Abstract
Clinically significant carbon dioxide embolism is a rare but potentially fatal complication of anesthesia administered during laparoscopic surgery. Its most common cause is inadvertent injection of carbon dioxide into a large vein, artery or solid organ. This error usually occurs during or shortly after insufflation of carbon dioxide into the body cavity, but may result from direct intravascular insufflation of carbon dioxide during surgery. Clinical presentation of carbon dioxide embolism ranges from asymptomatic to neurologic injury, cardiovascular collapse or even death, which is dependent on the rate and volume of carbon dioxide entrapment and the patient's condition. We reviewed extensive literature regarding carbon dioxide embolism in detail and set out to describe the complication from background to treatment. We hope that the present work will improve our understanding of carbon dioxide embolism during laparoscopic surgery.
Keywords: Carbon dioxide, embolism, laparoscopy, pneumoperitoneum
INTRODUCTION
Laparoscopy has become a routine method for diagnosis and treatment of gynecological and intra-abdominal diseases. To do so requires insufflation of carbon dioxide for accurate visualization and operative manipulation. Consequently, carbon dioxide embolism may arise therefrom. Carbon dioxide embolism is a rare but potentially serious complication of laparoscopic procedures.1 It is caused by entrapment of carbon dioxide in an injured vein, artery or solid organ, and results in blockage of the right ventricle (RV) or pulmonary artery.2 There have been reports of carbon dioxide emboli occurring in various procedures including laparoscopic appendectomy,3 laparoscopic cholecystectomy,4-7 endoscopy,8 hysteroscopy,9 and other gynecological laparoscopic surgeries.10-13 Recently, carbon dioxide emboli have also been reported to occur during laparoscopic nephrectomy,14 laparoscopic hepatectomy,15 endoscopic vein harvesting,16,17 endoscopic thyroidectomy,18 and laparoscopic radical prostatectomy.19
Here we provide an extensive literature review regarding carbon dioxide embolism in detail, and describe the incidence, pathophysiology, clinical signs, diagnosis, prevention, and treatment of carbon dioxide embolism during laparoscopic surgery.
INCIDENCE
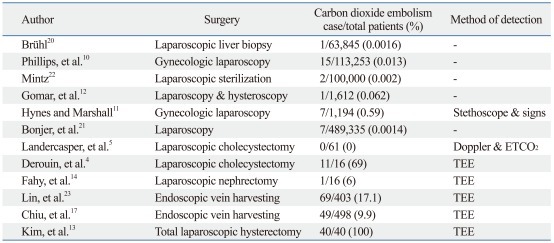
Although the occurrence of clinically significant carbon dioxide embolism is rare, the incidence of any kind of carbon dioxide embolism is varied (Table 1). Brühl20 reviewed 63,845 laparoscopies for liver biopsies and found only one case of gas embolism among 1,594 serious complications. In 1974, Phillips, et al.10 reported 15 probable carbon dioxide emboli among 113,253 gynecologic laparoscopies performed in one year. Hynes and Marshall11 found seven cases of carbon dioxide embolism out of 1,194 gynecologic laparoscopic procedures, representing an incidence of 0.59%. In a recent meta-analysis of nearly 500,000 closed-entry laparoscopies, carbon dioxide emboli occurred in seven out of 489,335 laparoscopic procedures (0.001%).21 Mintz22 reported three fatal carbon dioxide emboli in a review of 100,000 laparoscopies in France in 1977. Notwithstanding, clinically significant carbon dioxide emboli may be fatal, and the reported mortality rate is 28%.3
Table 1.
The Incidence of Carbon Dioxide Embolism during Laparoscopic Procedures
TEE, transesophageal echocardiography; ETCO2, end-tidal carbon dioxide.
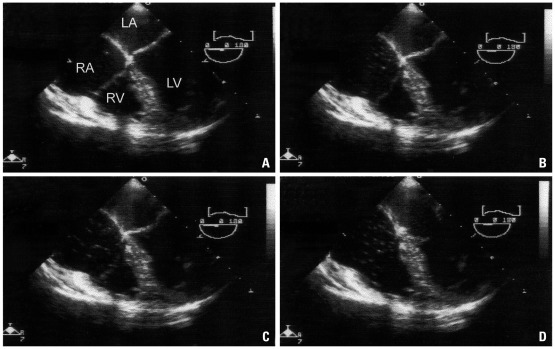
Recently, transesophageal echocardiography (TEE) has been used to monitor for carbon dioxide embolism. Fahy, et al.14 also detected one episode of gas embolism with TEE out of 16 healthy kidney donors during laparoscopic nephrectomy. Lin, et al.23 identified carbon dioxide emboli in 69 out of 403 patients (17.1%) during endoscopic saphenous vein harvesting during coronary artery bypass surgery with TEE monitoring. Minimal, moderate, and massive carbon dioxide emboli occurred in 53 (13.1%), 14 (3.5%), and 2 (0.5%) patients, respectively. Recently, in our lab, Kim, et al.13 reported the incidence and grade of carbon dioxide embolism during total laparoscopic hysterectomy using TEE. Gas embolism was observed in all patients undergoing total laparoscopic hysterectomy, and 37.5% of patients had grades higher than III (Fig. 1). No patient in this study showed hemodynamic instability or electrocardiogram changes at the time of venous air embolism (VAE) occurrence. Most instances of VAE during total laparoscopic hysterectomy (TLH) occurred during transection of the round ligament and dissection of the broad ligament.
Fig. 1.
Carbon dioxide emboli detected by transesophageal echocardiography in a patient undergoing total laparoscopic hysterectomy; mid-esophageal four chamber view. (A) A single gas bubble in the right atrium (RA), right ventricle (RV), and right ventricle outflow tract (RVOT) (grade I). (B) gas bubbles filling less than half the diameter of RA, RV, and RVOT (grade II). (C) gas bubbles filling more than half the diameter of RA, RV, and RVOT (grade III). (D) gas bubbles completely filling the diameter of RA, RV, and RVOT (grade IV). Permission from Kim, et al.13
In several cases, clinical carbon dioxide as a diagnosis was only reported in the medical or hospital record when some provocative medical intervention occurred. Less significant cases of embolism may only have been historically maintained as a comment in the specific anesthetic record, which were likely not individually reviewed in a large case series. Thus, the real "clinical incidence" of carbon dioxide embolism is likely higher than that reported by these series.
PATHOPHYSIOLOGY, CLINICAL SIGNS AND SYMPTOMS
Carbon dioxide is the most widely used insufflation gas. Most serious cases of carbon dioxide embolism reported in the literature occurred at the beginning of the procedure due to misplacement of the Veres needle directly into a vein or parenchymal organ. Smaller amounts of carbon dioxide may also enter circulation through an opening in injured vessels, either in the abdominal wall or at the operative site,13,24 which may be one of the mechanisms that can explain the late onset of carbon dioxide embolism.
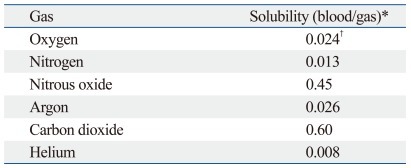
Carbon dioxide is well-suited for creating a pneumoperitoneum because it is chemically inert, colorless, inexpensive, readily available, and less combustible than air. Carbon dioxide is highly soluble in blood (Table 2),25 which allows rapid absorption into the blood stream across the peritoneum.26 At the same time, carbon dioxide can cause hypercapnea, metabolic acidosis, cardiorespiratory compromise, and greater postoperative pain as well as having adverse effects on intraperitoneal immune function, even increasing the risk of port-site tumor metastases in experimental models.27 Consequently, alternative gases have been investigated to reduce negative side effects.26-30
Table 2.
The Solubility of Medical Gases
*Solubility in mL gas/mL solvent with 100% gas and at 17℃.
†Not including that bound to hemoglobin.
The clinical effects of carbon dioxide embolism depend on the balance between the volume of carbon dioxide entering circulation and the amount of carbon dioxide that is removed.27 Dion, et al.31 reported that a mean of 300 mL carbon dioxide was necessary to cause the death of a 35 kg dog. If this figure is extrapolated to a 70 kg human, a volume of 600 mL could potentially cause death. However, a study that infused carbon dioxide into the left jugular vein in 89 dogs (5.1-9.7 kg) extrapolated that the lethal dose for 50% of the subjects (LD50) for a 70 kg human was 1,750 mL carbon dioxide.28 Mayer, et al.32 described a mortality of 60% at a continuous intravenous carbon dioxide infusion rate of 1.2 mL/kg/min, which is equivalent to a rate of 72 mL/min for a 60 kg person. That volume is only 5% of the volume of carbon dioxide that may be infused into a vein, intentionally cannulated by a Veres needle, in one minute at a low-flow rate.32
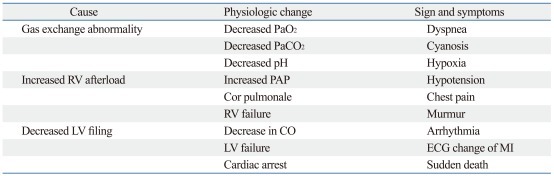
Carbon dioxide embolism can manifest itself through a "gas lock" effect, causing obstruction to RV ejection, right and left cardiac failure, paradoxical embolism with or without patent foramen ovale, arrhythmia, pulmonary hypertension, systemic hypotension, and cardiovascular collapse.16 Because of its high blood solubility, carbon dioxide causes similar but less marked effects than those produced by air. Carbon dioxide embolism does not produce the bronchoconstriction or changes in pulmonary compliance that are caused by air embolism.33 Clinically, carbon dioxide embolism can present with systemic hypotension, dyspnea, cyanosis, tachycardia or bradycardia, arrhythmia, or asystole. A "mill-wheel" murmur also can be ausculated. Carbon dioxide embolism may increase or decrease end-tidal carbon dioxide tension.24 Other effects include elevated pulmonary arterial pressure (PAP), elevated central venous pressure, hypoxemia, and increased arterial partial pressure of carbon dioxide (Table 3).27
Table 3.
The Physiological Changes, Signs and Symptoms of Carbon Dioxide Embolism
CO, cardiac output; ECG, electrocardiogram; LV, left ventricle; MI, myocardial infarct; PAP, pulmonary artery pressure; RV, right ventricle.
The relationship between carbon dioxide embolism and the interplay of different pressures (intro-abdominal, thoracic, cardiac, and venous) existing during laparoscopic surgeries has not been extensively studied. Peritoneal insufflation to intra-abdominal pressures higher than 10 mm Hg induces significant alterations of hemodynamics.34,35 Such hemodynamic changes might be caused by pneumoperitoneum, patient position, hypercapnia, or the carbon dioxide embolism itself. Adequate peritoneal insufflation below 10 mm Hg may decrease the incidence of carbon dioxide embolism.
DIAGNOSIS
TEE
TEE has been established as the most sensitive method for detecting intravenous injection of carbon dioxide as small as 0.1 mL/kg when compared with ETCO2, pulmonary artery pressure, and precordial auscultation, which has a similar threshold of 0.5 mL/kg.36 In another study, TEE detected gas emboli of 0.02 mL (0.0007 mL/kg) boluses, and was 50 to 100 times as sensitive to the presence of emboli, after bolus injection, as ETCO2.37 Studies with TEE probes identified subclinical gas emboli in 11 of 16 patients (68%) undergoing laparoscopic cholecyctectomy,4 but no emboli were reported when precordial Doppler ultrasonography was used in 61 patients undergoing the same procedure.5 Dion, et al.31 identified a change in pulmonary artery pressure in dogs only after injection of a 100 mL bolus of air, whereas TEE identified all bolus injections of 15 mL of air. TEE monitoring has also been increasingly used for the diagnosis for carbon dioxide embolism during endoscopic saphenous vein harvesting in coronary artery bypass surgery.16,17,23
Because administration of fluid and pharmacologic agents during surgical intervention causes some turbulent flow, which stimulates gas bubbles in the right atrium, the traditional TEE view of the right atrium is not ideal for monitoring for the appearance of carbon dioxide bubbles. Therefore, the transgastric inferior vena cava view has been utilized to solve this problem.17,23 TEE has clearly demonstrated carbon dioxide originating from the inferior vena cava, as well as gas in the RV and main pulmonary artery, documented RV failure, interventricular septal shift toward the left ventricle, and a decrease in left ventricular dimensions.16
The disadvantages of TEE include high cost, technical complexity, and the need for constant operator attention.38
Transesophageal Doppler
Transesophageal Doppler has recently emerged as an optimal means of gas embolus detection. It is easier and much less expensive to use routinely, and it is nearly as sensitive as TEE. During laparoscopic cholecystectomy in pigs, transesophageal Doppler was found to be a highly sensitive monitor that provided earlier detection of carbon dioxide emboli at lower doses than ETCO2 monitoring. This method demonstrated 100% sensitivity in detecting 0.1 mL/kg emboli.38
Precordial Doppler
Precordial Doppler was initially introduced as a simple and highly sensitive device for detecting venous air emboli.39 However, although Wadhwa, et al.40 monitored 100 patients undergoing laparoscopic procedures with a Doppler ultrasonic instrument, they reported no incidence of carbon dioxide embolism. In another study,5 no carbon dioxide embolism was detected on precordial Doppler ultrasonography in 61 patients undergoing laparoscopic cholecystectomy. The Doppler transducer is usually placed in the parasternal area at the fourth intercostal space overlying the RA. Despite the precordial Doppler's ease of placement and sensitivity, it is an imperfect method because it is not quantitative, and its false negative rate due to position of probe.41
ETCO2
ETCO2 monitoring has been suggested as a sensitive and noninvasive means of detecting gas embolism.42 However, carbon dioxide embolism can cause either an increase or a decrease in ETCO2.24 Several reports have described a rise in ETCO2,11,42 where a continuously recorded ETCO2 concentration increased abruptly from 3.8 to 4.2% in carbon dioxide embolism patients who were diagnosed on the basis of a sudden abrupt onset of systolic and diastolic murmurs during laparoscopy.42
Diakun43 detailed the temporal association of cardiovascular collapse with an abrupt increase in ETCO2. Most reports, however, described significant decreases in ETCO2 with clinical episodes of carbon dioxide embolism.6-8,23,44 A small transient rise in ETCO2 follows larger boluses, probably caused by an increase in the carbon dioxide dissolved in the blood. Rapid decrease in ETCO2 is caused by obstruction of some of the pulmonary vasculature by emboli, expanding the ventilatory dead space.37 Couture, et al.36 found that, regardless of the mode of administration of carbon dioxide (bolus or infusion), the first response to carbon dioxide embolism was a decrease in ETCO2.
Precordial or esophageal stethoscope
There have been several reports of "mill-wheel" precordial murmurs: a loud, harsh, churning, tickling, splashing metallic sound, which are usually noted concomitantly with cardiovascular collapse.11,12,45,46 However, these signs are inconsistent. A recent review of seven episodes of carbon dioxide embolism during laparoscopy revealed that a "mill-wheel" murmur was reported by fewer than half of the patients. It was not specifically sought out in the other cases because of the rapidity of cardiac events.3 Brundin, et al.9 reported that the typical metallic heart sounds were noted during hysteroscopy in 10% of cases.
PAP monitoring
In dogs, gas bubbles were seen in the right heart by TEE after bolus injection of 15 mL carbon dioxide, while it took a bolus injection of 100 mL carbon dioxide to cause a significant increase in PAP.31 The mean PAP rose by ≥3 mm Hg after bolus injection of 0.76±0.33 mL/kg of carbon dioxide in pigs.36 During laparoscopic liver resection in nine pigs after injection of 0.4 mL/kg of carbon dioxide, the mean PAP increased gradually over the entire four-hour study period. Pulmonary cardiac wedge pressure was fairly stable, varying between 6 and 8 mm Hg. Pulmonary venous resistance increased during the first 20 min, then remained stable. Cardiac output decreased during the first 15 min, then stayed constant during the rest of the study period.47
Schmandra, et al.15 observed a rise in mean PAP during laparoscopic liver resection, but the changes were not significant. There was, however, a significant increase in pulmonary cardiac wedge pressure with a significant decrease in cardiac output. Mayer, et al.32 reported an immediate three-fold increase above the baseline in mean PAP upon intravenous infusion of carbon dioxide, particularly in the high infusion rate group. Assuming that this increase in mean PAP can be entirely explained by a mechanical obstruction, mean PAP more closely approximates the residual degree of pulmonary vascular obstruction and circulatory compromise. The mean PAP returned to the basal level 9.9±3.3 min after a 1 mL/kg injection of carbon dioxide, which was shorter than the time required for the mean PAP to return to basal levels after a 1 mL/kg injection of air (15.3±2.1 min).48
PREVENTION
Preventive measures can be taken at the time of surgery in an effort to avoid carbon dioxide emboli. To avoid massive infusion of carbon dioxide intravenously, correct positioning of the Veres needle must be assured. This can be accomplished by aspiration prior to insufflation or test inflation of a few mL of carbon dioxide. Use of low insufflation pressure during laparoscopic surgery is also very important to preventing carbon dioxide emboli.3
An increase in central venous pressure to a level permanently exceeding the intraperitoneal pressure may be appropriate for reducing the risk of carbon dioxide embolism.15
The reverse Trendelenburg position has been known to decrease the incidence of gas embolism,49 and head-down positioning may also reduce the migration of gas bubbles to the head because the bubbles are naturally buoyant. Positive end-expiratory pressure may decrease the pressure gradient between a vessel opening and the heart, thus reducing the likelihood of gas entry.14
TREATMENT
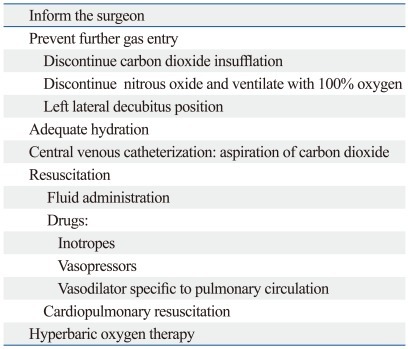
When a carbon dioxide embolism is suspected, a series of measures must be immediately performed (Table 4). Initially, insufflation should be discontinued with release of the pneumoperitoneum. The patient should be ventilated with 100% oxygen in order to wash out carbon dioxide and improve ventilation perfusion mismatch and hypoxemia. Discontinuing nitrous oxide will allow 100% oxygen to be administered, but will not reduce the size of a carbon dioxide embolus the way it would an air embolus. This difference is due to the similarity in the solubility of nitrous oxide and carbon dioxide in blood, which prevents significant diffusion of nitrous oxide into or out of the carbon dioxide bubbles.50 Hyperventilation increases carbon dioxide excretion.
Table 4.
Management of Carbon Dioxide Emboli
Aggressive volume expansion may reduce further gas entry by elevating central venous pressure.7 The patient should be placed in a steep-head down, left-lateral decubitus (Durant's) position in order to allow gas bubbles to rise to the apex of the RA and to prevent entry into the pulmonary artery.51 The Trendelenburg position can also decrease the risk of gas traveling to the head. Insertion of a Bunegin-Albin multi-orifice central venous or pulmonary artery catheter may allow aspiration of gas from the RA or RV, provide a quicker diagnosis, and significantly improve hemodynamic status by relieving gas lock in the RA or RV.8,52
Vital signs should be continuously assessed, and supportive measures and cardiopulmonary resuscitation should be initiated as needed to maintain oxygenation of vital organs.12,53-56 The most important causes of life-threatening carbon dioxide emboli are right heart failure caused by gas lock created by entrapped carbon dioxide bubbles in the heart, pulmonary vasoconstriction, and subsequent left ventricular failure due to left ventricular filling defects. The treatment of life-threatening carbon dioxide emboli includes administration of vasopressors and inotropic agents to maintain cardiac output and the use of intra-aortic balloon counterpulsation to maintain hemodynamic stability.23 Vasodilators can also be used to treat increased pulmonary vasoconstriction and to reduce RV afterload when hypotension is not present.2 Prostaglandin analogues or phosphodiesterase inhibitors may be considered for treatment of severe pulmonary hypertension. One case report described the use of inhaled epoprostenol to treat pulmonary hypertension caused by a carbon dioxide embolism.16
Cardiopulmonary bypass has also been successfully used to support patients with emboli.43 If a patient remains unstable, emergency thoracotomy with internal cardiac massage and aspiration should be considered to facilitate movement of the gas from the heart.7,8
Hyperbaric oxygen therapy is reportedly useful for treating carbon dioxide emboli, especially for the neurologic deficits caused by cerebral gas emboli. Hyperbaric compression reduces bubble size (one-third of the original volume at three atmospheres), restores blood flow, and limits detrimental effects of the gas-blood interface. Other potential beneficial effects include a reduction in intracranial pressure and increased tissue oxygenation via diffusion.57 Reust, et al.58 reported that repeated TEE showed clearance of large carbon dioxide bubbles in the coronary artery after hyperbaric oxygen therapy. Hyperbaric oxygen therapy for carbon dioxide emboli is less useful than for air emboli, because carbon dioxide is more soluble. Also, there is a higher pressure gradient between the blood and carbon dioxide bubbles (over 600 mm Hg), which encourages reabsorption.23 Complications of hyperbaric oxygen therapy include barotrauma (particularly to the ear and sinuses), pulmonary oxygen toxicity, decompression sickness, and seizures.59
ACKNOWLEDGEMENTS
We would like to thank the publisher of Anesthesiology and the authors (Kim et al., Anesthesiology 2009; 111) for giving us permission to adapt their figures (Fig. 1). Also, we would like to thank the authors (Mirski MA et al., Anesthesiology 2007; 106) of a major review article on venous air embolism for giving us inspiration and guidance.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Magrina JF. Complications of laparoscopic surgery. Clin Obstet Gynecol. 2002;45:469–480. doi: 10.1097/00003081-200206000-00018. [DOI] [PubMed] [Google Scholar]
- 2.Kim CS, Liu J, Kwon JY, Shin SK, Kim KJ. Venous air embolism during surgery, especially cesarean delivery. J Korean Med Sci. 2008;23:753–761. doi: 10.3346/jkms.2008.23.5.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cottin V, Delafosse B, Viale JP. Gas embolism during laparoscopy: a report of seven cases in patients with previous abdominal surgical history. Surg Endosc. 1996;10:166–169. doi: 10.1007/s004649910038. [DOI] [PubMed] [Google Scholar]
- 4.Derouin M, Couture P, Boudreault D, Girard D, Gravel D. Detection of gas embolism by transesophageal echocardiography during laparoscopic cholecystectomy. Anesth Analg. 1996;82:119–124. doi: 10.1097/00000539-199601000-00021. [DOI] [PubMed] [Google Scholar]
- 5.Landercasper J, Miller GJ, Strutt PJ, Olson RA, Boyd WC. Carbon dioxide embolization and laparoscopic cholecystectomy. Surg Laparosc Endosc. 1993;3:407–410. [PubMed] [Google Scholar]
- 6.Mattei P, Tyler DC. Carbon dioxide embolism during laparoscopic cholecystectomy due to a patent paraumbilical vein. J Pediatr Surg. 2007;42:570–572. doi: 10.1016/j.jpedsurg.2006.10.051. [DOI] [PubMed] [Google Scholar]
- 7.Cobb WS, Fleishman HA, Kercher KW, Matthews BD, Heniford BT. Gas embolism during laparoscopic cholecystectomy. J Laparoendosc Adv Surg Tech A. 2005;15:387–390. doi: 10.1089/lap.2005.15.387. [DOI] [PubMed] [Google Scholar]
- 8.Herron DM, Vernon JK, Gryska PV, Reines HD. Venous gas embolism during endoscopy. Surg Endosc. 1999;13:276–279. doi: 10.1007/s004649900963. [DOI] [PubMed] [Google Scholar]
- 9.Brundin J, Thomasson K. Cardiac gas embolism during carbon dioxide hysteroscopy: risk and management. Eur J Obstet Gynecol Reprod Biol. 1989;33:241–245. doi: 10.1016/0028-2243(89)90136-6. [DOI] [PubMed] [Google Scholar]
- 10.Phillips J, Keith D, Hulka J, Hulka B, Keith L. Gynecologic laparoscopy in 1975. J Reprod Med. 1976;16:105–117. [PubMed] [Google Scholar]
- 11.Hynes SR, Marshall RL. Venous gas embolism during gynaecological laparoscopy. Can J Anaesth. 1992;39:748–749. doi: 10.1007/BF03008249. [DOI] [PubMed] [Google Scholar]
- 12.Gomar C, Fernandez C, Villalonga A, Nalda MA. Carbon dioxide embolism during laparoscopy and hysteroscopy. Ann Fr Anesth Reanim. 1985;4:380–382. doi: 10.1016/S0750-7658(85)80111-8. [DOI] [PubMed] [Google Scholar]
- 13.Kim CS, Kim JY, Kwon JY, Choi SH, Na S, An J, et al. Venous air embolism during total laparoscopic hysterectomy: comparison to total abdominal hysterectomy. Anesthesiology. 2009;111:50–54. doi: 10.1097/ALN.0b013e3181a05ac7. [DOI] [PubMed] [Google Scholar]
- 14.Fahy BG, Hasnain JU, Flowers JL, Plotkin JS, Odonkor P, Ferguson MK. Transesophageal echocardiographic detection of gas embolism and cardiac valvular dysfunction during laparoscopic nephrectomy. Anesth Analg. 1999;88:500–504. doi: 10.1097/00000539-199903000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Schmandra TC, Mierdl S, Bauer H, Gutt C, Hanisch E. Transoesophageal echocardiography shows high risk of gas embolism during laparoscopic hepatic resection under carbon dioxide pneumoperitoneum. Br J Surg. 2002;89:870–876. doi: 10.1046/j.1365-2168.2002.02123.x. [DOI] [PubMed] [Google Scholar]
- 16.Martineau A, Arcand G, Couture P, Babin D, Perreault LP, Denault A. Transesophageal echocardiographic diagnosis of carbon dioxide embolism during minimally invasive saphenous vein harvesting and treatment with inhaled epoprostenol. Anesth Analg. 2003;96:962–964. doi: 10.1213/01.ANE.0000048827.03602.3F. [DOI] [PubMed] [Google Scholar]
- 17.Chiu KM, Lin TY, Wang MJ, Chu SH. Reduction of carbon dioxide embolism for endoscopic saphenous vein harvesting. Ann Thorac Surg. 2006;81:1697–1699. doi: 10.1016/j.athoracsur.2005.12.040. [DOI] [PubMed] [Google Scholar]
- 18.Kim SH, Park KS, Shin HY, Yi JH, Kim DK. Paradoxical carbon dioxide embolism during endoscopic thyroidectomy confirmed by transesophageal echocardiography. J Anesth. 2010;24:774–777. doi: 10.1007/s00540-010-0992-4. [DOI] [PubMed] [Google Scholar]
- 19.Hong JY, Kim WO, Kil HK. Detection of subclinical CO2 embolism by transesophageal echocardiography during laparoscopic radical prostatectomy. Urology. 2010;75:581–584. doi: 10.1016/j.urology.2009.04.064. [DOI] [PubMed] [Google Scholar]
- 20.Bruhl W. Complications of laparoscopy and liver biopsy under vision; the results of a survey. Ger Med Mon. 1967;12:31–32. [PubMed] [Google Scholar]
- 21.Bonjer HJ, Hazebroek EJ, Kazemier G, Giuffrida MC, Meijer WS, Lange JF. Open versus closed establishment of pneumoperitoneum in laparoscopic surgery. Br J Surg. 1997;84:599–602. [PubMed] [Google Scholar]
- 22.Mintz M. Risks and prophylaxis in laparoscopy: a survey of 100,000 cases. J Reprod Med. 1977;18:269–272. [PubMed] [Google Scholar]
- 23.Lin TY, Chiu KM, Wang MJ, Chu SH. Carbon dioxide embolism during endoscopic saphenous vein harvesting in coronary artery bypass surgery. J Thorac Cardiovasc Surg. 2003;126:2011–2015. doi: 10.1016/s0022-5223(03)01323-0. [DOI] [PubMed] [Google Scholar]
- 24.Gutt CN, Oniu T, Mehrabi A, Schemmer P, Kashfi A, Kraus T, et al. Circulatory and respiratory complications of carbon dioxide insufflation. Dig Surg. 2004;21:95–105. doi: 10.1159/000077038. [DOI] [PubMed] [Google Scholar]
- 25.Longnecker DE, Murphy FL. Introduction to anesthesia. 9th ed. Philadelphia: WB Saunders Co.; 1997. [Google Scholar]
- 26.Wolf JS, Jr, Carrier S, Stoller ML. Gas embolism: helium is more lethal than carbon dioxide. J Laparoendosc Surg. 1994;4:173–177. doi: 10.1089/lps.1994.4.173. [DOI] [PubMed] [Google Scholar]
- 27.Yau P, Watson DI, Lafullarde T, Jamieson GG. Experimental study of effect of embolism of different laparoscopy insufflation gases. J Laparoendosc Adv Surg Tech A. 2000;10:211–216. doi: 10.1089/109264200421603. [DOI] [PubMed] [Google Scholar]
- 28.Graff TD, Arbegast NR, Phillips OC, Harris LC, Frazier TM. Gas embolism: a comparative study of air and carbon dioxide as embolic agents in the systemic venous system. Am J Obstet Gynecol. 1959;78:259–265. doi: 10.1016/0002-9378(59)90169-3. [DOI] [PubMed] [Google Scholar]
- 29.Steffey EP, Johnson BH, Eger EI., 2nd Nitrous oxide intensifies the pulmonary arterial pressure response to venous injection of carbon dioxide in the dog. Anesthesiology. 1980;52:52–55. doi: 10.1097/00000542-198001000-00011. [DOI] [PubMed] [Google Scholar]
- 30.Fleming RY, Dougherty TB, Feig BW. The safety of helium for abdominal insufflation. Surg Endosc. 1997;11:230–234. doi: 10.1007/s004649900332. [DOI] [PubMed] [Google Scholar]
- 31.Dion YM, Lévesque C, Doillon CJ. Experimental carbon dioxide pulmonary embolization after vena cava laceration under pneumoperitoneum. Surg Endosc. 1995;9:1065–1069. doi: 10.1007/BF00188988. [DOI] [PubMed] [Google Scholar]
- 32.Mayer KL, Ho HS, Mathiesen KA, Wolfe BM. Cardiopulmonary responses to experimental venous carbon dioxide embolism. Surg Endosc. 1998;12:1025–1030. doi: 10.1007/s004649900773. [DOI] [PubMed] [Google Scholar]
- 33.Khan MA, Alkalay I, Suetsugu S, Stein M. Acute changes in lung mechanics following pulmonary emboli of various gases in dogs. J Appl Physiol. 1972;33:774–777. doi: 10.1152/jappl.1972.33.6.774. [DOI] [PubMed] [Google Scholar]
- 34.Struthers AD, Cuschieri A. Cardiovascular consequences of laparoscopic surgery. Lancet. 1998;352:568–570. doi: 10.1016/S0140-6736(97)11478-7. [DOI] [PubMed] [Google Scholar]
- 35.Koivusalo AM, Lindgren L. Effects of carbon dioxide pneumoperitoneum for laparoscopic cholecystectomy. Acta Anaesthesiol Scand. 2000;44:834–841. doi: 10.1034/j.1399-6576.2000.440709.x. [DOI] [PubMed] [Google Scholar]
- 36.Couture P, Boudreault D, Derouin M, Allard M, Lepage Y, Girard D, et al. Venous carbon dioxide embolism in pigs: an evaluation of end-tidal carbon dioxide, transesophageal echocardiography, pulmonary artery pressure, and precordial auscultation as monitoring modalities. Anesth Analg. 1994;79:867–873. doi: 10.1213/00000539-199411000-00009. [DOI] [PubMed] [Google Scholar]
- 37.O'Sullivan DC, Micali S, Averch TD, Buffer S, Reyerson T, Schulam P, et al. Factors involved in gas embolism after laparoscopic injury to inferior vena cava. J Endourol. 1998;12:149–154. doi: 10.1089/end.1998.12.149. [DOI] [PubMed] [Google Scholar]
- 38.Mann C, Boccara G, Fabre JM, Grevy V, Colson P. The detection of carbon dioxide embolism during laparoscopy in pigs: a comparison of transesophageal Doppler and end-tidal carbon dioxide monitoring. Acta Anaesthesiol Scand. 1997;41:281–286. doi: 10.1111/j.1399-6576.1997.tb04680.x. [DOI] [PubMed] [Google Scholar]
- 39.Glenski JA, Cucchiara RF, Michenfelder JD. Transesophageal echocardiography and transcutaneous O2 and CO2 monitoring for detection of venous air embolism. Anesthesiology. 1986;64:541–545. doi: 10.1097/00000542-198605000-00001. [DOI] [PubMed] [Google Scholar]
- 40.Wadhwa RK, McKenzie R, Wadhwa SR, Katz DL, Byers JF. Gas embolism during laparoscopy. Anesthesiology. 1978;48:74–76. doi: 10.1097/00000542-197801000-00011. [DOI] [PubMed] [Google Scholar]
- 41.Schubert A, Deogaonkar A, Drummond JC. Precordial Doppler probe placement for optimal detection of venous air embolism during craniotomy. Anesth Analg. 2006;102:1543–1547. doi: 10.1213/01.ane.0000198665.84248.61. [DOI] [PubMed] [Google Scholar]
- 42.Shulman D, Aronson HB. Capnography in the early diagnosis of carbon dioxide embolism during laparoscopy. Can Anaesth Soc J. 1984;31:455–459. doi: 10.1007/BF03015425. [DOI] [PubMed] [Google Scholar]
- 43.Diakun TA. Carbon dioxide embolism: successful resuscitation with cardiopulmonary bypass. Anesthesiology. 1991;74:1151–1153. [PubMed] [Google Scholar]
- 44.Huang YY, Wu HL, Tsou MY, Zong HJ, Guo WY, Chan KH, et al. Paradoxical carbon dioxide embolism during pneumoperitoneum in laparoscopic surgery for a huge renal angiomyolipoma. J Chin Med Assoc. 2008;71:214–217. doi: 10.1016/S1726-4901(08)70107-2. [DOI] [PubMed] [Google Scholar]
- 45.Yacoub OF, Cardona I, Jr, Coveler LA, Dodson MG. Carbon dioxide embolism during laparoscopy. Anesthesiology. 1982;57:533–535. doi: 10.1097/00000542-198212000-00017. [DOI] [PubMed] [Google Scholar]
- 46.Clark CC, Weeks DB, Gusdon JP. Venous carbon dioxide embolism during laparoscopy. Anesth Analg. 1977;56:650–652. doi: 10.1213/00000539-197709000-00010. [DOI] [PubMed] [Google Scholar]
- 47.Jersenius U, Fors D, Rubertsson S, Arvidsson D. The effects of experimental venous carbon dioxide embolization on hemodynamic and respiratory variables. Acta Anaesthesiol Scand. 2006;50:156–162. doi: 10.1111/j.1399-6576.2006.00933.x. [DOI] [PubMed] [Google Scholar]
- 48.Drummond JC, Prutow RJ, Scheller MS. A comparison of the sensitivity of pulmonary artery pressure, end-tidal carbon dioxide, and end-tidal nitrogen in the detection of venous air embolism in the dog. Anesth Analg. 1985;64:688–692. [PubMed] [Google Scholar]
- 49.Fong J, Gadalla F, Druzin M. Venous emboli occurring caesarean section: the effect of patient position. Can J Anaesth. 1991;38:191–195. doi: 10.1007/BF03008143. [DOI] [PubMed] [Google Scholar]
- 50.Nichols SL, Tompkins BM, Henderson PA. Probable carbon dioxide embolism during laparoscopy; case report. Wis Med J. 1981;80:27–29. [PubMed] [Google Scholar]
- 51.Durant TM, Oppenheimer MJ, Lynch PR, Ascanio G, Webber D. Body position in relation to venous air embolism: a roentgenologic study. Am J Med Sci. 1954;227:509–520. [PubMed] [Google Scholar]
- 52.Colley PS, Artru AA. Bunegin-Albin catheter improves air retrieval and resuscitation from lethal venous air embolism in upright dogs. Anesth Analg. 1989;68:298–301. [PubMed] [Google Scholar]
- 53.Duncan C. Carbon dioxide embolism during laparoscopy: a case report. AANA J. 1992;60:139–144. [PubMed] [Google Scholar]
- 54.Ostman PL, Pantle-Fisher FH, Faure EA, Glosten B. Circulatory collapse during laparoscopy. J Clin Anesth. 1990;2:129–132. doi: 10.1016/0952-8180(90)90068-e. [DOI] [PubMed] [Google Scholar]
- 55.Nishiyama T, Hanaoka K. Gas embolism during hysteroscopy. Can J Anaesth. 1999;46:379–381. doi: 10.1007/BF03013233. [DOI] [PubMed] [Google Scholar]
- 56.Staffieri F, Lacitignola L, De Siena R, Crovace A. A case of spontaneous venous embolism with carbon dioxide during laparoscopic surgery in a pig. Vet Anaesth Analg. 2007;34:63–66. doi: 10.1111/j.1467-2995.2006.00291.x. [DOI] [PubMed] [Google Scholar]
- 57.McGrath BJ, Zimmerman JE, Williams JF, Parmet J. Carbon dioxide embolism treated with hyperbaric oxygen. Can J Anaesth. 1989;36:586–589. doi: 10.1007/BF03005390. [DOI] [PubMed] [Google Scholar]
- 58.Reust RS, Diener BC, Stroup JS, Haraway GD. Hyperbaric treatment of arterial CO2 embolism occuring after laparoscopic surgery: a case report. Undersea Hyperb Med. 2006;33:317–320. [PubMed] [Google Scholar]
- 59.Gabb G, Robin ED. Hyperbaric oxygen. A therapy in search of diseases. Chest. 1987;92:1074–1082. doi: 10.1378/chest.92.6.1074. [DOI] [PubMed] [Google Scholar]