Abstract
Purpose
Several studies have demonstrated the superiority of endorectal coil magnetic resonance imaging (MRI) over pelvic phased-array coil MRI at 1.5 Tesla for local staging of prostate cancer. However, few have studied which evaluation is more accurate at 3 Tesla MRI. In this study, we compared the accuracy of local staging of prostate cancer using pelvic phased-array coil or endorectal coil MRI at 3 Tesla.
Materials and Methods
Between January 2005 and May 2010, 151 patients underwent radical prostatectomy. All patients were evaluated with either pelvic phased-array coil or endorectal coil prostate MRI prior to surgery (63 endorectal coils and 88 pelvic phased-array coils). Tumor stage based on MRI was compared with pathologic stage. We calculated the specificity, sensitivity and accuracy of each group in the evaluation of extracapsular extension and seminal vesicle invasion.
Results
Both endorectal coil and pelvic phased-array coil MRI achieved high specificity, low sensitivity and moderate accuracy for the detection of extracapsular extension and seminal vesicle invasion. There were statistically no differences in specificity, sensitivity and accuracy between the two groups.
Conclusion
Overall staging accuracy, sensitivity and specificity were not significantly different between endorectal coil and pelvic phased-array coil MRI.
Keywords: Prostatic neoplasms, magnetic resonance imaging, neoplasm staging, comparative study
INTRODUCTION
Prostate cancer remains a major health concern among the male population. Currently, a rapid increase in prostate-specific antigen (PSA) screening has resulted in an increased detection rate of small cancers and an increased incidence of this disease. In the evaluation of patients with prostate cancer, the most important factor affecting a patient's prognosis and choice of management method is the disease stage at the time of diagnosis.1 For several decades, various imaging modalities have been assessed for staging of prostate cancer. Among these imaging modalities, magnetic resonance imaging (MRI), with its excellent soft-tissue contrast, provides high-resolution images of the prostate and surrounding structures.2-4
Although there has been much debate regarding whether MRI should be used routinely for the diagnosis of prostate cancer, for over two decades, MRI has improved our ability to delineate localized versus locally advanced prostate cancer.5 However, it has poor sensitivity for the detection of microscopic spread. Hence, various MR techniques, such as endorectal coil, higher Tesla and diffusion-weighted MRI, have been proposed to improve the morphological imaging quality of prostate cancers. To date, several studies have suggested that MRI using an endorectal coil (ERC) is the most promising technique for the detection and staging of prostate cancer. Especially, at standard clinical field strengths of 1.5 Tesla, an ERC is necessary to obtain a sufficiently high signal-to-noise ratio (SNR) with subsequent spatial resolution, which allows for more reliable cancer delineation in a clinically reasonable timeframe.6-8 However, the use of an ERC is more time-consuming, entails higher costs and causes greater discomfort in the patient.9,10 Moreover, at higher magnetic field strengths, such as 3 Tesla, SNR increases in standard MRI, and the need for an ERC for the detection or localization of prostate cancer at this magnetic field strength has not yet been resolved.11 Therefore, it is important to determine whether an ERC remains necessary or whether a pelvic phased-array coil (PAC) could suffice for staging of prostate cancer at 3 Tesla MRI. Thus, we compared the prostate cancer local-staging performance of ERC and PAC MRI at 3 Tesla along with histopathologic findings as a reference standard.
MATERIALS AND METHODS
Patient characteristics
Between January 2005 and May 2010, 151 consecutive patients, who met our inclusion criteria and had biopsy-proven prostate cancer, underwent radical prostatectomy at our institution. Of these patients, 33 patients underwent open radical retropubic prostatectomy, while 118 patients underwent robotic-assisted laparoscopic surgery. All surgeries were performed by one oncologic urologist who had experience in more than 100 cases of radical prostatectomies. Exclusion criteria were contraindications for MRI (e.g., pacemaker and metal cerebral clips) as well as severe claustrophobia. Additionally, patients who received neoadjuvant hormonal therapy or radiotherapy after MRI examination and patients who had undergone a prostate biopsy and MRI within 3 weeks of each other were also excluded. If a patient had difficulty or was contraindicated for ERC insertion (e.g., prior anorectal surgery, inflammatory bowel disease, or high anal sphincter tension), the patient underwent MRI with a PAC.
MR imaging analysis
All patients were randomly scheduled to undergo 3 Tesla MRI (Signa Excite, GE Medical Systems, Waukesha, WI, USA) with either an ERC or a PAC prior to radical prostatectomy. In the MRI examination using a PAC (Pelvic Array, GE Healthcare, Waukesha, WI, USA), a commercially available eight-element standard PAC was placed around the pelvic area with the patient in the supine position. After localizing images were obtained, T2-weighted fast spin-echo image series in the transverse, sagittal, and coronal planes were obtained. Radiofrequency power deposition from the spin-echo train was reduced using hyperechoes.12 The T2-weighted imaging scan parameters were as follows: TR/TE, 3700/104 ms; slice thickness, 4 mm; interslice gap, 0.4 mm; 320×224 matrix; FOV, 160×160 mm; number of excitations, 3; and scan time, 8 minutes 20 seconds.
In the MRI examination using an ERC (MR Innerva, Medrad, Pittsburgh, PA, USA), a commercially available balloon-covered expandable ERC was inserted with the patient in the left lateral decubitus position. The balloon was then inflated with 40-60 mL of de-mineralized water. The patient was then placed in the supine position, and bowel movements were suppressed with an intramuscular injection of 1 mg of glucagon. An ERC localization image series was obtained, and subsequently, T2-weighted fast spin-echo images were obtained with the use of hyperechoes in the transverse, sagittal, and coronal planes. Sequence parameters were as follows: TR/TE, 3700/104 ms; slice thickness, 4 mm; interslice gap, 0.4 mm; 320×224 matrix; FOV, 160×160 mm; number of excitations, 3; and scan time, 8 minutes 20 seconds. Of these 151 patients, 63 patients underwent prostate MRI with an ERC, and 88 patients underwent prostate MRI with a PAC. Radiologic interpretations were made by consensus of two radiologists. One radiologist had more than 10 years of experience with prostate MRI with the use of a PAC at 1.5 Tesla and 3 years of experience with the use of an ERC at 1.5 Tesla. The other radiologist had 2 years of experience with prostate MRI with the use of both ERC and PAC at 1.5 Tesla. The criteria for the diagnosis of extraprostatic extension included a bulge in the contour of the prostate, obliteration of the rectoprostatic angle, thickening or disruption of the prostatic capsule, an infiltrative strand in the periprostatic fat, or asymmetry of the neurovascular bundle. Seminal vesicle invasion was defined by the presence of abnormal tissue with low signal intensity within the seminal vesicle or dilatation of the seminal vesicle with asymmetry on T2-weighted images. T1-weighted images were used to rule out false-positive findings caused by post-biopsy hemorrhage; if a low signal intensity lesion on a T2-weighted image matched a high signal intensity, then this area was considered a biopsy hematoma. Furthermore, SNRs of the entire prostate region for ERC and PAC MRI groups were calculated.
Histopathologic examination
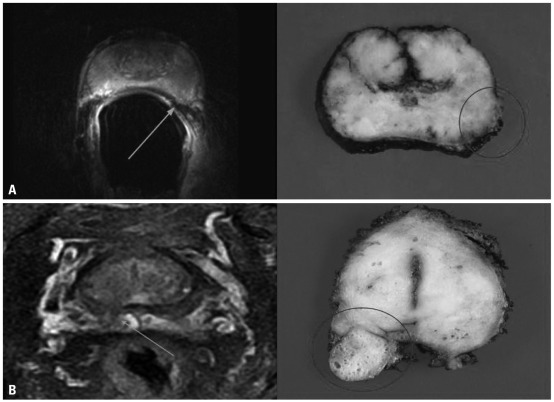
After excision, prostatectomy specimens were fixed overnight in 10% neutral buffered formaldehyde and coated with India ink. Four-millimeter-interval whole-mount sections were cut at a plane likewise to the transverse MRI plane. All sections were routinely embedded in paraffin. Tissue sections of 5 µm were prepared and stained with hematoxylin-eosin. The exact localization, volume, Gleason score, extent of each cancer focus, and radical distance of each extracapsular penetration were determined by a genitourinary histopathologist with 13 years of experience who was blinded to the MRI results. The MRI findings were compared with the histopathologic findings of the radical prostatectomy specimen in each patient (Fig. 1).
Fig. 1.
Comparison of a T2-weighted fast spin-echo magnetic resonance image at 3 Tesla and a corresponding axial whole-mount-section histopathologic slice (A) example of a tumor with a 2-mm radial distance of extracapsular extension (arrow) (prostate-specific antigen level, 7.8 ng/mL; final Gleason score, 3+4; stage, pT3a) that was detected with ERC MRI in a 61-year-old man, and histopathologic examination confirmed the presence of extracapsular entension at the left lateral side (circle) (B) example of a tumor with a 8-mm radial distance of extracapsular extension (arrow) (prostate-specific antigen level, 10.6 ng/mL; final Gleason score, 4+3; stage, pT3a) that was detected with PAC MRI in a 64-year-old man, and histopathologic examination confirmed the presence of extracapsular entension at the right dorsal side (circle). ERC, endorectal coil; PAC, phased-array coil; MRI, magnetic resonance imaging.
Statistical analysis
Sensitivity, specificity, positive prediction values, negative prediction values, and overall accuracy in prediction of extracapsular extension and seminal vesicle invasion were calculated by dichotomizing the readings. Calculated SNR values of each sequence were expressed as the mean±SD and compared using Student's t-test. Statistical significance differences in sensitivity, specificity, positive prediction value, negative prediction value, and accuracy between the ERC MRI group and PAC MRI group were determined using the Chi square test. All statistical analyses were conducted using SPSS 12.0 for Windows, and statistical significance was accepted at a p-value less than 0.05.
RESULTS
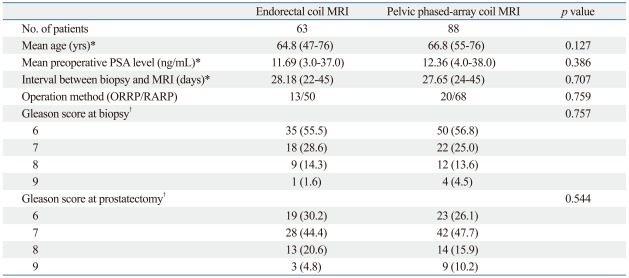
Table 1 summarizes the clinicopathologic data of the ERC MRI and PAC MRI groups. The mean age, mean preoperative PSA level, average time interval between prostate biopsy and MRI examination, ratio of open/robotic surgery, Gleason score at biopsy, and prostatectomy were not significantly different between the two groups. Of 63 patients, ERC MRI discovered suspected extraprostatic extension in 11 patients and suspected seminal vesicle invasion in 6 patients, while histopathologic analysis revealed 33 patients with extraprostatic extension and 13 patients with seminal vesicle invasion. Of 88 patients, PAC MRI discovered suspected extraprostatic extension in 15 patients and suspected seminal vesicle invasion in 9 patients, while histopathologic analysis revealed 48 patients with extraprostatic extension and 21 patients with seminal vesicle invasion. The sensitivity, specificity, and overall accuracy for the detection of extraprostatic extension were 33.3%, 96.6%, and 63.5% for ERC MRI and 31.3%, 97.5%, and 61.4% for PAC MRI, respectively (Table 2). Additionally, the sensitivity, specificity, and overall accuracy for the detection of seminal vesicle invasion were 46.2%, 92.0%, and 82.5% for ERC MRI and 42.9%, 92.5%, and 80.7% for PAC MRI, respectively (Table 2). Statistically, there were no significant differences in sensitivity, specificity, and accuracy between the two groups. The sensitivity of both ERC and PAC MRI for the detection of extraprostatic extension and seminal vesicle invasion tended to increase in the intermediate/high risk group (Table 3).
Table 1.
Clinicopathologic Characteristics of the Patients
MRI, magnetic resonance imaging; PSA, prostate specific antigen; ORRP, open retropubic radical prostatectomy; RARP, robotic-assisted laparoscopic radical prostatectomy.
*Data in parentheses are ranges.
†Data in parentheses are percentages.
Table 2.
Comparison of Local Staging Performance between Endorectal Coil and External Surface Coil MRI for Detecting Extraprostatic Extension and Seminal Vesicle Invasion of Prostate Cancer
MRI, magnetic resonance imaging; ECE, extracapsular extension; SVI, seminal vesicle invasion.
Data in parentheses are percentages.
Table 3.
Local Staging Performance of the Patients Stratified in the Low and Intermediate/high Risk Groups for Preoperative Endorectal Coil and External Surface Coil MRI
ERC, endorectal coil; PAC, pelvic phased-array coil; ECE, extracapsular extension; SVI, seminal vesicle invasion; PSA, prostate-specific antigen.
Data in parentheses are percentages.
*Low risk: clinical stage T1c to T2a and PSA ≤10 and Gleason score 6.
†Intermediate/high risk: clinical stage ≥T2b or PSA >10 or Gleason score ≥7.
The SNR of ERC MRI was 14.75±3.92 and 11.53±3.44 for PAC MRI. Although the SNR was slightly higher in the ERC MRI group, there was no statistically significant difference (p=0.081).
DISCUSSION
In the past, the use of computed tomography and MRI to evaluate the local extent of prostate cancer was not routinely recommended because of low sensitivity and accompanying low cost-effectiveness.13-15 However, with continuous developments in better technology, MRI has become increasingly implemented for the diagnosis and staging of prostate cancer. Recent studies reported that dynamic contrast-enhanced MRI are useful for the staging work-up of prostate cancer.16-18 In addition, proton MR spectroscopic imaging and functional MRI can further improve the detection rate of cancer while enhancing the assessment of tumors' aggressiveness, volume, and extent.16-19 Especially, endorectal coil MRI seems to be the most accurate imaging modality available for the evaluation of extensiveness of prostate cancer, replacing pelvic phased-array MRI of the prostate due to higher spatial and contrast resolution, improving image quality.8,20 For the detection of extraprostatic extension, the sensitivity, specificity, and accuracy of endorectal coil MRI has been reported to range from 13-71%, 47-97%, and 58-91%, respectively, whereas for the detection of seminal vesicle invasion, the sensitivity, specificity, and accuracy of endorectal MRI has been reported to range from 33-71%, 83-99%, and 80-95%, respectively.21-23 In our study, the sensitivity, specificity, and accuracy of endorectal coil MRI for the detection of extraprostatic extension was 33.3%, 96.6%, and 63.5%, respectively, and the sensitivity, specificity, and accuracy of the detection of seminal vesicle invasion was 46.2%, 92.0%, and 82.5%, respectively.
This wide range of specificities and sensitivities of MRI in the assessment of extraprostatic extension and seminal vesicle invasion is due to a combination of factors including considerable interobserver variability14,24 as well as the lack of standardized diagnostic criteria.25,26 In addition, the oncologic characteristics of preoperative patients are also important factors. Recent studies demonstrated that sensitivity was higher in poorly differentiated prostate cancer and/or intermediate and high-risk groups.7,27 Low-risk groups and well-differentiated cancer groups may not usually have extraprostatic extension. However, they have a greater chance of microscopic invasion rather than gross invasion, if they indeed do have extraprostatic invasion. Further, MRI still has a limitation in the detection of microscopic spread. A large population in the low-risk group (Gleason score 6 and less than 10 of preoperative serum PSA level) and a high upgrading rate in Gleason scores after radical prostatectomy may be one of the main factors of low sensitivity in our study. In addition, low sensitivity may be attributable to post-biopsy changes. While the amount of hemorrhaging often varies, it can be profound and involve large segments of the peripheral zone. Hematoma and edema can mimic not only tumor and capsular penetration but also seminal vesicle invasion as well.
High diagnostic specificity indicates less chance of false-positives and thus helps to ensure that few patients will be deprived of potentially curative surgery. It is accepted that tests with high specificity, even if accompanied by low sensitivity, offer a more cost-effective approach for patients being considered for surgery in the management of prostate cancer.28 Moreover, most errors in assessing extracapsular extension by MRI lie with false-negative results, as MRI even at 3 Tesla has difficulty detecting microscopic invasion of the capsule, which is noted in pathologic examinations. However, microscopic invasion of the capsule without seminal vesicle invasion or a positive surgical margin may not change the disease-free survival rate from that associated with pathologically organ-confined disease treated with radical prostatectomy.29
Although several studies demonstrated significant improvements in the quality of MR imaging of the prostate with the use of an ERC, ERC MRI requires more time, involves higher costs, and can causes greater discomfort in the patient; even further, it cannot be performed in patients with prior anorectal surgery, inflammatory bowel disease, and high anal sphincter tone. In contrast, the use of a PAC alone for signal reception can save time and costs as well as cause less discomfort in the patient. In addition, higher magnetic field strengths of 3 Tesla can increase the SNR and can improve the image quality of PAC MRI. In our study, there were no significant differences in sensitivity, specificity, positive prediction value, negative prediction value, and overall accuracy as well as SNR for the detection of extracapsular extension and seminal vesicle invasion between ERC and PAC MRI.
The present study has some limitations. This study was retrospectively designed and performed at single center. In addition, study method of comparing two modalities was limited to comparing two groups of patients rather than two distinct diagnostic methods in the same patient. This methodological flaw might have biased the results, affecting their validity.
In conclusion, both ERC and PAC prostate MRI at 3 Tesla have high specificity and low sensitivity for the detection of extracapsular extension and seminal vesicle invasion. Overall staging accuracy, sensitivity, and specificity were not significantly different between the two groups. Therefore, 3 Tesla PAC MRI can be an effective alternative for the local staging of prostate cancer.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Epstein JI, Partin AW, Sauvageot J, Walsh PC. Prediction of progression following radical prostatectomy. A multivariate analysis of 721 men with long-term follow-up. Am J Surg Pathol. 1996;20:286–292. doi: 10.1097/00000478-199603000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Hricak H, Williams RD, Spring DB, Moon KL, Jr, Hedgcock MW, Watson RA, et al. Anatomy and pathology of the male pelvis by magnetic resonance imaging. AJR Am J Roentgenol. 1983;141:1101–1110. doi: 10.2214/ajr.141.6.1101. [DOI] [PubMed] [Google Scholar]
- 3.Bryan PJ, Butler HE, LiPuma JP, Haaga JR, El Yousef SJ, Resnick MI, et al. NMR scanning of the pelvis: initial experience with a 0.3 T system. AJR Am J Roentgenol. 1983;141:1111–1118. doi: 10.2214/ajr.141.6.1111. [DOI] [PubMed] [Google Scholar]
- 4.Phillips ME, Kressel HY, Spritzer CE, Arger PH, Wein AJ, Marinelli D, et al. Prostatic disorders: MR imaging at 1.5 T. Radiology. 1987;164:386–392. doi: 10.1148/radiology.164.2.2440074. [DOI] [PubMed] [Google Scholar]
- 5.Nishimoto K, Nakashima J, Hashiguchi A, Kikuchi E, Miyajima A, Nakagawa K, et al. Prediction of extraprostatic extension by prostate specific antigen velocity, endorectal MRI, and biopsy Gleason score in clinically localized prostate cancer. Int J Urol. 2008;15:520–523. doi: 10.1111/j.1442-2042.2008.02042.x. [DOI] [PubMed] [Google Scholar]
- 6.Engelbrecht MR, Jager GJ, Laheij RJ, Verbeek AL, van Lier HJ, Barentsz JO. Local staging of prostate cancer using magnetic resonance imaging: a meta-analysis. Eur Radiol. 2002;12:2294–2302. doi: 10.1007/s00330-002-1389-z. [DOI] [PubMed] [Google Scholar]
- 7.Park SY, Kim JJ, Kim TH, Lim SH, Han DH, Park BK, et al. The role of endorectal magnetic resonance imaging in predicting extraprostatic extension and seminal vesicle invasion in clinically localized prostate cancer. Korean J Urol. 2010;51:308–312. doi: 10.4111/kju.2010.51.5.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hricak H, White S, Vigneron D, Kurhanewicz J, Kosco A, Levin D, et al. Carcinoma of the prostate gland: MR imaging with pelvic phased-array coils versus integrated endorectal--pelvic phased-array coils. Radiology. 1994;193:703–709. doi: 10.1148/radiology.193.3.7972810. [DOI] [PubMed] [Google Scholar]
- 9.Lee SH, Park KK, Choi KH, Lim BJ, Kim JH, Lee SW, et al. Is endorectal coil necessary for the staging of clinically localized prostate cancer? Comparison of non-endorectal versus endorectal MR imaging. World J Urol. 2010;28:667–672. doi: 10.1007/s00345-010-0579-6. [DOI] [PubMed] [Google Scholar]
- 10.Heijmink SW, Fütterer JJ, Hambrock T, Takahashi S, Scheenen TW, Huisman HJ, et al. Prostate cancer: body-array versus endorectal coil MR imaging at 3 T--comparison of image quality, localization, and staging performance. Radiology. 2007;244:184–195. doi: 10.1148/radiol.2441060425. [DOI] [PubMed] [Google Scholar]
- 11.Rouvière O, Hartman RP, Lyonnet D. Prostate MR imaging at high-field strength: evolution or revolution? Eur Radiol. 2006;16:276–284. doi: 10.1007/s00330-005-2893-8. [DOI] [PubMed] [Google Scholar]
- 12.Hennig J, Scheffler K. Hyperechoes. Magn Reson Med. 2001;46:6–12. doi: 10.1002/mrm.1153. [DOI] [PubMed] [Google Scholar]
- 13.Rifkin MD, Zerhouni EA, Gatsonis CA, Quint LE, Paushter DM, Epstein JI, et al. Comparison of magnetic resonance imaging and ultrasonography in staging early prostate cancer. Results of a multi-institutional cooperative trial. N Engl J Med. 1990;323:621–626. doi: 10.1056/NEJM199009063231001. [DOI] [PubMed] [Google Scholar]
- 14.Tempany CM, Zhou X, Zerhouni EA, Rifkin MD, Quint LE, Piccoli CW, et al. Staging of prostate cancer: results of Radiology Diagnostic Oncology Group project comparison of three MR imaging techniques. Radiology. 1994;192:47–54. doi: 10.1148/radiology.192.1.8208963. [DOI] [PubMed] [Google Scholar]
- 15.Levran Z, Gonzalez JA, Diokno AC, Jafri SZ, Steinert BW. Are pelvic computed tomography, bone scan and pelvic lymphadenectomy necessary in the staging of prostatic cancer? Br J Urol. 1995;75:778–781. doi: 10.1111/j.1464-410x.1995.tb07390.x. [DOI] [PubMed] [Google Scholar]
- 16.Kayhan A, Fan X, Oto A. Dynamic contrast-enhanced magnetic resonance imaging in prostate cancer. Top Magn Reson Imaging. 2009;20:105–112. doi: 10.1097/RMR.0b013e3181c0e2fa. [DOI] [PubMed] [Google Scholar]
- 17.Engelbrecht MR, Huisman HJ, Laheij RJ, Jager GJ, van Leenders GJ, Hulsbergen-Van De Kaa CA, et al. Discrimination of prostate cancer from normal peripheral zone and central gland tissue by using dynamic contrast-enhanced MR imaging. Radiology. 2003;229:248–254. doi: 10.1148/radiol.2291020200. [DOI] [PubMed] [Google Scholar]
- 18.Buckley DL, Roberts C, Parker GJ, Logue JP, Hutchinson CE. Prostate cancer: evaluation of vascular characteristics with dynamic contrast-enhanced T1-weighted MR imaging--initial experience. Radiology. 2004;233:709–715. doi: 10.1148/radiol.2333032098. [DOI] [PubMed] [Google Scholar]
- 19.Claus FG, Hricak H, Hattery RR. Pretreatment evaluation of prostate cancer: role of MR imaging and 1H MR spectroscopy. Radiographics. 2004;24(Suppl 1):S167–S180. doi: 10.1148/24si045516. [DOI] [PubMed] [Google Scholar]
- 20.Schnall MD, Imai Y, Tomaszewski J, Pollack HM, Lenkinski RE, Kressel HY. Prostate cancer: local staging with endorectal surface coil MR imaging. Radiology. 1991;178:797–802. doi: 10.1148/radiology.178.3.1994421. [DOI] [PubMed] [Google Scholar]
- 21.Ikonen S, Kärkkäinen P, Kivisaari L, Salo JO, Taari K, Vehmas T, et al. Magnetic resonance imaging of clinically localized prostatic cancer. J Urol. 1998;159:915–919. [PubMed] [Google Scholar]
- 22.Nakashima J, Tanimoto A, Imai Y, Mukai M, Horiguchi Y, Nakagawa K, et al. Endorectal MRI for prediction of tumor site, tumor size, and local extension of prostate cancer. Urology. 2004;64:101–105. doi: 10.1016/j.urology.2004.02.036. [DOI] [PubMed] [Google Scholar]
- 23.Rørvik J, Halvorsen OJ, Albrektsen G, Ersland L, Daehlin L, Haukaas S. MRI with an endorectal coil for staging of clinically localised prostate cancer prior to radical prostatectomy. Eur Radiol. 1999;9:29–34. doi: 10.1007/s003300050622. [DOI] [PubMed] [Google Scholar]
- 24.Mullerad M, Hricak H, Wang L, Chen HN, Kattan MW, Scardino PT. Prostate cancer: detection of extracapsular extension by genitourinary and general body radiologists at MR imaging. Radiology. 2004;232:140–146. doi: 10.1148/radiol.2321031254. [DOI] [PubMed] [Google Scholar]
- 25.Yu KK, Hricak H, Alagappan R, Chernoff DM, Bacchetti P, Zaloudek CJ. Detection of extracapsular extension of prostate carcinoma with endorectal and phased-array coil MR imaging: multivariate feature analysis. Radiology. 1997;202:697–702. doi: 10.1148/radiology.202.3.9051019. [DOI] [PubMed] [Google Scholar]
- 26.Outwater EK, Petersen RO, Siegelman ES, Gomella LG, Chernesky CE, Mitchell DG. Prostate carcinoma: assessment of diagnostic criteria for capsular penetration on endorectal coil MR images. Radiology. 1994;193:333–339. doi: 10.1148/radiology.193.2.7972739. [DOI] [PubMed] [Google Scholar]
- 27.Fütterer JJ, Engelbrecht MR, Jager GJ, Hartman RP, King BF, Hulsbergen-Van de Kaa CA, et al. Prostate cancer: comparison of local staging accuracy of pelvic phased-array coil alone versus integrated endorectal-pelvic phased-array coils. Local staging accuracy of prostate cancer using endorectal coil MR imaging. Eur Radiol. 2007;17:1055–1065. doi: 10.1007/s00330-006-0418-8. [DOI] [PubMed] [Google Scholar]
- 28.Coakley FV, Qayyum A, Kurhanewicz J. Magnetic resonance imaging and spectroscopic imaging of prostate cancer. J Urol. 2003;170(6 Pt 2):S69–S75. doi: 10.1097/01.ju.0000094958.23276.c4. [DOI] [PubMed] [Google Scholar]
- 29.Wieder JA, Soloway MS. Incidence, etiology, location, prevention and treatment of positive surgical margins after radical prostatectomy for prostate cancer. J Urol. 1998;160:299–315. [PubMed] [Google Scholar]