Abstract
Purpose
Rett syndrome is a severe neurodevelopmental disorder in females. Most have mutations in the methyl-CpG-binding protein 2 (MECP2) gene (80-90%). Epilepsy is a significant commonly accompanied feature in Rett syndrome. Our study was aimed at comprehensive analysis of genetic and clinical features in Rett syndrome patients, especially in regards to epileptic features.
Materials and Methods
We retrospectively reviewed 20 patients who were diagnosed with MECP2 mutations at Severance Children's Hospital between January 1995 and July 2010. All patients met clinical criteria for Rett syndrome. Evaluations included clinical features, epilepsy classification, electroencephalography analysis, and treatment of seizures.
Results
Ages ranged from 3.6 to 14.3 years (7.7±2.6). Fourteen different types of MECP2 mutations were found, including a novel in-frame mutation (1153-1188 del36). Fourteen of these patients (70.0%) had epilepsy, and the average age of seizure onset was 3.0±1.8 years. Epilepsy was diverse, including partial seizure in four patients (28.5%), secondarily generalized seizure in six (42.8%), generalized tonic seizure in two (14.3%), Lennox-Gastaut syndrome in one (7.1%), and myoclonic status in non-progressive encephalopathy in one (7.1%). Motor functions were delayed so that only 10 patients (50.0%) were able to walk independently: five (35.8%) in the epilepsy group and five (83.3%) in the non-epilepsy group. Average developmental scale was 33.5±32.8 in the epilepsy group and 44.4±21.2 in the non-epilepsy group. A clear genotype-phenotype correlation was not found.
Conclusion
There is a tendency for more serious motor impairment and cognitive deterioration in Rett syndrome patients with epilepsy.
Keywords: Rett syndrome, epilepsy, MECP2 mutation
INTRODUCTION
Rett syndrome is an X-linked dominant severe neurodevelopmental disorder, first described in 1966 by Dr. Andreas Rett. It affects one in 10,000-15,000 female births worldwide and is the second most common cause of mental retardation in girls. Patients show normal development after birth during the first six months or more, but thereafter show progressive regression in cognition, language, and purposeful hand skills. Deceleration in head circumference and stereotypic hand movement are typically characteristic. Seizure, scoliosis, gait apraxia, and breathing disturbances are also common.1 Among these symptoms, seizure occurs in 70-90% of Rett syndrome patients, usually in later stages of the disease after regression.1 Epilepsy can be a major factor in both disease prognosis as well as quality of life.
Mutations in the methyl-CpG-binding protein 2 (MECP2) gene were recognized as causes of Rett syndrome in 1999,2 identified in 90-95% cases with typical Rett syndrome.3 The MECP2 gene is located at the q28 locus on the X chromosome and functions as a regulator of gene transcription in neurons that are important in synapses and neuronal plasticity.4,5
In this study, we analyzed MECP2 mutations in 20 Korean Rett syndrome patients and characterized the patients' clinical features, in particular, with respect to epilepsy.
MATERIALS AND METHODS
Study patients
A retrospective review was performed on patients who visited Severance Children's Hospital in clinical suspicion of Rett syndrome from January 1995 to July 2010. We identified twenty patients who met the revised diagnostic criteria for classical and variant Rett syndrome defined by Hagberg, et al.1 and revealed MECP2 mutations therein.
Clinical evaluation
Demographic data concerning the patients' age, gender, and birth history were collected from medical records, and clinical symptoms, including hand movement, language, ambulatory capacity, and seizure profile, were analyzed. The results of the MECP2 mutation analyses, electroencephalography (EEG), extended video EEG monitoring, and brain magnetic resonance imaging (MRI) were reviewed. All 20 patients underwent brain MRI, 19 underwent EEG, and 6 underwent additional extended video EEG monitoring. Concerning epilepsy, we recorded the age of seizure onset, the type of seizures, as well as the administration of antiepileptic drugs and dietary therapy. Epilepsy classification was documented according to the criteria of the International League Against Epilepsy (ILAE).6 EEG features were analyzed based on background activity and epileptiform discharges. Background activity was categorized according to the EEG classification system proposed by Synek.7 The determining factor of improvement in seizure frequency was defined as a reduction greater than 50%.
Statistical analysis of nonparametric measures was conducted using SAS version 9.2 (Statistical Analysis System, Institute Inc., Cary, NC, USA). Griffiths Mental Development Scales, a head circumference below the 10th percentile, ambulation ability, EEG background and epileptiform discharges were compared between an epilepsy and a non-epilepsy group. Mann-Whitney U-test was performed to analyze developmental scale, and the other categorical variables were analyzed using Chi-square test or Fisher's exact test.
Mutation analysis
Analyses of MECP2 mutations were performed by polymerase chain reaction (PCR) and direct sequencing. Patients' genomic DNAs were extracted from peripheral blood, and four exons of the MECP2 gene were amplified by PCR. The primer sequence was designed by the Primer3 program. The PCR conditions were as follows: the total volume was 20 µL; the weight of genomic DNA was 100 ng; the concentrations of each primer and deoxynucleotide triphosphate were 10 pM and 200 µM respectively; 2X reaction buffer (500 mM KCl, 2X 100 mM Tris HCl: pH 9.0, 1% Trion X-100) and 1 U Taq polymerase (Cosmo, Seoul, Korea) were used; during the first step, denaturation was performed at 94℃ for 5 minutes and repeated 35 times for 1 minute at 94℃; annealing was done at 72℃ for 30 seconds; and the last step, extension, was done at 72℃ for 7 minutes. For the analysis of the amplified products, agarose gel electrophoresis at 135 V was done for 10 minutes. We purified PCR products using the Cosmo PCR purification kit (Cosmo) and performed DNA sequencing using a DNA sequencer (Life Technologies, Foster City, CA, USA) and the Life Technologies PRISM dye terminator cycle sequencing reaction kit (PerkinElmer, Waltham, MA, USA) with the same sequencing primer used in the PCR process. Results were then compared with the normal DNA sequence (X99686).
RESULTS
Clinical features
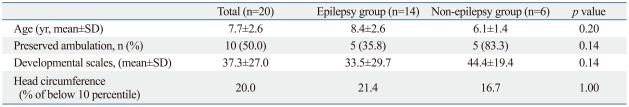
The ages of 20 patients ranged from 3.6 to 14.3 years (7.7±2.6 years). The mean follow-up duration was 46 months (range, 15-120 months). Demographic and clinical features are presented in Table 1. All patients were female. No patient had perinatal brain damage. Brain MRI showed no specific abnormalities except for brain volume reduction. Fourteen (70.0%) out of twenty patients, whom we grouped as the epilepsy group, had epilepsy. The other six patients were grouped in the non-epilepsy group. Psychomotor developmental delay was observed in all subjects to various degrees. Nineteen patients could not generate any meaningful words. Only one exceptional patient was able to make about ten words. Due to delayed motor function, only ten patients were able to walk independently: five patients (35.8%) in the epilepsy group and five patients (83.3%) in the non-epilepsy group (p=0.14). The average developmental scale score was 33.5±32.8 in the epilepsy group and 44.4±21.2 in the non-epilepsy group (Mann-Whitney U-test, p=0.14). Developmental scale scores ranged from 6.7 to 100.0. The patient who scored 100.0 (E2) had late deterioration after she had fully developed. Patients whose head circumference was below the 10th percentile accounted for 20.0% of all patients, 21.4% of the epilepsy group and 16.7% of the non-epilepsy group (p=1.00). All patients showed stereotypical "washing hand" movements.
Table 1.
Statistical Analysis between the Epilepsy and Non-Epilepsy Group
SD, standard deviation.
MECP2 mutations
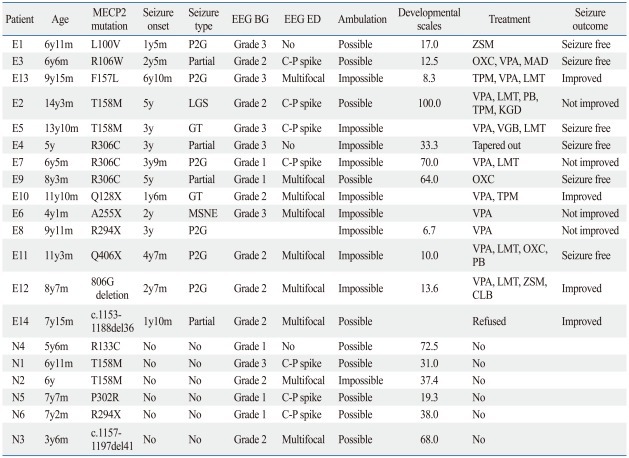
We identified MECP2 mutations in all 20 Rett syndrome patients. The mutations consisted of 14 distinct types, including seven missense, four nonsense, and three frameshift mutations. The missense mutations were T158M, R306C, L100V, R106W, F157L, R133C, and P302R, all of which were reported in previous Rett syndrome studies. The nonsense mutations consisted of R294X, A255X, Q128X, and Q406X. Four of five patients who showed nonsense mutations had epilepsy. There were three frameshift mutations which were 806delG, 1153del36, and 1157del41. The 1153del36 mutation was a novel mutation. Among fourteen mutations, T158M (20.0%), R306C (15.0%) and R294X (10.0%) were most frequent.
Characteristics of epilepsy
The mean age of the epilepsy group was 8.4±2.6 years and that of the non epilepsy group was 6.1±1.4 years. The average age of seizure onset was 3.0±1.8 years. In the epilepsy group, three patients (21.4%) had their first seizure between 1 and 2 years of age, three patients (21.4%) between 2 and 3 years, five patients (35.7%) between 3 and 5 years, and three patients (21.4%) above 5 years.
Various types of seizures were presented in the epilepsy group. Secondary generalized seizures were found in six patients (42.8%), partial seizures in four (28.5%), generalized seizures in two (14.3%), Lennox-Gastaut syndrome (LGS) in one (7.1%), and myoclonic status in non-progressive encephalopathy (MSNE) in one (7.1%). Four patients (28.6%) had seizures daily; two patients (14.3%) had less than seven seizures per week; five (35.4%) had seizures less than four times a month; and three patients (21.4%) rarely had seizures.
EEG showed abnormalities in most patients, including those in the non-epilepsy group. Only one patient in the non-epilepsy group (N4) showed a normal EEG pattern, and two patients (E1 and E4) in the epilepsy group showed no epileptiform discharges after effective anti-epileptic drug (AED) treatment. In patients with abnormal EEG, slow background and epileptiform discharges were prominent in centro-parietal areas. EEG characteristics are explained in Table 2. Categorized EEG background severity and appearance of epileptiform discharges did not show any statistical difference between both groups (p=1.00, p=1.00).
Table 2.
Characteristics of the Population
MECP2, methyl-CpG-binding protein 2; BG, background; ED, epileptiform discharges; E, epilepsy group; N, non-epilepsy group; P2G, partial seizure with secondary generalization; LGS, Lennox-Gastaut syndrome; GT, generalized tonic; MSNE, myoclonic status in non-progressive encephalopathy; C-P, centro-parietal; CLB, clobazam; LMT, lamotrigine; OXC, oxcarbazepine; PB, Phenobarbital; TPM, topiramate; VPA, valproic acid; ZSM, zonisamide; KGD, ketogenic diet; MAD, modified Atkins diet; EEG, electroencephalography; VGB, vigabatrin.
Background abnormality was classified by the dominant wave proposed by Synek.7 Grade 1: dominant alpha activity with some scattered theta activity, Grade 2: dominant theta activity, generally reactive, Grade 3: dominant widespread delta activity or small amplitude, diffuse, irregular delta activity, non reactive. The determining factor of improvement in seizure outcomes was defined as a reduction greater than 50%.
Thirteen patients (92.9%) in the epilepsy group were treated with AEDs. The average number of AEDs used was 2.2 (range: 1-4). The most frequently used drug was valproic acid, which was used in 10 patients (76.9%), followed by lamotrigine in six patients (46.2%). Two patients (14.3%) were placed on ketogenic diet therapy. The overall results of the treatments were favorable. Six patients (42.9%) became seizure free, four patients (28.6%) showed improvement with seizure frequency reduction rates above 50%, and four patients (28.6%) showed reduction rates under 50% (Table 2).
DISCUSSION
Epilepsy is a significant symptom in Rett syndrome in regards to its negative effects on quality of life and disease morbidity.8 In our study, the incidence of epilepsy in Rett syndrome patients was 70.0%. A recent USA study reported an epilepsy incidence of 60% in 602 patients9 and an Israeli study reported an incidence of 72% in 97 patients.10 Seizures in Rett syndrome have been described as benign.9,12 They usually appear in stage III (usually after 3 years of age) and rarely occur after the age of twenty.1,9 In comparison to our study, the discrepancy in the reported incidences is thought to be related to a difference in the range of the patients' ages, as there was a demonstratively younger patient age range (3.6-14.3 years) in our study compared to those in the USA study (0.7-64 years).
An occurrence of epilepsy in Rett syndrome is closely linked to early neurodevelopmental outcomes and poor functional abilities such as ambulation and hand use.11 We observed a tendency of lower ability of ambulation and poorer development in our epilepsy group than in our non-epilepsy group (Table 1), though statistical correlation was not found in both aspects.
EEG patterns in Rett syndrome were described in the early years of research, and a definite diagnostic pattern remains to be established. EEG can not only vary from one patient to another but also within different stages of disease progression. EEG usually shows normal patterns up to the age of two years old and subsequently deteriorates to the loss of age-expected developmental features along with the appearance of abnormal patterns and epileptiform discharges in centro-parietal or temporal regions.13-16 In our study, we compared EEG abnormalities in frequencies of main background activities and epileptiform discharges recorded in the epilepsy group with those in the non-epilepsy group. In two epileptic patients (E1, E4), epileptiform activities disappeared after effective treatments, although abundant slow background activities persisted.
Seizure types were presented diversely including partial as well as generalized seizures, and epileptic syndromes. Epileptic syndromes including LGS and MSNE were presented in one patient each. MSNE, recently classified as a developmental disorder by ILAE,9 is an epileptic encephalopathy, characterized by myoclonic absences and rhythmic myoclonias. These are followed by brief silent period related to subcontinuous delta-theta activity in the central areas and rhythmic delta waves with superimposed spikes mainly in the parieto-occipital regions.17 This status is accompanied by worsening of neuropsychological development.18 However, cases of MSNE in Rett syndrome has been rarely reported with only one in 29 case studies by Caraballo, et al.17 Particularly, Rett syndrome may present many movement disorders such as hand stereotypes, tremor, chorea, myoclonus, ataxia, and dystonia. In order to avoid misdiagnosing myoclonic status as a movement disorder, video EEG monitoring accompanied with EMG is essential for proper diagnosis.
The choice of AEDs was mainly influenced by the type of seizure. Commonly used drugs in our study were similar to those in other reports by Nissenkorn, et al.10 and Jian, et al.11 Valproate (10/14, 71.4%) and lamotrigine (6/14, 42.8%) were most frequently used in our patients. Carbamazepine, commonly used in Rett syndrome in other studies,9,12 was not used for this population. Oxcarbazepine and topiramate were each used in three patients. Two patients were placed on a ketogenic diet after unsuccessful multiple AED treatments, and one of them became seizure-free.
We found fourteen types of mutations in our patients' MECP2 genes. Common mutations included T158M (4), R306C (3), and R294X (2). Common type of mutations in this study was missense mutation (12/20, 60.0%), followed by nonsense mutation (5/20, 25.0%) and frameshift mutation (3/20, 15.0%). These results were similar to those of other studies from USA,11 Japan,19 and China.20 Two patients with nucleotide deletions of 36 and 41 base pairs did not share clinical features. One had epilepsy and one did not. The 1157del41 was previously recognized as an MECP2 mutation in a Korean population by Chae, et al.,21 and the clinical features thereof were similar to our patient, in respect to the non-epilepsy presenter. The 1153del36 mutation was a newly indentified de novo mutation found in our study. The patient of this mutation was characterized by typical features of Rett syndrome with an early onset of partial seizure at the age of 1 year and 10 months. Her seizures were self-remitted without AEDs though her neurodevelopmental function was still severely compromised. Glaze, et al.9 reported a higher incidence of seizures (74%) with the T158M mutation and a lower incidence of seizures (49%) with the R255X mutation. We were not able to make such a correlation between the mutation genotype and the prevalence or severity of seizures. This may be due to our small sample size.
Because of the limited sample size and short follow-up duration, a genotype-phenotype correlation was not made. However, our results will be helpful in understanding epilepsy in Rett syndrome patients. In addition, we were able to find a tendency of more serious motor impairment and cognitive deterioration in Rett syndrome patients with epilepsy. Further studies on MECP2 gene function and mutations on a larger scale will provide a better understanding of the pathogenesis and natural courses of Rett syndrome.
ACKNOWLEDGEMENTS
This work was supported by a National Research Foundation grant funded by the Korea government (MEST) (2010-0020353).
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Hagberg B, Hanefeld F, Percy A, Skjeldal O. An update on clinically applicable diagnostic criteria in Rett syndrome. Comments to Rett Syndrome Clinical Criteria Consensus Panel Satellite to European Paediatric Neurology Society Meeting, Baden Baden, Germany, 11 September 2001. Eur J Paediatr Neurol. 2002;6:293–297. doi: 10.1053/ejpn.2002.0612. [DOI] [PubMed] [Google Scholar]
- 2.Amir RE, Van den Veyver IB, Wan M, Tran CQ, Francke U, Zoghbi HY. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet. 1999;23:185–188. doi: 10.1038/13810. [DOI] [PubMed] [Google Scholar]
- 3.Neul JL, Fang P, Barrish J, Lane J, Caeg EB, Smith EO, et al. Specific mutations in methyl-CpG-binding protein 2 confer different severity in Rett syndrome. Neurology. 2008;70:1313–1321. doi: 10.1212/01.wnl.0000291011.54508.aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aber KM, Nori P, MacDonald SM, Bibat G, Jarrar MH, Kaufmann WE. Methyl-CpG-binding protein 2 is localized in the postsynaptic compartment: an immunochemical study of subcellular fractions. Neuroscience. 2003;116:77–80. doi: 10.1016/s0306-4522(02)00586-9. [DOI] [PubMed] [Google Scholar]
- 5.Chen WG, Chang Q, Lin Y, Meissner A, West AE, Griffith EC, et al. Derepression of BDNF transcription involves calcium-dependent phosphorylation of MeCP2. Science. 2003;302:885–889. doi: 10.1126/science.1086446. [DOI] [PubMed] [Google Scholar]
- 6.Engel J., Jr Report of the ILAE classification core group. Epilepsia. 2006;47:1558–1568. doi: 10.1111/j.1528-1167.2006.00215.x. [DOI] [PubMed] [Google Scholar]
- 7.Synek VM. Prognostically important EEG coma patterns in diffuse anoxic and traumatic encephalopathies in adults. J Clin Neurophysiol. 1988;5:161–174. doi: 10.1097/00004691-198804000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Ben Zeev Ghidoni B. Rett syndrome. Child Adolesc Psychiatr Clin N Am. 2007;16:723–743. doi: 10.1016/j.chc.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 9.Glaze DG, Percy AK, Skinner S, Motil KJ, Neul JL, Barrish JO, et al. Epilepsy and the natural history of Rett syndrome. Neurology. 2010;74:909–912. doi: 10.1212/WNL.0b013e3181d6b852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nissenkorn A, Gak E, Vecsler M, Reznik H, Menascu S, Ben Zeev B. Epilepsy in Rett syndrome---the experience of a National Rett Center. Epilepsia. 2010;51:1252–1258. doi: 10.1111/j.1528-1167.2010.02597.x. [DOI] [PubMed] [Google Scholar]
- 11.Jian L, Nagarajan L, de Klerk N, Ravine D, Christodoulou J, Leonard H. Seizures in Rett syndrome: an overview from a one-year calendar study. Eur J Paediatr Neurol. 2007;11:310–317. doi: 10.1016/j.ejpn.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steffenburg U, Hagberg G, Hagberg B. Epilepsy in a representative series of Rett syndrome. Acta Paediatr. 2001;90:34–39. doi: 10.1080/080352501750064842. [DOI] [PubMed] [Google Scholar]
- 13.Glaze DG, Frost JD, Jr, Zoghbi HY, Percy AK. Rett's syndrome. Correlation of electroencephalographic characteristics with clinical staging. Arch Neurol. 1987;44:1053–1056. doi: 10.1001/archneur.1987.00520220051016. [DOI] [PubMed] [Google Scholar]
- 14.Verma NP, Chheda RL, Nigro MA, Hart ZH. Electroencephalographic findings in Rett syndrome. Electroencephalogr Clin Neurophysiol. 1986;64:394–401. doi: 10.1016/0013-4694(86)90072-6. [DOI] [PubMed] [Google Scholar]
- 15.Niedermeyer E, Rett A, Renner H, Murphy M, Naidu S. Rett syndrome and the electroencephalogram. Am J Med Genet Suppl. 1986;1:195–199. doi: 10.1002/ajmg.1320250522. [DOI] [PubMed] [Google Scholar]
- 16.Trauner DA, Haas RH. Electroencephalographic abnormalities in Rett syndrome. Pediatr Neurol. 1987;3:331–334. doi: 10.1016/0887-8994(87)90003-8. [DOI] [PubMed] [Google Scholar]
- 17.Caraballo RH, Cersósimo RO, Espeche A, Arroyo HA, Fejerman N. Myoclonic status in nonprogressive encephalopathies: study of 29 cases. Epilepsia. 2007;48:107–113. doi: 10.1111/j.1528-1167.2006.00902.x. [DOI] [PubMed] [Google Scholar]
- 18.Elia M. Myoclonic status in nonprogressive encephalopathies: an update. Epilepsia. 2009;50(Suppl 5):41–44. doi: 10.1111/j.1528-1167.2009.02119.x. [DOI] [PubMed] [Google Scholar]
- 19.Fukuda T, Yamashita Y, Nagamitsu S, Miyamoto K, Jin JJ, Ohmori I, et al. Methyl-CpG binding protein 2 gene (MECP2) variations in Japanese patients with Rett syndrome: pathological mutations and polymorphisms. Brain Dev. 2005;27:211–217. doi: 10.1016/j.braindev.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 20.Pan H, Wang YP, Bao XH, Meng HD, Zhang Y, Wu XR, et al. MECP2 gene mutation analysis in Chinese patients with Rett syndrome. Eur J Hum Genet. 2002;10:484–486. doi: 10.1038/sj.ejhg.5200827. [DOI] [PubMed] [Google Scholar]
- 21.Chae JH, Hwang YS, Kim KJ. Mutation analysis of MECP2 and clinical characterization in Korean patients with Rett syndrome. J Child Neurol. 2002;17:33–36. doi: 10.1177/088307380201700108. [DOI] [PubMed] [Google Scholar]