Abstract
Purpose
Tumor marker concentrations in a given specimen measured by different analyzers vary according to assay methods, epitopes for antibodies used, and reagent specificities. Although great effort in quality assessment has been instituted, discrepancies among results from different analyzers are still present. We evaluated the assay performance of the UniCel™ DxI 800 automated analyzer in measuring the alpha-fetoprotein (AFP), carcinoembryonic antigen (CEA), carbohydrate antigen (CA) 125, CA 15-3 and CA 19-9 tumor markers.
Materials and Methods
The linearity and precision performance of the five tumor marker assays were evaluated, and concentrations of the respective markers as measured by DxI were compared to those measured by other conventional analyzers (ADVIA Centaur™ and Vitros™ ECi) using 200 specimens collected from 100 healthy persons and 100 patients with respective cancers.
Results
The linear fits for all five tumor markers were statistically acceptable (F=4648 for AFP, F=15846 for CEA, F=6445 for CA 125, F=2285 for CA 15-3, F=7459 for CA 19-9; p<0.0001 for all). The imprecision of each tumor marker assay was less than 5% coefficient of variation, except for low and high concentrations of AFP. The results from UniCel™ DxI 800 were highly correlated with those from other analyzers.
Conclusion
Our results demonstrate that UniCel™ DxI 800 has good linearity and precision performance for the tumor markers assayed in this study. However, there were discrepancies between assaying methods. Efforts to standardize tumor marker assays should be undertaken, and the redetermination of cut-off levels is necessary when developing methods of analyzing tumor markers.
Keywords: Alpha-fetoprotein (AFP), CA 125, CA 15-3, CA 19-9, carcinoembryonic antigen (CEA), tumor markers
INTRODUCTION
Tumor markers are substances produced by tumors or by the host in response to the presence of a tumor, and are used to differentiate a tumor from normal tissue. Such substances are found in cells, tissues, or body fluids and can be measured qualitatively or quantitatively by chemical, immunological, or molecular biological methods.1 For many malignancies, the determination of serum tumor markers plays an important role in clinical research and diagnosis. Currently, tumor markers are widely used during therapy in order to perceive an indication of response to therapy and to distinguish between remission and progression. Tumor markers also provide important information towards recognizing recurrences and metastases at an early point in disease progression.2-7
Carcinoembryonic antigen (CEA) is a highly glycosylated cell surface glycoprotein with a molecular weight of 150-300 kDa, which can be detected at high levels in colon epithelial cells during embryonic development. Levels of CEA are significantly lower in adult colon tissue, but become elevated when inflammation or tumors arise in any endodermal tissue, including that of the gastrointestinal tract, respiratory tract, pancreas, and breast.1,8-11 Alpha-fetoprotein (AFP) is a glycoprotein with a molecular mass of 70 kDa, which is synthesized in large quantities during embryonic development of the fetal yolk sac and liver, and it can be used as a marker for hepatocellular or germ cell (nonseminoma) carcinoma, except in pregnant individuals.1,7 Carbohydrate antigen 15-3 (CA 15-3) was initially detected by the murine monoclonal antibody (MAb) DF3, prepared against the human breast carcinoma cell line MCF-7. The circulating DF3-reactive antigen is a heterogenous molecule with a molecular mass of 300 to 450 kDa. In previous studies, CA 15-3 was useful in monitoring therapy and predicting the progression of metastatic breast cancer.1,2,12 The carbohydrate antigen 125 (CA 125) is a glycoprotein with about a 200 kDa molecular weight which is recognized by the monoclonal antibody OC 125, and measurement of the CA 125 antigen can aid in the management of patients diagnosed with ovarian cancer.1,13 Carbohydrate antigen 19-9 (CA 19-9) is a marker for both colorectal and pancreatic carcinoma, and is used in monitoring patients with these cancers during palliative chemotherapy in conjunction with imaging tests.1,4,10
Early detection of cancer offers the best chance for a cure. Unfortunately, most cancers do not produce symptoms until carcinogenesis has progressed; however, most tumor markers are used to monitor treatment responses and recurrences of cancers. Therefore, more sensitive, specific, and reproducible detection methods would be helpful for managing patients with cancers. However, the concentration of a tumor marker in a given specimen, determined with assays from different manufacturers, can vary due to differences in assay methods, types of antibodies and epitopes used, and reagent specificity. Although precision has improved from the use of nonisotopic immunoassays and the institution of quality assessment efforts, discrepancies still arise among results from different analyzers. Accordingly, standardized reagents for tumor marker assays are needed to ensure the reliability of the results from different assays, and the standardization thereof is also important for commutability among results from different assays in order to deal with increased patient mobility between hospitals.
With the increased incidence and prevalence of cancers, the workload on tumor marker assays in clinical laboratories has also increased. The UniCel™ DxI 800 Access Immunoassay System (DxI) is an automated instrument that can handle a large volume of various test items with high throughput, and only a few studies have tested the performance characteristics of this analyzer so far.14-16 In this study, we aimed to evaluate the analytical performance of this immunoassay analyzer in measuring five tumor markers (AFP, CEA, CA 15-3, CA 125, and CA 19-9).
MATERIALS AND METHODS
Linearity and precision performance of the UniCel™ DxI 800 Access Immunoassay System (Beckman Coulter Inc., Brea, CA, USA) in measuring the five aforementioned tumor markers were evaluated. The results of the respective tumor marker levels as measured by DxI were compared with AFP, CEA, and CA 125 levels measured by the ADVIA Centaur™ XP Immunoassay System (Centaur) (Siemens Healthcare Diagnostics Inc., Deerfield, IL, USA) and with CA 15-3 and CA 19-9 concentrations measured by the Vitros™ ECi Immunodiagnostic System (ECi) (Ortho Clinical Diagnostics Ltd., Auckland, New Zealand). All assays were performed according to the manufacturers' instructions.
Precision performance evaluation
Imprecision of the assays by DxI was assessed based on guidelines from the Clinical and Laboratory Standards Institute (CLSI) document EP4-A2, using commercially available quality control materials of three levels (MAS T-Marker; Medical Analysis Systems, Camarillo, CA, USA) and pooled sera for the respective markers. Two daily runs of duplicate testing were conducted per day for 20 days, with a minimum of 2 hours between runs.
Linearity of the assays
Tests for validating the linearity of the assays were performed based on the CLSI document EP6-A. Pooled serum samples with high and low concentrations were mixed to make six equally spaced samples for their respective tumor markers. Four intermediate concentration pools were mixed as follows: 0.8 Low (L)+0.2 High (H), 0.6 L+0.4 H, 0.4 L+0.6 H, and 0.2 L+0.8 H. All pools were assayed in quadruplicate for their respective markers, and the mean was compared with the expected values.
Method comparison
Correlation between the levels of tumor markers as measured by DxI and Centaur or ECi were evaluated in 200 specimens for respective tumor markers (total 2000 tests with 1000 samples). All sera, which were requested for tumor marker testing, were collected and assayed for the respective tumor markers with DxI and other comparative instruments on the same day. The samples with measured concentrations over the analytical measurement range were re-tested after dilution according to the manufacturers' instructions.
Statistical analysis
All statistical analyses were performed using the Analyse-it Method Evaluation Edition version 2.22 software (Analyse-it Software Ltd., Leeds, UK). Comparisons between assays were estimated with regression equations calculated by Passing and Bablok regression analysis and with the mean-difference plots of Bland and Altman.17-19
RESULTS
Precision performances
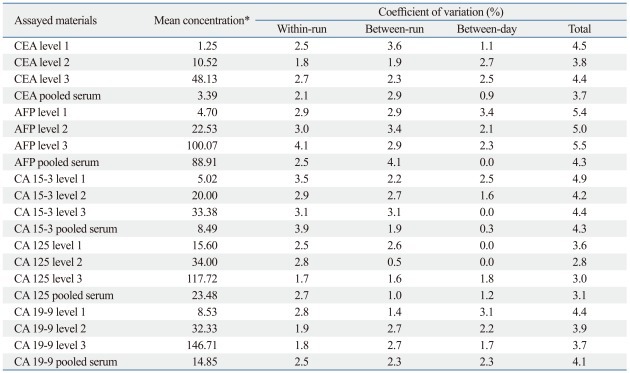
The within-run, between-run, between-day, and total precision performances of five tumor markers are summarized in Table 1. Within-run imprecision ranged from 1.7% to 4.1% coefficient of variation (CV), and between-run and between-day imprecision was between 0.5% and 3.6% CV and between 0.0% and 3.4% CV, respectively. Total imprecision ranged from 2.8% to 5.5% CV for all assessed levels of tumor markers.
Table 1.
Precision Performances of UniCel™ DxI 800 in Measuring Five Tumor Markers
CEA, carcinoembryonic antigen; AFP, alpha-fetoprotein; CA, carbohydrate antigen.
*Units are µg/L for CEA and AFP, and kU/L for CA 15-3, CA 125, and CA 19-9.
Linearity of the assays
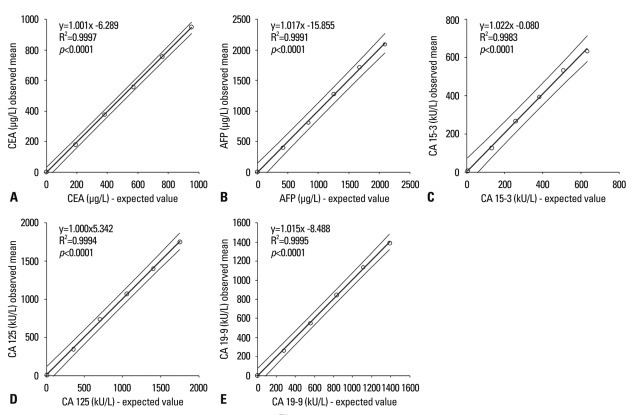
Linearity of the five tumor marker assays (CEA, AFP, CA 15-3, CA 125, and CA 19-9) were evaluated with 6 levels of serum samples prepared from mixing high and low pooled sera, of which the concentrations ranged from 951.41 to 0.80 µg/L for CEA, from 2092.91 to 1.86 µg/L for AFP, from 633.45 to 5.55 kU/L for CA 15-3, from 1752.53 to 7.68 kU/L for CA 125, and from 1389.73 to 0.80 kU/L for CA 19-9. The results from DxI showed excellent linear responses in measuring the concentrations of the five tumor markers (Fig. 1). The linear fits for all five tumor markers tested were accepted statistically at the level of p<0.05 (where x is expected values and y is observed means, y=1.017x-15.855, R2=0.9991, F=4648 for AFP; y=1.001x+6.289, R2=0.9997, F=15846 for CEA; y=1.000x+5.342, R2=0.9994, F=6445 for CA 125; y=1.022x-0.080, R2=0.9983, F=2285 for CA 15-3; y=1.015x-8.488, R2=0.9995, F=7459 for CA 19-9; p<0.001 for all).
Fig. 1.
Linearity of the five tumor marker assays performed by UniCel™ DxI 800. The observed mean tumor marker levels showed excellent linear responses to the expected values (p<0.001). (A) The linearity range of the CEA assay was from 0.80 to 951.41 µg/L (R2=0.9997). (B) The AFP assay was linear in the range between 1.86 and 2092.91 µg/L (R2=0.9991). (C) The CA 15-3 assay showed linear responses to the expected concentrations in the range of 5.55 to 633.45 kU/L (R2=0.9983). (D) The CA 125 assay was verified to be linear at the concentrations between 7.68 and 1752.53 kU/L (R2=0.9994). (E) Linearity of the CA 19-9 assay was validated in the range of 0.80 to 1389.73 kU/L (R2=0.9995). CEA, carcinoembryonic antigen; AFP, alpha-fetoprotein; CA, carbohydrate antigen.
Comparison between the assays
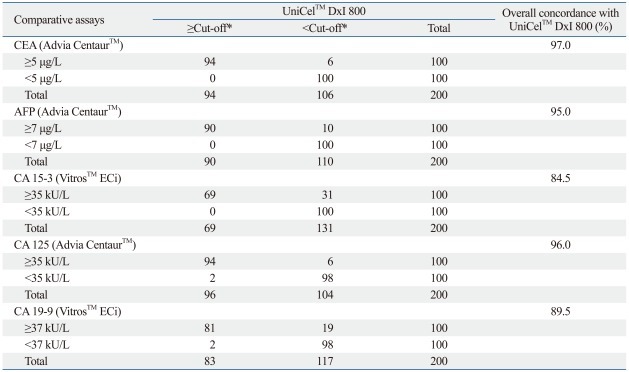
Comparison of the assays performed by DxI and Centaur or ECi demonstrated varying agreement, with slopes ranging from 0.507 to 1.118, intercepts ranging from -1.689 to 1.512, and correlation coefficients (r) ranging from 0.806 to 0.995 (Table 2). The results from the CEA measured by DxI showed the highest degree of agreement with those measured by Centaur (slope=0.910, r=0.995). CA 15-3 levels were poorly agreed upon between DxI and ECi, with a slope of 0.507 and correlation coefficients of 0.806. The results of the samples for all five assays were classified as normal or elevated, and the analytic concordance for each tumor marker was assessed using the cut-offs recommended by the manufacturers (Table 3). The overall concordance rates between DxI and Centaur or ECi ranged from 84.5% to 97.0%.
Table 2.
Parameters of Passing and Bablok Regression between UniCel™ DxI 800 and the Other Systems
CI, confidence interval; CEA, carcinoembryonic antigen; AFP, alpha-fetoprotein; CA, carbohydrate antigen.
*Units are µg/L for CEA and AFP, and kU/L for CA 15-3, CA 125, and CA 19-9.
Table 3.
Analytic Concordance between UniCel™ DxI 800 and the Other Analyzers
CEA, carcinoembryonic antigen; AFP, alpha-fetoprotein; CA, carbohydrate antigen.
*CEA, 5.0 µg/L; AFP, 7.4 µg/L; CA 15-3, 31.3 kU/L; CA 125, 35.0 kU/L; CA 19-9, 35.0 kU/L.
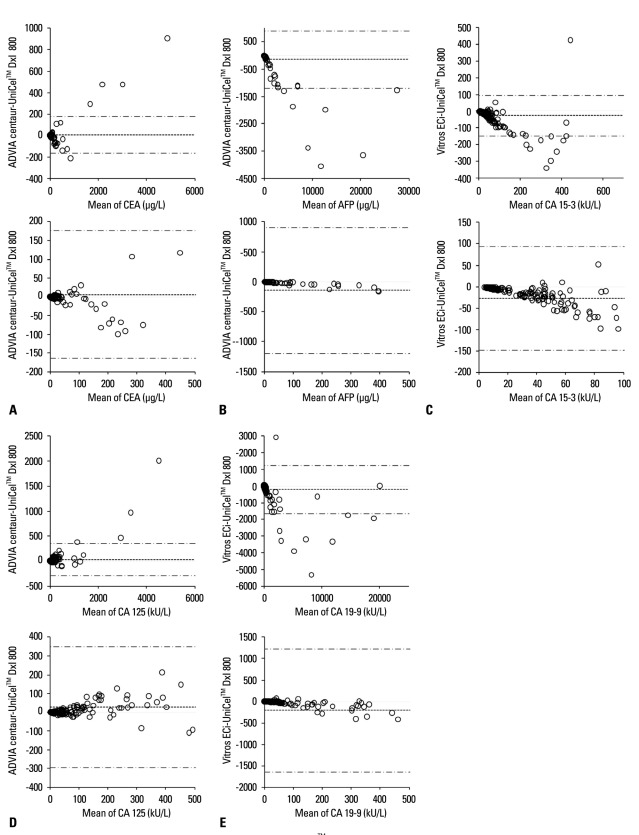
When the results for each marker were analyzed using difference plots (Fig. 2), CEA and CA 125 concentrations showed mean differences of 30 or less between DxI and Centaur, and CA 15-3 levels demonstrated a mean difference of -27.59 kU/L between DxI and ECi. The results of CA 19-9 measured by ECi and AFP measured by Centaur exhibited large mean differences of -209.59 kU/L and -145.39 µg/L, respectively, from those measured by DxI.
Fig. 2.
Comparison of the results of five tumor markers measured by the UniCel™ DxI 800 with those measured by other instruments using Bland and Altman difference plots. The solid line indicates the mean difference between the methods, and the dashed lines indicate the upper and lower 95% confidence limits of the difference between the methods. (A) The mean difference of CEA was 5.62 µg/L [95% confidence interval (CI), -6.53 to 17.77 µg/L]. (B) The mean difference of AFP was -145.39 µg/L (95% CI, -220.47 to -70.32 µg/L). (C) The mean difference of CA 15-3 was -27.59 kU/L (95% CI, -36.24 to -18.94 kU/L). (D) The mean difference of CA 125 was 26.73 kU/L (95% CI, 3.72 to 49.74 kU/L). (E) The mean difference of CA 19-9 was -209.59 kU/L (95% CI, -312.58 to -106.60 kU/L). CEA, carcinoembryonic antigen; AFP, alpha-fetoprotein; CA, carbohydrate antigen.
DISCUSSION
For many malignancies, serum tumor markers play important roles in patient management. Tumor markers are the biochemical or immunological indicators of differentiating clinical status in patients with malignancies. Many well-known markers can be elevated in noncancerous conditions, and although the presence of tumor markers is not diagnostic of cancer, it is thought that blood levels of tumor markers reflect tumor activity and the size of a mass. In clinical practice, tumor markers are useful in evaluating the progression of disease status after surgical or cytotoxic therapies have been undertaken and in monitoring subsequent treatment modalities.1-7,20 Therefore, precise and accurate assays for tumor markers are important in the management of patients with cancer.
In this study, we assessed the analytical performances of the UniCel™ DxI 800 Access Immunoassay System in measuring tumor marker concentrations. The results on the linearity and imprecision of the assays by DxI were highly acceptable and in accordance with previous reports.12,14-16,21-23 In addition, we compared the results from DxI for CEA, AFP, and CA 125 with those measured by Centaur, and for CA 15-3 and CA 19-9 with those measured by ECi, as our laboratory currently uses Centaur and ECi, which were intended to be replaced with DxI, for assaying these respective tumor markers. The extensive differences between analyzer systems, which are shown in Fig. 2, could be caused by different antibodies utilized by the assaying systems or by unique circumstances of the samples.2,12,24 In the case of CA 15-3, the respective assays utilize different CA 15-3 MAbs as the Ma552 antibody targeted epitope in the GVT-SAPDTRAPP region on the MUC1 glycoprotein core, and the DF3M antibody targeted epitope Ma695 on a carbohydrate. Different antibodies recognize different parts of the molecule, and heterogeneity or conformational alteration of the antigens may explain inter-method differences, in part. Although the results of AFP and CA 19-9 measured by DxI and the other systems were well correlated according to their correlation coefficients, they showed relatively large mean differences (Fig. 2). Samples tested for these two markers were considered to have higher antigen levels than the other markers, and the mean differences between higher values were generally larger. Therefore, the large mean differences between the results of AFP and CA 19-9 measured by DxI and the other systems seem to be due to the high antigen levels of the samples tested in our study.
In addition, CA 15-3 and CA 19-9 measured by DxI and ECi in this study showed relatively lower concordance rates and correlation coefficients among the results between the two analyzers, and the results for CEA, AFP, and CA 125 measured by DxI were more comparable to those by Centaur. The concordance between assay systems can vary according to the evaluated tumor markers and researchers.12,15,16,21,22 Therefore, even though the results, on average, agreed fairly well across the assays, when replacing tumor marker assays for clinical use, parallel tests by old and new methods are recommended to establish a new baseline in the management of patients.
These days, the incidence and prevalence of tumors are increasing due to advances in the technology of cancer detection and longer average life expectancies. Accordingly, the number of specimens for tumor marker testing has increased, and in the case of our laboratory, more than 18000 tumor marker tests are performed monthly. Because of this assay workload, one instrument cannot sufficiently analyze all five tumor markers, presently, and we have adopted the use of more than four immunoassay analyzers in our lab, including those compared in this study. From sample aspiration to results, the incubation times are 20 minutes for AFP and CEA and 50 minutes for CA 125 with the Centaur system, and 45 minutes for CA 19-9 and 50 minutes for CA 15-3 with the ECi system on average. Compared with those two analyzers, DxI has similar incubation times as follows: 20 minutes for AFP and CEA, 45 minutes for CA 19-9, and 50 minutes in CA 125 and CA 15-3. However, quicker results may be derived using the DxI system, which can analyze all five tumor markers and load 120 samples at once.
In conclusion, our study demonstrated that the UniCel™ DxI 800 system has high analytical performance. In spite of efforts to harmonize the results from different analyzers developed by different manufacturers, discrepant results remain among analytical methods. These differences may result from the application of different antibodies by different assays and suppliers. Additional efforts to standardize tumor marker assays are greatly necessitated, and the establishment of reliable reference materials and methods are also needed. The substantial differences between methods also indicate that the redetermination of baselines and cut-off levels is necessary when replacing analyzers and methods for measuring tumor marker assays.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Burtis CA, Ashwood ER, Bruns DE. Tietz textbook of clinical chemistry and molecular diagnostics. 4th ed. St. Louis: Elsevier saunders; 2006. [Google Scholar]
- 2.Duffy MJ. Serum tumor markers in breast cancer: are they of clinical value? Clin Chem. 2006;52:345–351. doi: 10.1373/clinchem.2005.059832. [DOI] [PubMed] [Google Scholar]
- 3.Cheli CD, Morris DL, Neaman IE, Dai J, Allard WJ, Yeung KK. Measurement of four tumor marker antigens in the sera of pregnant women. J Clin Lab Anal. 1999;13:35–39. doi: 10.1002/(SICI)1098-2825(1999)13:1<35::AID-JCLA7>3.0.CO;2-R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Connor S, Bosonnet L, Alexakis N, Raraty M, Ghaneh P, Sutton R, et al. Serum CA19-9 measurement increases the effectiveness of staging laparoscopy in patients with suspected pancreatic malignancy. Dig Surg. 2005;22:80–85. doi: 10.1159/000085297. [DOI] [PubMed] [Google Scholar]
- 5.Bendardaf R, Lamlum H, Pyrhönen S. Prognostic and predictive molecular markers in colorectal carcinoma. Anticancer Res. 2004;24:2519–2530. [PubMed] [Google Scholar]
- 6.Ishigami S, Natsugoe S, Nakashima H, Tokuda K, Nakajo A, Okumura H, et al. Biological aggressiveness of alpha-fetoprotein (AFP)-positive gastric cancer. Hepatogastroenterology. 2006;53:338–341. [PubMed] [Google Scholar]
- 7.Toyoda H, Kumada T, Kiriyama S, Sone Y, Tanikawa M, Hisanaga Y, et al. Prognostic significance of simultaneous measurement of three tumor markers in patients with hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2006;4:111–117. doi: 10.1016/s1542-3565(05)00855-4. [DOI] [PubMed] [Google Scholar]
- 8.Delwiche R, Zamcheck N, Marcon N. Carcinoembryonic antigen in pancreatitis. Cancer. 1973;31:328–330. doi: 10.1002/1097-0142(197302)31:2<328::aid-cncr2820310209>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 9.Loewenstein MS, Zamcheck N. Carcinoembryonic antigen (CEA) levels in benign gastrointestinal disease states. Cancer. 1978;42(3 Suppl):1412–1418. doi: 10.1002/1097-0142(197809)42:3+<1412::aid-cncr2820420805>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 10.Gebauer G, Müller-Ruchholtz W. Carcinoembryonic antigen and CA19-9: implications of quantitative marker measurement in tissues for prognosis of colorectal cancer. Cancer Detect Prev. 2001;25:344–351. [PubMed] [Google Scholar]
- 11.Dungchai W, Siangproh W, Lin JM, Chailapakul O, Lin S, Ying X. Development of a sensitive micro-magnetic chemiluminescence enzyme immunoassay for the determination of carcinoembryonic antigen. Anal Bioanal Chem. 2007;387:1965–1971. doi: 10.1007/s00216-006-0899-y. [DOI] [PubMed] [Google Scholar]
- 12.Slev PR, Rawlins ML, Roberts WL. Performance characteristics of seven automated CA 15-3 assays. Am J Clin Pathol. 2006;125:752–757. doi: 10.1309/G6X6-PR75-26FA-KV0E. [DOI] [PubMed] [Google Scholar]
- 13.Crombach G, Zippel HH, Würz H. Clinical significance of cancer antigen 125 (CA 125) in ovarian cancer. Cancer Detect Prev. 1985;8:135–139. [PubMed] [Google Scholar]
- 14.Yagmur E, Driesch R, Gressner AM, Kiefer P. Technical evaluation of the Beckman Coulter OV-Monitor (CA 125 antigen) immunoassay. Clin Chem Lab Med. 2006;44:420–422. doi: 10.1515/CCLM.2006.083. [DOI] [PubMed] [Google Scholar]
- 15.Deinzer M, Faissner R, Metzger T, Kaminski WE, Löhr M, Neumaier M, et al. Comparison of two different methods for CA19-9 antigen determination. Clin Lab. 2010;56:319–325. [PubMed] [Google Scholar]
- 16.Lee JH, Park Y, Suh B, Song SM, Kwon OH, Kim JH. Performance characteristics of the UniCel DxI 800 immunoassay for the maternal serum quadruple test, including median values for each week of gestation, in Korean women. Korean J Lab Med. 2010;30:126–132. doi: 10.3343/kjlm.2010.30.2.126. [DOI] [PubMed] [Google Scholar]
- 17.Passing H, Bablok A new biometrical procedure for testing the equality of measurements from two different analytical methods. Application of linear regression procedures for method comparison studies in clinical chemistry, Part I. J Clin Chem Clin Biochem. 1983;21:709–720. doi: 10.1515/cclm.1983.21.11.709. [DOI] [PubMed] [Google Scholar]
- 18.Passing H, Bablok W. Comparison of several regression procedures for method comparison studies and determination of sample sizes. Application of linear regression procedures for method comparison studies in Clinical Chemistry, Part II. J Clin Chem Clin Biochem. 1984;22:431–445. doi: 10.1515/cclm.1984.22.6.431. [DOI] [PubMed] [Google Scholar]
- 19.Bland JM, Altman DG. Comparing methods of measurement: why plotting difference against standard method is misleading. Lancet. 1995;346:1085–1087. doi: 10.1016/s0140-6736(95)91748-9. [DOI] [PubMed] [Google Scholar]
- 20.Nicolini A, Carpi A, Michelassi C, Spinelli C, Conte M, Miccoli P, et al. "Tumour marker guided" salvage treatment prolongs survival of breast cancer patients: final report of a 7-year study. Biomed Pharmacother. 2003;57:452–459. doi: 10.1016/j.biopha.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 21.Mongia SK, Rawlins ML, Owen WE, Roberts WL. Performance characteristics of seven automated CA 125 assays. Am J Clin Pathol. 2006;125:921–927. doi: 10.1309/NBA3-12W0-LANR-XYH9. [DOI] [PubMed] [Google Scholar]
- 22.Stern P, Friedecky B, Bartos V, Bezdickova D, Vavrova J, Uhrova J, et al. Comparison of different immunoassays for CA 19-9. Clin Chem Lab Med. 2001;39:1278–1282. doi: 10.1515/CCLM.2001.205. [DOI] [PubMed] [Google Scholar]
- 23.Ognibene A, Drake CJ, Jeng KY, Pascucci TE, Hsu S, Luceri F, et al. A new modular chemiluminescence immunoassay analyser evaluated. Clin Chem Lab Med. 2000;38:251–260. doi: 10.1515/CCLM.2000.037. [DOI] [PubMed] [Google Scholar]
- 24.Liew M, Groll MC, Thompson JE, Call SL, Moser JE, Hoopes JD, et al. Validating a custom multiplex ELISA against individual commercial immunoassays using clinical samples. Biotechniques. 2007;42:327–328. 330–333. doi: 10.2144/000112332. [DOI] [PubMed] [Google Scholar]