Abstract
Purpose
To investigate the changes and correlations of the serum inflammation factors levels and left ventricular (LV) structure and function in patients with acute ST segment elevation myocardial infarction (STEMI).
Materials and Methods
A prospective study was performed on 70 STEMI patients and 70 control subjects. Serum levels of interleukin-6 (IL-6), soluble CD40 ligand (sCD40L), metalloproteinase-9 (MMP-9) and tissue inhibitor of metalloproteinase-1 (TIMP-1) were measured by sandwich enzyme-linked immunosorbent assay (ELISA), and cardiac structure and function were assessed by echocardiography at admission and 3-year follow-up.
Results
We found that the levels of serum IL-6, sCD40L and MMP-9 increased steadily among control subjects, remote myocardial infarction and acute STEMI patients, and the level of TIMP-1 elevated remarkly at 3-year follow-up visit in STEMI. The admission level of serum MMP-9 positively correlated with LV end-diastolic and end-diastole volume (r=0.294, p=0.022; r=0.269, p=0.036, respectively), and TIMP-1 positively correlated with E/A ratio (r=0.278, p=0.044) at 3-year follow-up.
Conclusion
The study indicates that admission levels of serum MMP-9 and TIMP-1 closely correlated with left ventricular structure and function, which may be involved in the process of post-infarction remodeling of myocardium.
Keywords: Acute ST segment elevation myocardial infarction, metalloproteinase-9, tissue inhibitor of metalloproteinase-1, left ventricular remodeling
INTRODUCTION
There is increased recognition that inflammation plays a key role in the pathogenesis of atherosclerosis and its complications.1 Recently, attention has been focused on the potential role of circulating inflammatory markers as risk predictors to indentify patients who are more likely to suffer a clinical event. In patients with myocardial infarction, the progressive left ventricular (LV) dilation, that is, ventricular remodeling, is linked intimately to adverse prognosis. A simple plasma marker that can allow early risk stratification would benefit patients in need of intensive therapy. The cardiac extracellular matrix (ECM), the connective tissue scaffold on which cellular elements are arranged, plays a vital role in the maintenance of myocardial structure and function, particularly that of the LV. Physiological integrity of ECM structure is largely under the control of the matrix metalloproteinase (MMPs) family of endopeptidases, and a fine balance between MMPs and tissue inhibitors of MMPs (TIMPs) is essential for maintaining the integrity of the highly organized and dynamic matrix that provides structural support for myocytes and a medium for non-myocytes, proteins, and signaling molecules.2 Previous studies reported that the activation of MMPs that degrade the extracellular matrix has been linked to adverse LV remodeling post-myocardial infarction.3,4 Interleukin-6 (IL-6) and soluble CD40 ligand (sCD40L) also have been shown to be predictors of adverse outcome in patients with coronary artery disease (CAD).5,6 However, a limitation of the previous studies is that inflammation markers were measured only at a single time point, and changes during follow-up were not measured. In this study, the circulating serum levels of the inflammatory markers (IL-6, sCD40L, MMP-9 and TIMP-1) were determined and echocardiographic assessment was carried out in patients with acute ST-segment elevation myocardial infarction (STEMI) at admission and 3-year follow-up visit, and we then evaluated the changes of the above inflammatory markers levels during 3-years follow-up period and the correlations between the above inflammatory markers with LV structure and function post-myocardial infarction.
MATERIALS AND METHODS
Study subjects
A prospective, cohort study was performed on 70 STEMI patients with first STEMI who were admitted to our institute within 6 h of symptoms onset, and 70 age- and gender-matched subjects as control, who had no CAD. Acute STEMI was defined as the presence of typical prolonged (>30 min) chest pain accompanied by typical ST segment elevation ≥0.2 mV in two or more contiguous leads on the standard 12-lead electrocardiogram (ECG) and abnormal increase of MB fraction of creatine kinase greater than twice the normal upper limit. Patients with equivocal or uninterpretable ECGs (i.e. left bundle branch block, paced rhythm, or persistent ST-segment elevation after a previous myocardial infarction) were not included in the study.
The present study did not include patients with a history of hematological, neoplastic, renal, liver or thyroid disease, or patients receiving treatment with anti-inflammatory drugs. Patients with acute or chronic infections and autoimmune disease were also excluded from the study. The study protocol was approved by the ethics committee of our institution, and written informed consent was obtained from all participating subjects.
Clinical data collection
Demographic data, lifestyle, environmental factors, medical history and treatment were collected in the hospital. Modifiable risk factor reassessment, event or complications, and current therapy were recorded at 3-year follow-up visit. A person who reported smoking at least one cigarette per day for at least one year were defined as current smokers. Indicate the negative life events occurred in the past 6 months (i.e. serious illness or death in family member, divorce or separation, forced to change job, feelings of insecurity at work, serious financial trouble, and being legally prosecuted). Height, weight and waist circumference were measured, and body mass index was calculated as the weight in kilograms divided by the square of the height in meters.
Laboratory procedures
In STEMI patients, peripheral venous blood was drawn immediately after admission and at the 3-year follow-up visit. In control subjects, blood samples were collected after an overnight fast at baseline. Sample after clotting were centrifuged at 2500 rpm for 10 min, and the serum was frozen and stored at -70℃ until analyzed. Sandwich enzyme-linked immunosorbent assay was performed for measuring concentrations of serum IL-6, MMP-9 and TIMP-1, using Quantikine R&D Systems commercial kits, and of serum sCD40L using Bender Medsystems commercial kits. The lower detection limits were 0.7 pg/mL for IL-6, 0.156 ng/mL for MMP-9, 0.08 ng/mL for TIMP-1 and 0.095 ng/mL for sCD40L. The average inter- and intra-assay coefficients of variation were <10% for all assays.
Echocardiographic assessment
M-mode and 2-dimensional echocardiography and Doppler ultrasound assessment were carried out at the time of admission and 3-year follow-up visit, by a single operator using a HP 7500 or IE 33 scanner. The study required recording of 10 cycles of two-dimensional (2D) parasternal long- and short-axis LV views and 10 cycles of M-mode with optimal cursor beam orientation in each view. If the 2D guided M-mode beam could not be optimally oriented, a 2D long-axis view was used to obtain linear measurements of the LV cavity [LV end-diastolic diameter (LVEDD) and LV end-systolic diameter (LVESD)] and walls. LV mass was estimated using the anatomically validated formula of Devereux. The indices that adjusted LV mass (LVM) was LV mass index (LVMI), defined as LVM/body surface area. The relative wall thickness was calculated as 2 posterior wall/LVEDD. LV end-systolic volume (LVESV), LV end-diastolic volume (LVEDV), and LV ejection fraction (LVEF) were estimated using the biplanar modified Simpson's rule from apical 2 and 4 chamber views. LV diastolic function was evaluated by the following pulse-wave Doppler echocardiographic parameters: early (E)/late (A) ratio, indicating the pattern of LV diastolic filling, measured by pulse-wave Doppler flow pattern at mitral anulus traced electronically to measure peak velocities of early and late diastolic LV filling, that is, early (E) and late (A) transmitral peak flow velocities.
Statistical analysis
Data are expressed as means±SD or median and interquartile ranges, as appropriate. Unpaired or paired Student's t-test was used to evaluate differences in normally distributed continuous variables between the two groups. Because of non-normal distribution of inflammatory markers variables, they were natural log-transformed. The normality of sCD40L, MMP-9, TIMP-1 and MMP-9/TIMP-1 ratio was achieved, and one-way analysis of variance and Post Hoc test were used; Kruskal-Wallis and Mann-Whitney test were used for IL-6 among three groups since the variable was non-normally distributed even after log transformation. Qualitative data are presented as numbers (percentages), and Chi-square tests were performed for categorical variables. Correlation analysis between variables of the study was made by means of Spearman's correlation coefficient r for continuous variables with non-normal distribution. For all tests, a 2-tailed p<0.05 was considered statistically significant. All calculations were made using SPSS statistical software for Windows (version 12.0).
RESULTS
Clinical characteristics
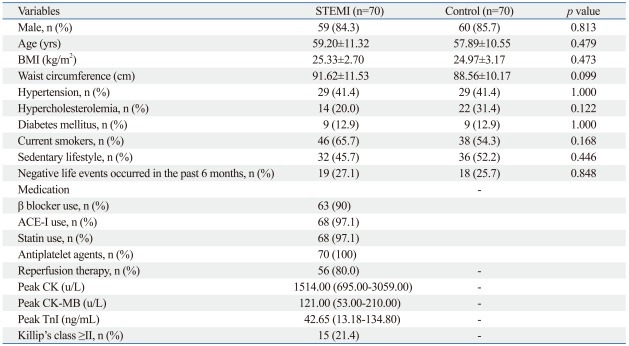
The baseline features of the study population are shown in Table 1. There were no significant differences in any of the clinical variables among the two patient groups.
Table 1.
Baseline Characteristics of the Study Population
BMI, body mass index; ACE-I, angiotensin-converting enzyme inhibitors; CK, creatine kinase; CK-MB, creatine kinase-myocardial fraction; Tn-I, Troponin I; STEMI, segment elevation myocardial infarction; Age, BMI and waist circumference are presented as mean±standard deviation; Peak CK, CK-MB and TnI are presented as medians (25th-75th percentile); categorical variables are presented as number (%).
Serum inflammatory markers levels
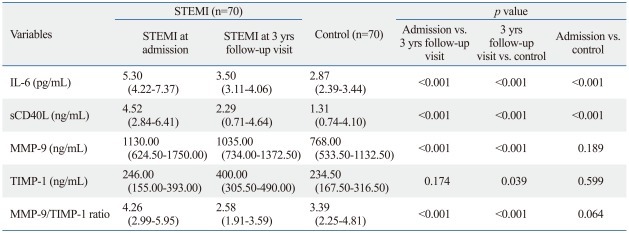
Significant differences in the levels of IL-6, sCD40L, MMP-9 and TIMP-1 were detected among the three studied groups, as shown in Table 2. The levels of IL-6 and sCD40L were found to be elevated in acute STEMI as compared to controls (both p<0.001); In patients with remote myocardial infarction (at 3-year follow-up visit), the levels of IL-6, sCD40L, MMP-9 and MMP-9/TIMP-1 ratio were significantly reduced compared admission levels (all p<0.001); The levels of IL-6, sCD40L, MMP-9, and TIMP-1 were still higher at 3-year follow-up visit in STEMI patients compared to controls (p<0.001, p<0.001, p<0.001 and p=0.039, respectively).
Table 2.
Serum Levels of Inflammatory Markers
IL-6, interleukin-6; sCD40L, soluble CD40 ligand; MMP-9, metalloproteinase-9; TIMP-1, tissue inhibitor of metalloproteinase-1; STEMI, segment elevation myocardial infarction.
Data are presented as medians (25th-75th percentile).
Echocardiographic assessment
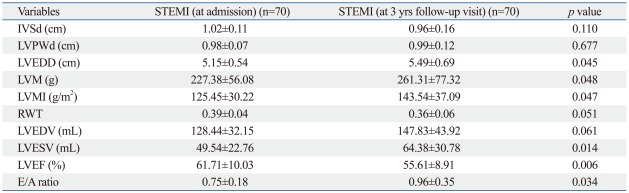
After 3-years, LVEDD, LVM, LVMI, LVESV and E/A ratio were significantly increased (p=0.045, p=0.048, p=0.047, p=0.014 and p=0.034, respectively), and LVEF was impaired (p=0.006), compared with baseline (Table 3).
Table 3.
Changes in Echocardiographic Parameters from Admission to 3 Years Follow-Up Visit in STEMI Patients
IVSd, interventricular septal thickness in diastole; LVPWd, left ventricular posterior wall thickness in diastole; LVEDD, left ventricular end-diastolic dimension; LVM, left ventricular mass; LVMI, left ventricular mass index; RWT, relative wall thickness; LVEDV, left ventricular end-diastolic volume; LVESV, left ventricular end-systolic volume; LVEF, left ventricular ejection fraction.
E, mitral peak flow velocity of early filling; A, peak flow velocity of late filling.
Data are presented as mean±standard deviation.
There was no association between the levels of IL-6, sCD40L, MMP-9, TIMP-1 and echocardiographic markers of LV structure or function at the same time. However, MMP-9 measured at admission showed weak association with great LVEDD and LVEDV (r=0.294, p=0.022 and r=0.269, p=0.036, respectively), and TIMP-1 positively correlated with E/A ratio at follow-up (r=0.278, p=0.044).
DISCUSSION
The main findings of this study are that the levels of serum IL-6, sCD40L and MMP-9 were steadily increased among control subjects, remote myocardial infarction and acute STEMI patients, that TIMP-1 was elevated, and MMP-9/TIMP-1 ratio decreased markedly in remote STEMI, that MMP-9 measured at admission showed a positive association with great LVEDD and LVEDV, and that TIMP-1 positively correlated with E/A ratio measured at subsequent follow-up period. The results suggest that elevated levels of IL-6, sCD40L, and MMP-9 and the imbalance of MMP-9/TIMP-1 system are involved in the process of plaque rupture, leading to the incidence of myocardial infarction, and that MMP-9/TIMP-1 may also be linked to the post-infarction remodeling of myocardium, probably leading to heart failure.
Acute coronary syndrome (ACS) is triggered by dysfunctional endothelial and atherosclerotic plaque instability. The stage of plaque instability is that pro-inflammatory cytokines and chemoattractants induce leukocyte chemoattraction to the endothelium, and together with other triggers such as the CD40L-CD40 co-stimulation system activate plaque monocytes (macrophages). The macrophages then produce MMPs that disintegrate extra-cellular plaque matrix, causing coronary plaque instability.7 IL-6 is a circulating cytokine known to be secreted from a number of different cells including activated macrophages and lymphocytes, which could stimulates hepatocytes to synthesize acute phase response proteins such as C-reactive protein and fibrinogen. The CD40L on T-cells binding to the CD40 receptor on macrophages is involved in the multiple stages of atherosclerosis and ACS, inducing macrophages to secrete tissue factor, up-regulating the expression of adhesion molecules, and activating secretion of MMPs. Previous study8,9 have shown, in agreement with our results, that the concentration of IL-6 and sCD40L are significantly elevated from stable CAD to ACS, as compared to control, possibly supporting their role in plaque instability and rupture.
MMPs are a tightly regulated group of zinc-dependent peptidases that participate in matrix turnover in both normal and pathological conditions.10 In the present study, we studied one member from the family of gelatinases, such as MMP-9, which has been found highly expressed in the shoulder regions of advanced atherosclerotic lesions and contributes to plaque instability.11 The main endogenous MMPs inhibitors are TIMPs, and TIMP-1 inhibits most MMPs except for MMP-2. Under normal circumstances, the activeity of MMPs and TIMPs maintain a balance between connective tissue synthesis and degradation. When the balance is disturbed, the undesired tissue destruction or remodeling takes place. Previous studes12-15 have reported that patients with ACS exhibited significantly higher levels of MMP-9 and TIMP-1 than those with stable angina and normal control subjects, and the higher levels were related to the presence of plaque rupture in the culprit lesion. In our pervious study,16 we found that acute STEMI patients exhibited significantly higher level of MMP-9 and slightly elevated TIMP-1 as compared to control, therefore, the MMP-9/TIMP-1 ratio was remarkably increased, indicating that an imbalance between MMP-9 and TIMP-1 in the micro-environment of the vulnerability atherosclerotic plaques may be responsible at least in part for plaque disruption eventually leading to occurrence of cardiovascular events. In the present study, we failed to find any significant difference of the MMP-9 and TIMP-1 levels between acute STEMI patients and control due to a small sample size. However, we found that the levels of MMP-9 and the ratio of MMP-9/TIMP-1 were significantly higher in acute STEMI patients than those in remote STEMI, which also add weight to our previous findings.
MMPs and TIMPs participate not only in the process of atherogenesis and atherosclerotic plaque destabilization, they may also influence LV dilation and function impairment after myocardial infarction.17-20 In the present study, we found that MMP-9 measured at admission showed a positive association with great LVEDD and LVEDV at 3-year follow-up visit, which add the additional weight to the above-described notion. We also found that TIMP-1 was elevated remarkly in remote myocardial, and that TIMP-1 at admission positively correlated with E/A ratio measured in the subsequent follow-up period. Thus, we speculate that TIMP-1, following the activation of MMP-9, plays a protective role during the phase of LV remodeling and function impairment. Indeed, MMPs inhibition has been postulated as a potential therapeutic intervention in patients with acute myocardial infarction. Beneficial effects of selective MMP inhibition on regional myocardial geometry post-myocardial infarction were shown in experiment animal models.21 Miyazaki, et al.22 found that LV remodeling might be suppressed in association with MMP-9 suppression in acute myocardial infarction patients treated with percutaneous coronary intervention and regular dose or half-dose-combination of renin-angiotensin system inhibitors. However, the broad spectrum MMP inhibitor PG-116800 failed to attenuate adverse LV remodeling after myocardial infarction in prevention of myocardial infarction early remodeling trial.23 Therefore, selective MMP inhibition seems to be the better solution.
The limitations of our study should be considered. First, although significant, the associations between MMP-9, TIMP-1 and LV remodeling were relatively weak, which can not be entirely due to relatively small sample size, nevertheless, a larger population is needed to confirm our findings. Second, we should study the change of the inflammatory markers during the period between admission and discharge, and then select the highest levels in the acute state. Ideally, control group should be the patients with stable CAD and healthy subjects, so as to observe the defferent levels of the inflammatory markers, and elucidate the role of inflammation in the different phases of CAD. Third, the development of ventricular remodeling and function impairment after myocardial infarction are strongly affected by the infracted area and drug treatment during follow-up period, and these factors should not be excluded in the study.
In conclusion, the admission levels of serum MMP-9 and TIMP-1 closely correlated with left ventricular structure and function, which may be involved in the process of post-infarction remodeling of myocardium.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 2.Jugdutt BI. Ventricular remodeling after infarction and the extracellular collagen matrix: when is enough enough? Circulation. 2003;108:1395–1403. doi: 10.1161/01.CIR.0000085658.98621.49. [DOI] [PubMed] [Google Scholar]
- 3.Kelly D, Cockerill G, Ng LL, Thompson M, Khan S, Samani NJ, et al. Plasma matrix metalloproteinase-9 and left ventricular remodelling after acute myocardial infarction in man: a prospective cohort study. Eur Heart J. 2007;28:711–718. doi: 10.1093/eurheartj/ehm003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kelly D, Khan S, Cockerill G, Ng LL, Thompson M, Samani NJ, et al. Circulating stromelysin-1 (MMP-3): a novel predictor of LV dysfunction, remodelling and all-cause mortality after acute myocardial infarction. Eur J Heart Fail. 2008;10:133–139. doi: 10.1016/j.ejheart.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fisman EZ, Benderly M, Esper RJ, Behar S, Boyko V, Adler Y, et al. Interleukin-6 and the risk of future cardiovascular events in patients with angina pectoris and/or healed myocardial infarction. Am J Cardiol. 2006;98:14–18. doi: 10.1016/j.amjcard.2006.01.045. [DOI] [PubMed] [Google Scholar]
- 6.Yan JC, Zhu J, Gao L, Wu ZG, Kong XT, Zong RQ, et al. The effect of elevated serum soluble CD40 ligand on the prognostic value in patients with acute coronary syndromes. Clin Chim Acta. 2004;343:155–159. doi: 10.1016/j.cccn.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 7.Gidron Y, Gilutz H, Berger R, Huleihel M. Molecular and cellular interface between behavior and acute coronary syndromes. Cardiovasc Res. 2002;56:15–21. doi: 10.1016/s0008-6363(02)00537-0. [DOI] [PubMed] [Google Scholar]
- 8.Tousoulis D, Antoniades C, Nikolopoulou A, Koniari K, Vasiliadou C, Marinou K, et al. Interaction between cytokines and sCD40L in patients with stable and unstable coronary syndromes. Eur J Clin Invest. 2007;37:623–628. doi: 10.1111/j.1365-2362.2007.01834.x. [DOI] [PubMed] [Google Scholar]
- 9.Wang J, Zhang S, Jin Y, Qin G, Yu L, Zhang J. Elevated levels of platelet-monocyte aggregates and related circulating biomarkers in patients with acute coronary syndrome. Int J Cardiol. 2007;115:361–365. doi: 10.1016/j.ijcard.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 10.Lijnen HR. Plasmin and matrix metalloproteinases in vascular remodeling. Thromb Haemost. 2001;86:324–333. [PubMed] [Google Scholar]
- 11.Brown DL, Hibbs MS, Kearney M, Loushin C, Isner JM. Identification of 92-kD gelatinase in human coronary atherosclerotic lesions. Association of active enzyme synthesis with unstable angina. Circulation. 1995;91:2125–2131. doi: 10.1161/01.cir.91.8.2125. [DOI] [PubMed] [Google Scholar]
- 12.Inokubo Y, Hanada H, Ishizaka H, Fukushi T, Kamada T, Okumura K. Plasma levels of matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 are increased in the coronary circulation in patients with acute coronary syndrome. Am Heart J. 2001;141:211–217. doi: 10.1067/mhj.2001.112238. [DOI] [PubMed] [Google Scholar]
- 13.Derosa G, D'Angelo A, Scalise F, Avanzini MA, Tinelli C, Peros E, et al. Comparison between metalloproteinases-2 and -9 in healthy subjects, diabetics, and subjects with acute coronary syndrome. Heart Vessels. 2007;22:361–370. doi: 10.1007/s00380-007-0989-6. [DOI] [PubMed] [Google Scholar]
- 14.Shu J, Ren N, Du JB, Zhang M, Cong HL, Huang TG. Increased levels of interleukin-6 and matrix metalloproteinase-9 are of cardiac origin in acute coronary syndrome. Scand Cardiovasc J. 2007;41:149–154. doi: 10.1080/14017430601164263. [DOI] [PubMed] [Google Scholar]
- 15.Fukuda D, Shimada K, Tanaka A, Kusuyama T, Yamashita H, Ehara S, et al. Comparison of levels of serum matrix metalloproteinase-9 in patients with acute myocardial infarction versus unstable angina pectoris versus stable angina pectoris. Am J Cardiol. 2006;97:175–180. doi: 10.1016/j.amjcard.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 16.Tan J, Hua Q, Gao J, Fan ZX. Clinical implications of elevated serum interleukin-6, soluble CD40 ligand, metalloproteinase-9, and tissue inhibitor of metalloproteinase-1 in patients with acute ST-segment elevation myocardial infarction. Clin Cardiol. 2008;31:413–418. doi: 10.1002/clc.20254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsunaga T, Abe N, Kameda K, Hagii J, Fujita N, Onodera H, et al. Circulating level of gelatinase activity predicts ventricular remodeling in patients with acute myocardial infarction. Int J Cardiol. 2005;105:203–208. doi: 10.1016/j.ijcard.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 18.Webb CS, Bonnema DD, Ahmed SH, Leonardi AH, McClure CD, Clark LL, et al. Specific temporal profile of matrix metalloproteinase release occurs in patients after myocardial infarction: relation to left ventricular remodeling. Circulation. 2006;114:1020–1027. doi: 10.1161/CIRCULATIONAHA.105.600353. [DOI] [PubMed] [Google Scholar]
- 19.Vanhoutte D, Schellings M, Pinto Y, Heymans S. Relevance of matrix metalloproteinases and their inhibitors after myocardial infarction: a temporal and spatial window. Cardiovasc Res. 2006;69:604–613. doi: 10.1016/j.cardiores.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 20.Kelly D, Khan SQ, Thompson M, Cockerill G, Ng LL, Samani N, et al. Plasma tissue inhibitor of metalloproteinase-1 and matrix metalloproteinase-9: novel indicators of left ventricular remodelling and prognosis after acute myocardial infarction. Eur Heart J. 2008;29:2116–2124. doi: 10.1093/eurheartj/ehn315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Apple KA, Yarbrough WM, Mukherjee R, Deschamps AM, Escobar PG, Mingoia JT, et al. Selective targeting of matrix metalloproteinase inhibition in post-infarction myocardial remodeling. J Cardiovasc Pharmacol. 2006;47:228–235. doi: 10.1097/01.fjc.0000200989.23987.b8. [DOI] [PubMed] [Google Scholar]
- 22.Miyazaki S, Kasai T, Miyauchi K, Miyazaki T, Akimoto Y, Takagi A, et al. Changes of matrix metalloproteinase-9 level is associated with left ventricular remodeling following acute myocardial infarction among patients treated with trandolapril, valsartan or both. Circ J. 2010;74:1158–1164. doi: 10.1253/circj.cj-09-0412. [DOI] [PubMed] [Google Scholar]
- 23.Hudson MP, Armstrong PW, Ruzyllo W, Brum J, Cusmano L, Krzeski P, et al. Effects of selective matrix metalloproteinase inhibitor (PG-116800) to prevent ventricular remodeling after myocardial infarction: results of the PREMIER (Prevention of Myocardial Infarction Early Remodeling) trial. J Am Coll Cardiol. 2006;48:15–20. doi: 10.1016/j.jacc.2006.02.055. [DOI] [PubMed] [Google Scholar]