Abstract
Phagocytosis or endocytosis by macrophages is critical to the uptake of fine particles, including nanoparticles, in order to initiate toxic effects in cells. Here, our data enhance the understanding of the process of internalization of silver nanoparticles by macrophages. When macrophages were pre-treated with inhibitors to phagocytosis, caveolin-mediated endocytosis, or clathrin-mediated endocytosis, prior to exposure to silver nanoparticles, Interleukin-8 (IL-8) production was inhibited. Although cell death was not reduced, the inflammatory response by macrophages was compromised by phagocytosis and endocytosis inhibitors.
Keywords: Silver nanoparticles, macrophages, endocytosis, phagocytosis, IL-8
INTRODUCTION
Since human exposure to nanomaterials has increased dramatically,1 concerns for the possible harmful effects of nanoparticles on cells have been raised. From their entry sites, such as the skin or respiratory tract, nanoparticles can translocate to other parts of the body.2,3 Macrophages are active phagocytic cells that are present in many tissues as resident macrophages, such as alveolar macrophages in the lungs or skin macrophages. The process of internalization of nanoparticles by phagocytic cells can be divided into phagocytosis/macropinocytosis, receptor-mediated endocytosis, and passive penetration.4,5 Phagocytosis and macropinocytosis are mediated by actin polymerization that causes cell membrane ruffling and can be inhibited by cytochalasin D.6 Endocytosis can be divided to clathrin-dependent endocytosis and caveolae-dependent endocytosis.7 Clathrin-dependent endocytosis can be inhibited by chlorpromazine, a cationic amphiphilic drug that prevents the recycling of clathrin.8 Caveolae-dependent endocytosis can be inhibited by nystatin,9 an antibiotic and sterol-binding agent that acts to remove membrane cholesterol, which is important for both the maintenance and sealing-off of the plasma membrane of caveolae.10
Therefore, our study was conducted to determine which internalization mechani-sm(s) is important to cellular uptake and cellular activation following exposure of macrophages to 5-nm silver nanoparticles. Using macrophages treated with silver nanoparticles at a concentration that does not induce cell death, Interleukin-8 (IL-8) production was examined. These results provide an understanding of the influence of silver nanoparticles on macrophages during the induction of immune responses or inflammation.
Silver nanoparticles suspended in water were provided by I&C (5-nm diameter, Seoul, Korea). Nanoparticles were round, polyvinylpyrrolidone-coated, and tested for contaminating endotoxin using a Pyrogene Recombinant Factor C Assay (Cambrex Bioscience, Walkersville, MD, USA), all of which were found as negative for endotoxin (less than 0.01 EU/mL). For cell culture, silver nanoparticles were prepared in RPMI 1640 medium with 2 mM L-glutamine supplemented with 10% fetal bovien serum (FBS), penicillin, and streptomycin (100 IU/mL each). Primary particle diameters were determined by transmission electron microscopy (TEM; model JEM-1011; JEOL, Peabody, MA, USA).
The human macrophage cell line U937 was cultured in RPMI 1640 containing 10% FBS and streptomycin/penicillin (100 IU/mL each) at 37℃ in a humidified 5% CO2 incubator. Although endotoxin was not detected in the silver nanoparticles used in this study, polymyxin B (InvivoGen, San Diego, CA, USA) was added at 10 ng/mL as an endotoxin neutralizer. U937 cells were treated for 1 hour with chlorpromazine (C8183; Sigma, St. Louis, MO, USA), cytochalasin D (C8273; Sigma), or nystatin (N6261; Sigma) at indicated concentrations prior to the addition of the nanoparticles. At the concentrations used in this study, the inhibitors were not toxic to U937 cells (data not shown). Cell viability was tested using a colorimetric Cell Counting Kit-8 (Dojindo Laboratories, Kyoto, Japan) that was based on colorimetric assays with the tetrazolium salt WST-8 [2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium, monosodium salt]. Optical densities were measured at 450 nm.
To assess IL-8 concentrations in cell culture supernatants, enzyme-linked immunosorbent assay (ELISA) was performed using human cytokine IL-8 assay kits (BD Biosciences, San Jose, CA, USA). Optical densities were measured with a microplate reader set to 450 nm. For real-time reverse transcription polymerase chain reaction (RT-PCR) analyses, cDNA was synthesized from total RNA via reverse transcription with oligo-dT primers (Invitrogen, San Diego, CA, USA). Primer pairs designed to amplify cDNA encoding IL-8 were prepared using the Universal ProbeLibrary Assay Design Center (Roche Applied Science, Indianapolis, IN, USA). The primer sequences for IL-8 were as follows: forward, 5'-GTG CAG TTT TGC CAA GGA GT-3' and reverse, 5'-CTC TGC ACC CAG TTT TCC TT-3'. PCR reactions were performed using FastStart DNA Master SYBR Green I reagents and 3 mM MgCl2 according to the manufacturer's instructions (Roche Applied Science) using a 7500 Real-Time PCR system (Applied Biosystems, Foster City, CA, USA). The PCR cycle parameters were: 2 minutes at 50℃, 10 minutes at 95℃, and 40 cycles of 95℃ for 10 seconds and 59℃ for 1 minute. Real-time RT-PCR data for each gene product was normalized against levels of glyceraldehyde 3-phosphate dehydrogenase. All transcript levels were reported as mean relative changes (mean±SD) compared to untreated controls from triplicate analyses. One-way analysis of variance was used for the analysis between controls and study groups.
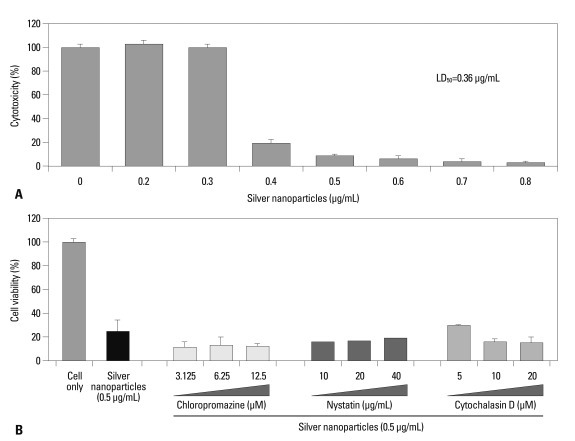
The viability of U937 cells treated with silver nanoparticles for 24 h declined abruptly when cells were treated with 0.3-0.4 µg/mL of nanoparticles (Fig. 1A). The LC50 for 5-nm silver nanoparticles was 0.36 µg/mL (Fig. 1A). Next, we examined the effects of endocytosis or phagocytosis of nanoparticles by macrophages on cell death. After treatment of U937 cells with 0.5 µg/mL of nanoparticles, cell death was not prevented by chlorpromazine, cytochalasin D, or nystatin at the indicated concentrations (Fig. 1B). These results suggest that a large portion of 5-nm silver nanoparticles enter cells by means other than endocytosis and phagocytosis. One mechanism of entry may be direct penetration of the cell membrane due to the extremely small size of the nanoparticles. Quantum dots11,12 can penetrate cell membranes as can gold nanoparticles.13,14 Gold nanoparticles 2.4 nm in diameter were found to localize in the nucleus via direct penetration, whereas gold nanoparticles 16-nm and larger did not enter the cells.13 Therefore, 5-nm silver nanoparticles may penetrate the lipid bilayer passively to enter cells as well as entering via endocytosis.
Fig. 1.
Cytotoxicity of silver nanoparticles in macrophages. (A) The cytotoxicity in U937 cells were assessed by CCK-8 assay. The LD50 of 5-nm silver nanoparticles was 0.36 µg/mL. (B) Each inhibitors were treaetd 1 hour before exposure to 0.5 µg/mL of 5-nm silver nanoparticles.
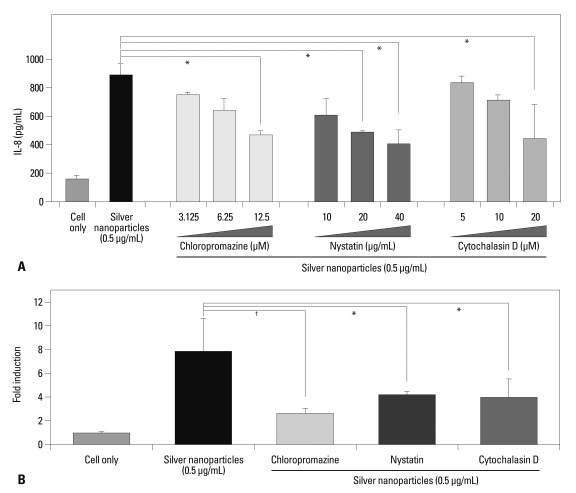
Tissue macrophages are distributed throughout the whole body and secrete a large pool of cytokines when they encounter foreign materials. Among the cytokines participating in innate immunity, IL-8 recruits neutrophils to acute inflammation sites. In a previous study, we demonstrated that silver nanoparticles triggered macrophages to release IL-8.15 Therefore, we chose IL-8 as an early responsive indicator for determining when macrophages are exposed to silver nanoparticles. As shown in Fig. 2A, chlorpromazine, cytochalasin D, and nystatin partially inhibited the production of IL-8 following exposure to silver nanoparticles at relatively higher concentrations for each of the inhibitors. These findings were confirmed by real-time RT-PCR assay (Fig. 2B).
Fig. 2.
Effects of inhibitors on IL-8 production induced by silver nanoparticles. Each inhibitors were added 1 hour before treatment of nanoparticles. (A) IL-8 in cell culture supernatants were assessed by ELISA 18 hours after exposure to 5-nm silver nanoparticles. (B) Real-time RT-PCR was performent. RNA was perpared from cells treated with 5-nm silver nanoparticles for 2 hours. Chloropromazine was treated at 12.5 µm, nystatin, at 40 µg/mL and cytochalasin D, at 20 µM. *p<0.05, †p<0.001.
In our study, a complex mechanism, rather than a dominant mechanism, was determined to be involved in the cellular uptake of nanoparticles and IL-8 production by macrophages following exposure to silver nanoparticles. The process of internalizing nanoparticles is composed of multiple steps. The uptake of quantum dots is dependent on environmental temperatures,16 indicating that a dynamic cellular process is involved. Additionally, the cellular uptake of quantum dots is dependent on cell type and cell differentiation stage, as shown in dendritic cells. Mature dendritic cells stimulated with lipopolysaccharide exhibited increased uptake of quantum dots compared with unstimulated dendritic cells.17 Our real-time RT-PCR data indicate that chlorpromazine inhibits IL-8 production by macrophages treated with nanoparticles to a greater extent than do cytochalasin D or nystatin. Therefore, clathrin-dependent endocytosis of nanoparticles may be more responsible for IL-8 production by macrophages than are caveolin-mediated endocytosis and phagocytosis.
In summary, our results demonstrate that multiple mechanisms, including endocytosis and phagocytosis, contribute to IL-8 production in macrophages following exposure to 5-nm silver nanoparticles. However, endocytosis or phagocytosis inhibitors were unable to inhibit the cytotoxicity triggered by these nanoparticles.
ACKNOWLEDGEMENTS
This research was supported by Nano · Material Technology Development Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2011-0019160).
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Yokel RA, Macphail RC. Engineered nanomaterials: exposures, hazards, and risk prevention. J Occup Med Toxicol. 2011;6:7. doi: 10.1186/1745-6673-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hackenberg S, Scherzed A, Kessler M, Hummel S, Technau A, Froelich K, et al. Silver nanoparticles: evaluation of DNA damage, toxicity and functional impairment in human mesenchymal stem cells. Toxicol Lett. 2011;201:27–33. doi: 10.1016/j.toxlet.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 3.Jang J, Lim DH, Choi IH. The impact of nanomaterials in immune system. Immune Netw. 2010;10:85–91. doi: 10.4110/in.2010.10.3.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tantra R, Knight A. Cellular uptake and intracellular fate of engineered nanoparticles: a review on the application of imaging techniques. Nanotoxicology. 2011;5:381–392. doi: 10.3109/17435390.2010.512987. [DOI] [PubMed] [Google Scholar]
- 5.Zhao F, Zhao Y, Liu Y, Chang X, Chen C, Zhao Y. Cellular uptake, intracellular trafficking, and cytotoxicity of nanomaterials. Small. 2011;7:1322–1337. doi: 10.1002/smll.201100001. [DOI] [PubMed] [Google Scholar]
- 6.Kerr MC, Teasdale RD. Defining macropinocytosis. Traffic. 2009;10:364–371. doi: 10.1111/j.1600-0854.2009.00878.x. [DOI] [PubMed] [Google Scholar]
- 7.Kumari S, Mg S, Mayor S. Endocytosis unplugged: multiple ways to enter the cell. Cell Res. 2010;20:256–275. doi: 10.1038/cr.2010.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hernaez B, Alonso C. Dynamin- and clathrin-dependent endocytosis in African swine fever virus entry. J Virol. 2010;84:2100–2109. doi: 10.1128/JVI.01557-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Santibanez JF, Blanco FJ, Garrido-Martin EM, Sanz-Rodriguez F, del Pozo MA, Bernabeu C. Caveolin-1 interacts and cooperates with the transforming growth factor-beta type I receptor ALK1 in endothelial caveolae. Cardiovasc Res. 2008;77:791–799. doi: 10.1093/cvr/cvm097. [DOI] [PubMed] [Google Scholar]
- 10.Greulich C, Diendorf J, Simon T, Eggeler G, Epple M, Köller M. Uptake and intracellular distribution of silver nanoparticles in human mesenchymal stem cells. Acta Biomater. 2011;7:347–354. doi: 10.1016/j.actbio.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 11.Wang T, Bai J, Jiang X, Nienhaus GU. Cellular uptake of nanoparticles by membrane penetration: a study combining confocal microscopy with FTIR spectroelectrochemistry. ACS Nano. 2012;6:1251–1259. doi: 10.1021/nn203892h. [DOI] [PubMed] [Google Scholar]
- 12.Dubavik A, Sezgin E, Lesnyak V, Gaponik N, Schwille P, Eychmüller A. Penetration of amphiphilic quantum dots through model and cellular plasma membranes. ACS Nano. 2012 Feb 13; doi: 10.1021/nn204930y. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 13.Lin J, Zhang H, Chen Z, Zheng Y. Penetration of lipid membranes by gold nanoparticles: insights into cellular uptake, cytotoxicity, and their relationship. ACS Nano. 2010;4:5421–5429. doi: 10.1021/nn1010792. [DOI] [PubMed] [Google Scholar]
- 14.Oh E, Delehanty JB, Sapsford KE, Susumu K, Goswami R, Blanco-Canosa JB, et al. Cellular uptake and fate of PEGylated gold nanoparticles is dependent on both cell-penetration peptides and particle size. ACS Nano. 2011;5:6434–6448. doi: 10.1021/nn201624c. [DOI] [PubMed] [Google Scholar]
- 15.Park J, Lim DH, Lim HJ, Kwon T, Choi JS, Jeong S, et al. Size dependent macrophage responses and toxicological effects of Ag nanoparticles. Chem Commun (Camb) 2011;47:4382–4384. doi: 10.1039/c1cc10357a. [DOI] [PubMed] [Google Scholar]
- 16.Zhang A, Guan Y, Xu LX. Theoretical study on temperature dependence of cellular uptake of QDs nanoparticles. J Biomech Eng. 2011;133:124502. doi: 10.1115/1.4005481. [DOI] [PubMed] [Google Scholar]
- 17.Zhang LW, Bäumer W, Monteiro-Riviere NA. Cellular uptake mechanisms and toxicity of quantum dots in dendritic cells. Nanomedicine (Lond) 2011;6:777–791. doi: 10.2217/nnm.11.73. [DOI] [PMC free article] [PubMed] [Google Scholar]