.4 ± 2.0# | 1.2 ± 0.5* |
| Heart | 4.1 ± 0.7 | 3.8 ± 0.6 | 4.0 ± 0.7 | 3.6 ± 0.8 | 3.9 ± 0.7 | 3.7 ± 0.8 | 1.1 ± 0.3# | 0.2 ± 0.1* |
| Kidney | 7.1 ± 1.0 | 6.2 ± 1.2 | 6.6 ± 1.5 | 6.4 ± 1.8 | 6.8 ± 1.3 | 6.3 ± 1.4 | 3.1 ± 0.8# | 1.3 ± 0.1* |
| Ferritin (% positive staining/area) | ||||||||
| Liver | 7.3 ± 2.0 | 6.9 ± 1.7 | 6.8 ± 1.4 | 7.5 ± 2.0 | 7.5 ± 1.1 | 7.1 ± 2.0 | 15.2 ± 1.4# | 2.1 ± 0.6* |
| Heart | 1.8 ± 0.4 | 1.7 ± 0.3 | 1.9 ± 0.4 | 1.7 ± 0.5 | 1.4 ± 0.5 | 1.6 ± 0.6 | 3.1 ± 0.4# | 0.2 ± 0.1* |
| Kidney | 3.6 ± 0.5 | 3.0 ± 0.5 | 2.7 ± 0.7 | 3.3 ± 0.5 | 3.6 ± 0.3 | 3.4 ± 0.6 | 6.1 ± 0.7# | 0.2 ± 0.1* |
p < 0.01 versus all groups
p < 0.01 versus ISSFERP, ISSFERI, ISSFERO, ISSENCI, ISSBACK, ISSLIB
On day 29, cardiomyocytes of all ISS groups showed a significantly larger (p<0.01) area for iron staining (Prussian blue) compared to that of the cardiomyocytes of the ISORIG and control groups. Only small ferritin deposits were observed in all of the ISS groups, whereas ferritin deposits were significantly larger (p<0.01) in the ISORIG group (Table 5).
A significant (p<0.01) positive staining for iron (Prussian blue) was detected in the proximal tubular epithelial cells of all the ISS groups compared with the ISORIG and control groups on day 29. The ISORIG group showed a larger area for ferritin deposits in the proximal tubular epithelial cells compared to all of the ISS and control groups (Table 5).
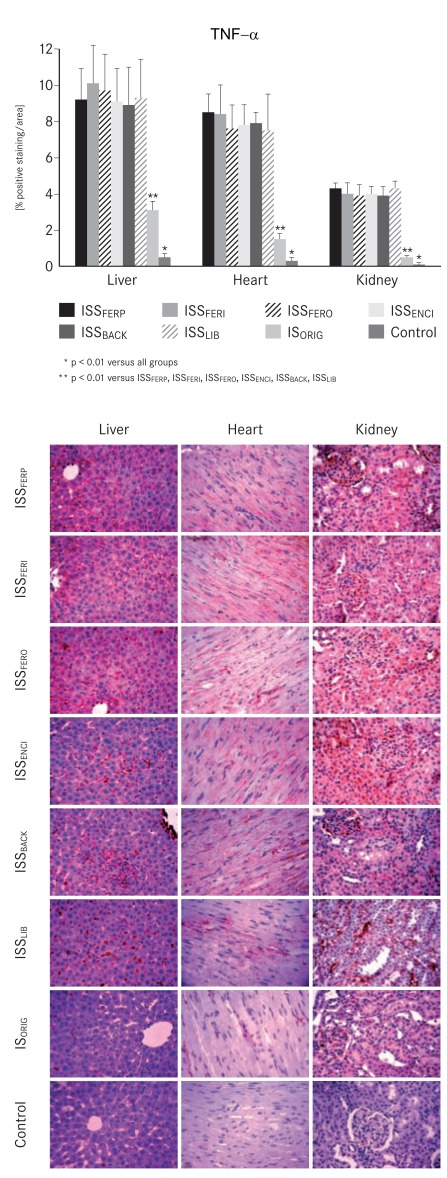
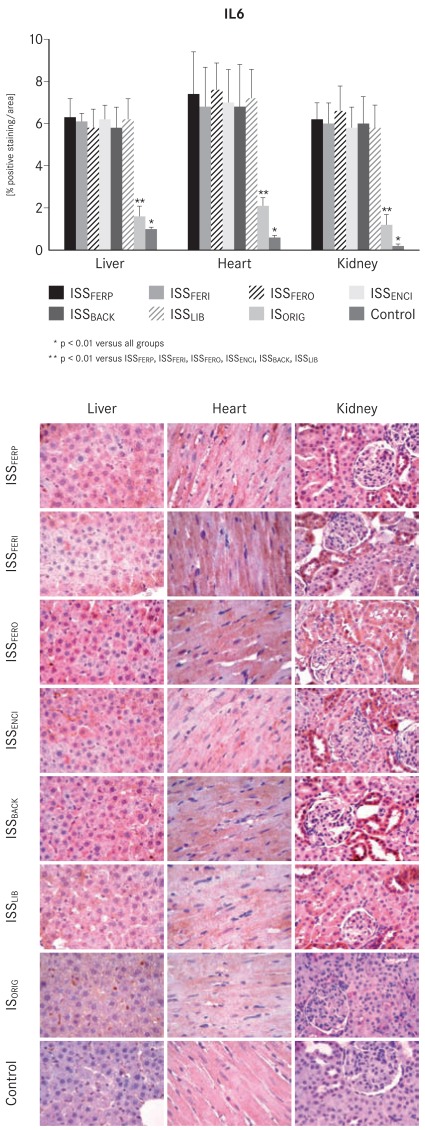
Upon completion of the experiments, the levels of both inflammatory markers TNF-α and IL6 were significantly increased (p<0.01) in the liver, heart and kidney samples of the ISS groups compared with the ISORIG and control groups (Figs. 4 and 5).
Fig. (4).
Bar chart and corresponding micrographs showing tumor necrosis factor-alpha (TNF-α) immunostaining in liver, heart and kidney samples taken from the ISORIG, ISS and control groups on day 29.
Fig. (5).
Bar chart and corresponding micrographs showing interleukin-6 (IL6) immunostaining in liver, heart and kidney samples taken from the ISORIG, ISS and control groups on day 29.
DISCUSSION
The iron sucrose originator complex has been in clinical use for decades representing a good therapeutic option for correction of ID and IDA in more than 80 countries [2]. Together with erythropoiesis-stimulating agents it has become an integral part of IDA management associated with various clinical conditions. Clinical experience with ISORIG is based on patient exposure of more than 12 million patient years (as of 30 June 2011). ISORIG is regarded as a product with a good clinical profile [36, 37], particularly as it carries a low potential risk of hypersensitivity reactions [38].
Iron sucrose is a complex made of a polynuclear iron(III)-oxyhydroxide core stabilized by a sucrose ligand [39]. As such, it is a polymeric compound which belongs to the class of non-biological complex drugs and therefore is very different from conventional small molecule active pharmaceutical ingredients (APIs). Polymeric compounds are composed of a large number of molecules of slightly different molecular weights and are characterized by a distinct molecular weight distribution. Thus, characterization of polymeric compounds requires a set of analytical methods different from those used for conventional APIs. Due to the complexity of the process to synthesize iron sucrose, the manufacturing process largely defines the quality of the product [23, 39].
Recently, a number of ISS preparations have entered the market [23-25]. However, little is known about their safety profile as non-clinical or clinical data are not largely available. In this study, the toxicologic effects of six ISS preparations marketed in Asian countries (ISSFERP, ISSFERI, ISSFERO, ISSENCI, ISSBACK, ISSLIB) on hemodynamic and oxidative stress parameters, inflammatory markers, tissue histologies and biochemical processes were evaluated versus ISORIG and control.
Similar to the results of the current study, our previous studies showed that the ISSs we investigated differed from ISORIG in terms of their stability and molecular structure [24, 25]. These structural differences are indicative of differences in the manufacturing process [23] and might be responsible for the negative effects of various ISS preparations that we have observed in our non-clinical studies [24, 25]. Interestingly, these subtle structural differences can escape physico-chemical characterization [23]. Recently, Meier et al. published a study in which they performed a toxicological characterization of a new ISS using a simplified version of the rat model used in the present study (shortened to an eight-day protocol). In contrast to the results presented here, their study found that the ISS had physico-chemical and toxicological properties comparable to those of ISORIG [40]. However, in a previous non-clinical study, we identified an ISS that had physico-chemical properties in compliance with the USP values for iron sucrose, but nevertheless induced a higher degree of oxidative stress and tissue damage than ISORIG [41]. Thus, even full compliance with USP-specified physico-chemical parameters does not ensure a complete characterization of an iron sucrose complex. Moreover, an observational clinical study in hemodialysis patients recently demonstrated that switching from ISORIG to an ISS led to a significant decrease in Hb levels and iron indices [26]. These findings highlight the increased need for appropriate clinical and non-clinical studies to determine the toxicological and clinical profiles of these ISS products prior to their approval [23, 42]. Consistent with this recommendation, the European Medicines Agency (EMA) recently recommended that non-clinical comparative assessments of target tissue concentrations should be performed in support of the evaluation of copies of intravenous nanoparticle iron medicinal products compared to the originator. In making their recommendation, the EMA recognized that assessments in humans based on plasma concentrations alone may not be sufficient to ensure a comparable safety and efficacy profile of these products [42].
In the present study, the results of the physico-chemical analysis of the investigated ISSs showed that only one of them, namely ISSFERP, complied with the USP values for iron sucrose. Within the other ISSs, significant fluctuations in the physico-chemical parameters from the reference values for iron sucrose were evident. In addition, differences in the physico-chemical parameters were also seen between the different lots of ISSENCI and ISSBACK. Clearly, the manufacturing processes of these two ISSs are not well controlled and thus lead to products with variable structure, stability and quality. In contrast, the manufacturing process of ISORIG is highly consistent, as shown by the identical polarograms of three randomly chosen lots. The polarographic analysis does not only indicate the potential of a complex to undergo redox cycling (reduction potential), but it is also considered as a fingerprint for the structure of the complex. Lack of physico-chemical identity with the originator demonstrates that ISSFERI, ISSFERO, ISSENCI, ISSBACK, and ISSLIB do not possess pharmaceutical equivalence to ISORIG.
According to the non-clinical analysis, initial observations of the effects of the ISS compounds on SBP revealed marked hypotension. This was evident throughout the 4-week study in all ISS-treated animals, whereas SBP in the ISORIG and control groups was not affected. The vasodilatory effects associated with ISS administration highlight a potentially significant risk factor for translation to clinical practice and are consistent with the hypotensive effects reported in animals receiving ISS preparations in previous studies [24, 25].
Renal function, explored by CrCl, was not affected in rats treated with ISORIG, but it was significantly reduced in the ISS groups. The reduced CrCl was accompanied by a marked proteinuria, indicating not only impaired renal function but also some disturbance in glomerular epithelial cells (podocytes) and tubular epithelial cells, in ISS-treated animals. As in our previous studies [24, 25], these results suggest that ISS preparations have more deleterious effects on the kidneys compared to the ISORIG compound. Also parallel to our previous studies, ISS induced hepatic damage was indicated by elevated levels of liver enzymes (ALT, AST and ALP) in the ISS groups compared to the ISORIG and control groups throughout the study [24, 25, 33]. The hepatic injury associated with elevated levels of liver enzymes in the blood may raise concerns regarding the use of ISS compounds in the clinical setting.
The stability of an iron complex depends on the exact structure of the complex and, in particular, on the interaction between the polynuclear iron(III)-hydroxide core and the surrounding carbohydrate [11]. As expected, in this study, all i.v. iron treated animals showed a significant increase in serum iron and TSAT levels. However, the administration of the ISS preparations resulted in significantly higher serum iron and TSAT levels than administration of the ISORIG. Serum iron reflects the balance of iron flow in and out of the plasma pool [2, 43]. Therefore, higher serum iron levels induced by the ISS preparations suggest a quicker release of iron from these carbohydrate complexes causing transfer mechanisms of iron to be saturated, which leads to the generation of non-transferrin bound iron (NTBI). The more labile compounds release iron more quickly and to a greater extent, saturating transferrin, generating higher amounts of NTBI, and possibly causing oxidative stress, endothelial damage, inflammation and hemodynamic alterations [44].
NTBI is taken up unspecifically by the liver, endocrine tissue and heart where it may further catalyze reactions leading to oxidative stress and tissue damage [19, 20]. Therefore, it is not surprising that the markers for oxidative stress and the levels of antioxidant enzymes were significantly increased in the liver, heart and kidney tissues in all ISS-treated animals compared to the ISORIG-treated animals and the control group throughout the study. Furthermore, oxidative stress can also increase the risk of endothelial damage and inflammation [44, 45]. The levels of both pro-inflammatory markers TNF-α and IL6 were substantially higher in the ISS groups relative to IS ORIG and control groups at the end of the study. These results further confirm that ISORIG has a lower potential to induce oxidative stress and inflammation in this non-clinical model. From a clinical point of view, these findings may be important since i.v. iron therapy is often used to treat ID or IDA in CKD, CHF and cancer patients, who are already subject to increased levels of oxidative stress. This is particularly relevant for hemodialysis patients, who necessitate frequent administration of i.v. iron.
In vitro experiments have also shown differences among commonly used i.v. iron preparations. In particular, there is accumulating evidence that depending on the stability and the redox properties of the iron complex, iron preparations can influence cytokine activation, reactive oxygen species generation, and lymphocyte survival to different degrees [46-48].
In this study, the iron(III)/iron(II) reduction potentials of the different ISSs, except ISSFERP, showed great variations from the USP values for iron sucrose and that of ISORIG. The reduction potentials of ISSFERI, ISSFERO and ISSENCI were more positive than that of ISORIG, suggesting that these ISS preparations may undergo redox cycling under physiological conditions and thus cause oxidative stress. Previous studies also showed that other ISSs with a more positive reduction potential than that of ISORIG induced oxidative stress in an analogous non-clinical model [24, 25]. Interestingly, ISSFERI was found to exhibit two distinct reduction potentials whereas ISSENCI had different reduction potentials depending on the analyzed lot.
Cellular uptake, transient storage and subsequent utilization of iron are influenced in part by the type of iron preparation administered and in part by the dosage, treatment regimen and physiological status of the patient [49]. Iron from the ideal iron preparation is transiently deposited in the reticuloendothelial system and not in the parenchyma of the liver [11]. ISORIG shows exactly this pattern, with a more pronounced increase in iron and ferritin in the liver, and only a smaller increase relative to the control group in the heart and kidney. The presence of NTBI and oxidative stress in the livers of animals of the ISS groups was consistent with positive staining for iron not only in the Kupffer cells but also in the surrounding tissue. Accordingly, ferritin deposits, in particular in the liver, were reduced in the ISS groups suggesting that iron was stored in other cellular compartments rather than in the endogenous storage protein ferritin found predominantly in the liver.
The data from this study along with the results from three additional ISSs tested in two previously published studies [24, 25] show that various ISSs have slightly different toxicological patterns. In particular, the organs analyzed (liver, heart, and kidney) were not always affected to the same extent. For instance, most ISSs from this study showed a more pronounced effect on the kidneys (as seen from the proteinuria, IL6, iron and ferritin data), whereas significant differences in liver toxicity have been observed for two ISSs tested in a previous study [24].
Despite the significant differences that have been observed between ISORIG and the investigated ISSs, the clinical relevance of the toxicological model used in this study and the significance of the results obtained require further discussion. The i.v. administration route adopted in this study is similar to clinical practice since iron sucrose can be given by both injection as well as infusion. However, the single iron doses used in the clinic are usually lower than those used in this study. Regarding oxidative stress, several clinical studies have investigated changes in biomarkers of oxidative stress after i.v. iron administration and demonstrated an inverse relation between the stability of the iron complexes and the induction of oxidative stress [46, 47, 50-52]. Accordingly, differences in the physicochemical properties and stability of ISSs compared to ISORIG as demonstrated in the present study may also be expected to affect oxidative stress levels in the clinical setting. In addition, differences observed in the present study in the distribution of iron that resulted in the deposition of iron in the parenchymal tissues instead of the reticuloendothelial system may lead to a decrease in the amount of iron that is available for effective erythropoiesis and, therefore, partly explain the recently published clinical observation of a lower efficacy of an ISS in maintaining Hb and iron status parameters in hemodialysis patients after the switch from ISORIG [26].
Overall, the significant differences observed for most of the ISSs compared to the ISORIG, raise potential safety concerns on the interchangeabilty of these i.v. iron preparations and suggest that careful non-clinical and clinical evaluations of the safety and efficacy of ISSs should be performed before exposing vulnerable patients to potential additional sources of oxidative stress and inflammation.
CONCLUSION
The results from this study support those of previous comparative non-clinical ISS studies highlighting the extent and severity of the effects that variations in manufacturing procedures for iron sucrose preparations can cause. The physico-chemical characterization demonstrated that five of the six studied ISS preparations do not comply with the specifications of the USP Monograph for iron sucrose injection. Differences of variable degree in the molecular structures of these compounds arise from variations in the manufacturing process and may be responsible for the toxic effects that were observed in this non-clinical model. In contrast, polarographic analyses of three different ISORIG lots were identical and thus confirmed the high degree of consistency in the manufacturing process of ISORIG. Interestingly, ISSFERP, which met the physico-chemical reference values for iron sucrose, induced an elevated level of oxidative stress compared to ISORIG. Thus demonstrating that similar physico-chemical properties do not ensure similar toxicological effects. The reduced ability of the investigated ISSs to provide iron in the form that can be stored in ferritin, as well as the increased iron release indicated by higher oxidative stress marker levels, led to deleterious effects on hemodynamic, functional and inflammatory responses. In conclusion, all of the studied ISS preparations marketed in Asian countries showed different toxicological effects in rats compared to the iron sucrose originator and therefore, before administering such products in the clinical setting, it is suggested that therapeutic equivalence to the originator product ought to be proved to avoid exposing patients to potentially less safe preparations.
ACKNOWLEDGEMENT
Scientific writing support was provided by Dr. Taija Koskenkorva-Frank [Vifor (International) Ltd.].
ABBREVIATIONS
- ALP
= Alkaline phosphatase
- ALT
= Alanine aminotransferase
- AST
= Aspartate aminotransferase
- CHF
= Chronic heart failure
- CKD
= Chronic kidney disease
- CrCl
= Creatinine clearance
- Cu,Zn-SOD
= Cu,Zn superoxide dismutase
- GPx
= Glutathione peroxidase
- GSH
= Glutathione
- Hb
= Hemoglobin
- i.v.
= Intravenous
- ID
= Iron deficiency
- IDA
= Iron deficiency anemia
- IL6
= Interleukin-6
- ISORIG
= Iron sucrose originator
- ISS
= Iron sucrose similar
- ISSBACK
= Iron sucrose similar Fe-Back
- ISSENCI
= Iron sucrose similar Encifer®
- ISSFERI
= Iron sucrose similar Ferijet
- ISSFERO
= Iron sucrose similar Ferosoft®-S
- ISSFERP
= Iron sucrose similar Ferplex® SS
- ISSLIB
= Iron sucrose similar Fe-Lib
- NTBI
= Non-transferrin bound iron
- RBC
= Red blood cell
- SBP
= Systolic blood pressure
- TBARS
= Thiobarbituric acid reactive species
- TNF-α
= Tumor necrosis factor-alpha
- TSAT
= Transferrin saturation
- USP
= The United States Pharmacopeia
CONFLICT OF INTEREST
Vifor (International) Ltd. financially supported this study but did not contribute to the study design. Professor Jorge E. Toblli has received research grants and consultancy fees from Vifor Pharma Ltd. The other authors have no conflicts of interest to declare.
REFERENCES
- 1. de Benoist B, McLean E, Egli I, Cogswell M. Worldwide prevalence of anaemia 1993-2005: WHO global database of anaemia. Geneva: World Health Organisation; 2008. [Google Scholar]
- 2. Crichton R, Danielson B, Geisser P. Iron therapy With Special Emphasis on Intravenous Administration. 4th ed. Bremen: UNI-MED Verlag AG; 2008. [Google Scholar]
- 3. Huch R, Schaefer R. Iron Deficiency and Iron Deficiency Anaemia. New York: Thieme Medical Publishers; 2006. [Google Scholar]
- 4. Afzali B, Goldsmith D J. Intravenous iron therapy in renal failure: friend and foe? J. Nephrol. 2004;17:487–495. [PubMed] [Google Scholar]
- 5. Alleyne M, Horne M K, Miller J L. Individualized treatment for iron-deficiency anemia in adults. Am. J. Med. 2008;121:943–948. doi: 10.1016/j.amjmed.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jacobs P, Johnson G, Wood L. Oral iron therapy in human subjects, comparative absorption between ferrous salts and iron polymaltose. J. Med. 1984;15:367–377. [PubMed] [Google Scholar]
- 7. Nissenson A R, Charytan C. Controversies in iron management. Kidney Int. 2003;64(Suppl. 87):S64–S71. doi: 10.1046/j.1523-1755.64.s87.10.x. [DOI] [PubMed] [Google Scholar]
- 8. Silverberg D S, Wexler D, Blum M, Iaina A. The cardio renal anemia syndrome: correcting anemia in patients with resistant congestive heart failure can improve both cardiac and renal function and reduce hospitalizations. Clin. Nephrol. 2003;60(Suppl 1):S93–102. [PubMed] [Google Scholar]
- 9. Wilson A, Reyes E, Ofman J. Prevalence and outcomes of anemia in inflammatory bowel disease: a systematic review of the literature. Am. J. Med. 2004;116(Suppl 7A):44S–49S. doi: 10.1016/j.amjmed.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 10. Johnson D W. Intravenous versus oral iron supplementation in peritoneal dialysis patients. Perit. Dial. Int. 2007;27(Suppl 2):S255–S260. [PubMed] [Google Scholar]
- 11. Geisser P, Baer M, Schaub E. Structure/histotoxicity relationship of parenteral iron preparations. Drug Res. 1992;42:1439–1452. [PubMed] [Google Scholar]
- 12. Macdougall I C. Intravenous administration of iron in epoetin-treated haemodialysis patients--which drugs, which regimen? Nephrol. Dial. Transplant. 2000;15:1743–1745. doi: 10.1093/ndt/15.11.1743. [DOI] [PubMed] [Google Scholar]
- 13. Macdougall I C. Strategies for iron supplementation: oral versus intravenous. Kidney Int. 1999;55(Suppl 69):S61–S66. doi: 10.1046/j.1523-1755.1999.055suppl.69061.x. [DOI] [PubMed] [Google Scholar]
- 14. Toblli J E, Cao G, Olivieri L, Angerosa M. Comparison of the renal, cardiovascular and hepatic toxicity data of original intravenous iron compounds. Nephrol. Dial. Transplant. 2010;25:3631–3640. doi: 10.1093/ndt/gfq260. [DOI] [PubMed] [Google Scholar]
- 15. Chertow G M, Mason P D, Vaage-Nilsen O, Ahlmen J. Update on adverse drug events associated with parenteral iron. Nephrol. Dial. Transplant . 2006;21:378–382. doi: 10.1093/ndt/gfi253. [DOI] [PubMed] [Google Scholar]
- 16. Faich G, Strobos J. Sodium ferric gluconate complex in sucrose: safer intravenous iron therapy than iron dextrans. Am. J. Kidney Dis. 1999;33:464–470. doi: 10.1016/s0272-6386(99)70183-6. [DOI] [PubMed] [Google Scholar]
- 17. Locatelli F, Aljama P, Bárány P, Canaud B, Carrera F, Eckardt KU, Hörl WH, Macdougal IC, Macleod A, Wiecek A, Cameron S European Best Practice Guidelines Working Group. Revised European best practice guidelines for the management of anaemia in patients with chronic renal failure. Nephrol. Dial. Transplant. 2004;19( Suppl 2):ii1–47. doi: 10.1093/ndt/gfh1032. [DOI] [PubMed] [Google Scholar]
- 18. Ternes N, Scheiber-Mojdehkar B, Landgraf G, Goldenberg H, Sturm B. Iron availability and complex stability of iron hydroxyethyl starch and iron dextran a comparative in vitro study with liver cells and macrophages. Nephrol. Dial. Transplant. 2007;22: 2824–2830. doi: 10.1093/ndt/gfm315. [DOI] [PubMed] [Google Scholar]
- 19. Andrews N C, Schmidt P J. Iron homeostasis. Annu. Rev. Physiol. 2007;69:69–85. doi: 10.1146/annurev.physiol.69.031905.164337. [DOI] [PubMed] [Google Scholar]
- 20. Evans RW, Rafique R, Zarea A, Rapisarda C, Cammack R, Evans PJ, Porter JB, Hider R C. Nature of non-transferrin-bound iron: studies on iron citrate complexes and thalassemic sera. J. Biol. Inorg. Chem . 2008;13:57–74. doi: 10.1007/s00775-007-0297-8. [DOI] [PubMed] [Google Scholar]
- 21. Zanen AL, Adriaansen HJ, van Bommel EF, Posthuma R, Th de Jong G M. 'Oversaturation' of transferrin after intravenous ferric gluconate (Ferrlecit®) in haemodialysis patients. Nephrol. Dial. Transplant. 1996;11:820–824. doi: 10.1093/oxfordjournals.ndt.a027405. [DOI] [PubMed] [Google Scholar]
- 22. Michael B, Coyne DW, Fishbane S, Folkert V, Lynn R, Nissenson AR, Agarwal R, Eschbach JW, Fadem SZ, Trout JR, Strobos J, Warnock D G Ferrlecit Publication Committee. Sodium ferric gluconate complex in hemodialysis patients: adverse reactions compared to placebo and iron dextran. Kidney Int. 2002;61:1830–1839. doi: 10.1046/j.1523-1755.2002.00314.x. [DOI] [PubMed] [Google Scholar]
- 23. Schellekens H, Klinger E, Muhlebach S, Brin JF, Storm G, Crommelin DJ. The therapeutic equivalence of complex drugs. Regul. Toxicol. Pharmacol. 2011;59:176–183. doi: 10.1016/j.yrtph.2010.09.021. [DOI] [PubMed] [Google Scholar]
- 24. Toblli JE, Cao G, Oliveri L, Angerosa M. Differences between original intravenous iron sucrose and iron sucrose similar preparations. Drug Res. 2009;59:176–190. doi: 10.1055/s-0031-1296383. [DOI] [PubMed] [Google Scholar]
- 25. Toblli JE, Cao G, Oliveri L, Angerosa M. Differences between the original iron sucrose complex Venofer® and the iron sucrose similar Generis®, and potential implications. Port. J. Nephrol. Hypert. 2009;1:53–63. [Google Scholar]
- 26. Rottembourg J, Kadri A, Leonard E, Dansaert A, Lafuma A. Do two intravenous iron sucrose preparations have the same efficacy? Nephrol. Dial. Transplant. 2011;26:3262–3267. doi: 10.1093/ndt/gfr024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.United States Pharmacopeial Convention. Iron Sucrose Injection, Official monograph in: The United States Pharmacopeia. Vol. 31. Rockville: United States Pharmacopeial Convention; 2008. pp. 2449–2451. [Google Scholar]
- 28. Danielson BG. Structure, chemistry, and pharmacokinetics of intravenous iron agents. J. Am. Soc. Nephrol. 2004;15(Suppl 2):S93–S98. doi: 10.1097/01.ASN.0000143814.49713.C5. [DOI] [PubMed] [Google Scholar]
- 29. Toblli JE, Stella I, de Cavanagh E, Angerosa M, Inserra F, Ferder L. Enalapril prevents tubulointerstitial lesions by hyperoxaluria. Hypertension. 1999;33:225–231. doi: 10.1161/01.hyp.33.1.225. [DOI] [PubMed] [Google Scholar]
- 30. Yamakoshi K, Rolfe P, Murphy C. Current developments in non-invasive measurement of arterial blood pressure. J. Biomed. Eng. 1988;10:130–137. doi: 10.1016/0141-5425(88)90088-x. [DOI] [PubMed] [Google Scholar]
- 31. Akerboom T P, Sies H. Assay of glutathione, glutathione disulfide, and glutathione mixed disulfides in biological samples. Methods. Enzymol . 1981;7:373–382. doi: 10.1016/s0076-6879(81)77050-2. [DOI] [PubMed] [Google Scholar]
- 32. Rossi R, Cardaioli E, Scaloni A, Amiconi G, Di Simplicio P. Thiol groups in proteins as endogenous reductants to determine glutathione-protein mixed disulphides in biological systems. Biochim. Biophys. Acta. 1995;1243:230–238. doi: 10.1016/0304-4165(94)00133-i. [DOI] [PubMed] [Google Scholar]
- 33. Toblli JE, Cao G, Oliveri L, Elba V. Inflammatory response of generic iron sucrose complex preparations in kidney, liver and heart of normal rats. Nephrol. Dial. Transplant . 2006;21:iv160. [Google Scholar]
- 34. Toblli JE, Munoz MC, Cao G, Mella J, Pereyra L, Mastai R. ACE inhibition and AT1 receptor blockade prevent fatty liver and fibrosis in obese Zucker rats. Obesity (Silver Spring) 2008;16:770–776. doi: 10.1038/oby.2007.114. [DOI] [PubMed] [Google Scholar]
- 35. Toblli JE, Cao G, DeRosa G, Forcada P. Reduced cardiac expression of plasminogen activator inhibitor 1 and transforming growth factor beta1 in obese Zucker rats by perindopril. Heart. 2005;91:80–86. doi: 10.1136/hrt.2003.022707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kulnigg S, Stoinov S, Simanenkov V, Dudar LV, Karnafel W, Garcia LC, Sambuelli AM, D'Haens G, Gasche C. A novel intravenous iron formulation for treatment of anemia in inflammatory bowel disease: the ferric carboxymaltose (FERINJECT) randomized controlled trial. Am. J. Gastroenterol. 2008;103:1182–1192. doi: 10.1111/j.1572-0241.2007.01744.x. [DOI] [PubMed] [Google Scholar]
- 37. Schröder O, Mickisch O, Seidler U, de Weerth A, Dignass AU, Herfarth H, Reinshagen M, Schreiber S, Junge U, Schrott M, Stein J. Intravenous iron sucrose versus oral iron supplementation for the treatment of iron deficiency anemia in patients with inflammatory bowel disease--a randomized, controlled, open-label, multicenter study. Am. J. Gastroenterol. 2005;100:2503–2509. doi: 10.1111/j.1572-0241.2005.00250.x. [DOI] [PubMed] [Google Scholar]
- 38. Bailie GR. Differences in spontaneously reported hypersensitivity and serious adverse events for intravenous iron preparations: comparison of Europe and North America. Drug Res. 2011;61:267–275. doi: 10.1055/s-0031-1296198. [DOI] [PubMed] [Google Scholar]
- 39. Geisser P. Safety and efficacy of iron(III)-hydroxide polymaltose complex / a review of over 25 years experience. Drug Res. 2007;57:439–452. doi: 10.1055/s-0031-1296693. [DOI] [PubMed] [Google Scholar]
- 40. Meier T, Schropp P, Pater C, Leoni AL, Khov-Tran VV, Elford P. Physicochemical and toxicological characterization of a new generic iron sucrose preparation. Drug Res. 2011;61:112–119. doi: 10.1055/s-0031-1296176. [DOI] [PubMed] [Google Scholar]
- 41. Toblli JE, Cao G, Giani J, Dominici F, Angerosa M. Different effects of European iron sucrose similar preparations and originator iron sucrose on nitrosative stress, apoptosis, oxidative stress, biochemical and inflammatory markers in rats. XLVIII ERAEDTA Congress. 2011 abstract SuO028. [Google Scholar]
- 42.European Medicines Agency. Reflection paper on non-clinical studies for generic nanoparticle iron medicinal product applications. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2011/04/WC500105048.pdf . 2011. [Accessed 17 Aug 2011].
- 43. Van Wyck DB, Anderson J, Johnson K. Labile iron in parenteral iron formulations: a quantitative and comparative study. Nephrol. Dial. Transplant. 2004;19:561–565. doi: 10.1093/ndt/gfg579. [DOI] [PubMed] [Google Scholar]
- 44. Fishbane S. Safety in iron management. Am. J. Kidney Dis. 2003;41:18–26. doi: 10.1016/s0272-6386(03)00373-1. [DOI] [PubMed] [Google Scholar]
- 45. Kartikasari AE, Georgiou NA, Visseren FL, van Kats-Renaud H, van Asbeck BS, Marx J J. Endothelial activation and induction of monocyte adhesion by nontransferrin-bound iron present in human sera. FASEB J. 2006;20:353–355. doi: 10.1096/fj.05-4700fje. [DOI] [PubMed] [Google Scholar]
- 46. Pai AB, Boyd AV, McQuade CR, Harford A, Norenberg J P, Zager P G. Comparison of oxidative stress markers after intravenous administration of iron dextran, sodium ferric gluconate, and iron sucrose in patients undergoing hemodialysis. Pharmacotherapy. 2007;27:343–350. doi: 10.1592/phco.27.3.343. [DOI] [PubMed] [Google Scholar]
- 47. Pai AB, Conner T, McQuade CR, Olp J, Hicks P. Non-transferrin bound iron, cytokine activation and intracellular reactive oxygen species generation in hemodialysis patients receiving intravenous iron dextran or iron sucrose. Biometals. 2011;24:603–613. doi: 10.1007/s10534-011-9409-6. [DOI] [PubMed] [Google Scholar]
- 48. Gupta A, Zhuo J, Zha J, Reddy S, Olp J, Pai A. Effect of different intravenous iron preparations on lymphocyte intracellular reactive oxygen species generation and subpopulation survival. BMC Nephrol. 2010;11:16–20. doi: 10.1186/1471-2369-11-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Caperna T J, Failla M L, Steele N C, Richards M P. Accumulation and metabolism of iron-dextran by hepatocytes, Kupffer cells and endothelial cells in the neonatal pig liver. J. Nutr. 1987;117:312–320. doi: 10.1093/jn/117.2.312. [DOI] [PubMed] [Google Scholar]
- 50. Stefánsson B V, Haraldsson B, Nilsson U. Acute oxidative stress following intravenous iron injection in patients on chronic hemodialysis: a comparison of iron-sucrose and iron-dextran. Nephron Clin. Pract. 2011;118:c249–256. doi: 10.1159/000321645. [DOI] [PubMed] [Google Scholar]
- 51. Michelis R, Sela S, Kristal B. Intravenous iron-gluconate during haemodialysis modifies plasma beta2-microglobulin properties and levels. Nephrol. Dial. Transplant . 2005;20:1963–1969. doi: 10.1093/ndt/gfh907. [DOI] [PubMed] [Google Scholar]
- 52. Borawski J, Gozdzikiewicz J, Abramowicz P, Naumnik B, Mysliwiec M. Endothelial injury markers with high-dose intravenous iron therapy in renal failure. Clin. Appl. Thromb. Hemost. 2004;10:403–406. doi: 10.1177/107602960401000416. [DOI] [PubMed] [Google Scholar]