Abstract
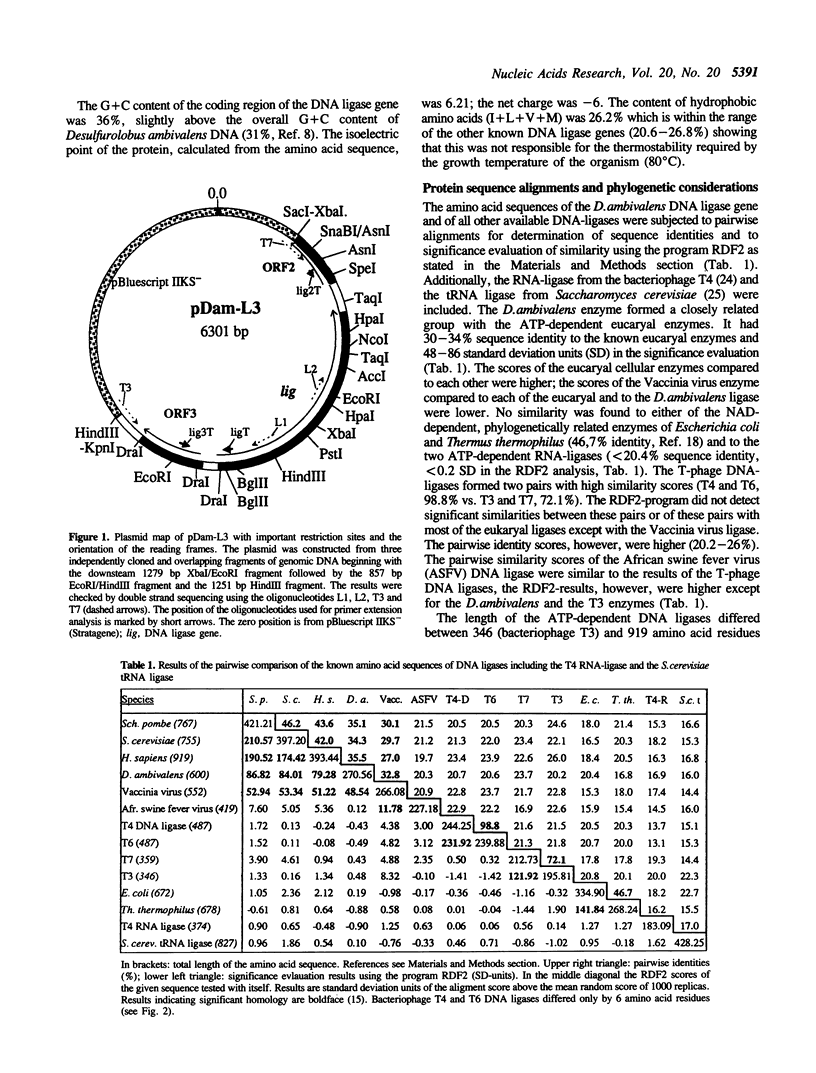
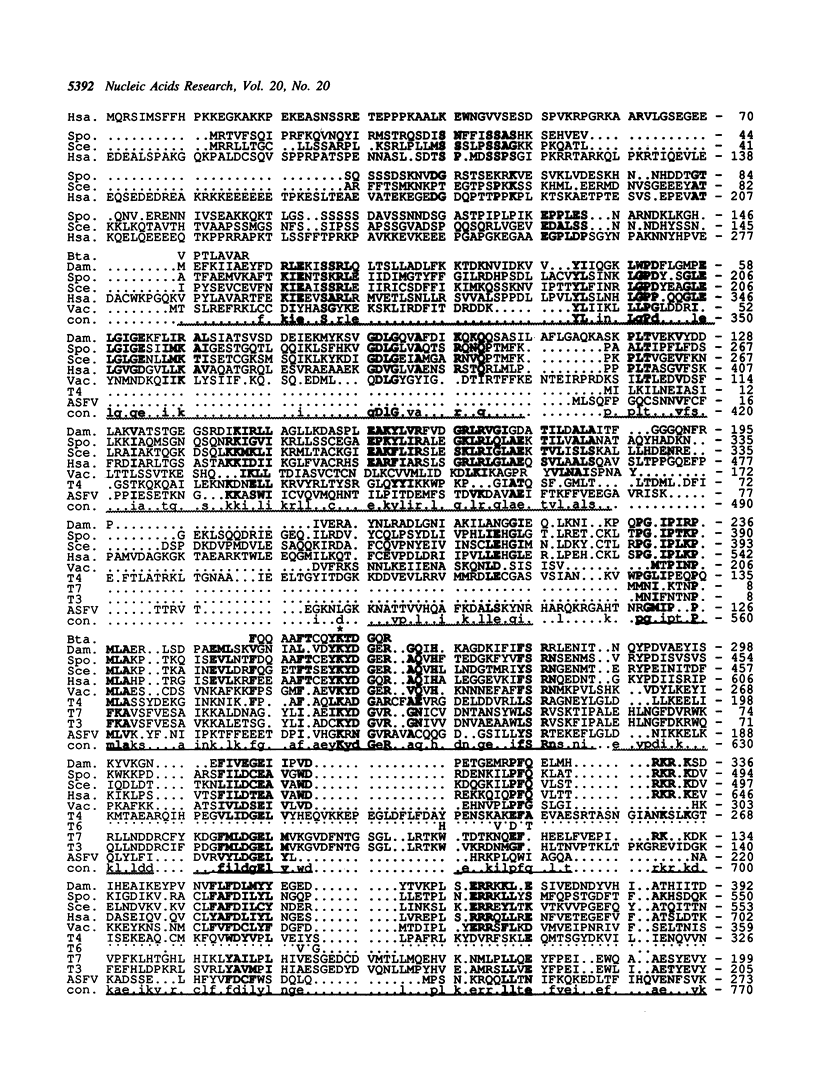
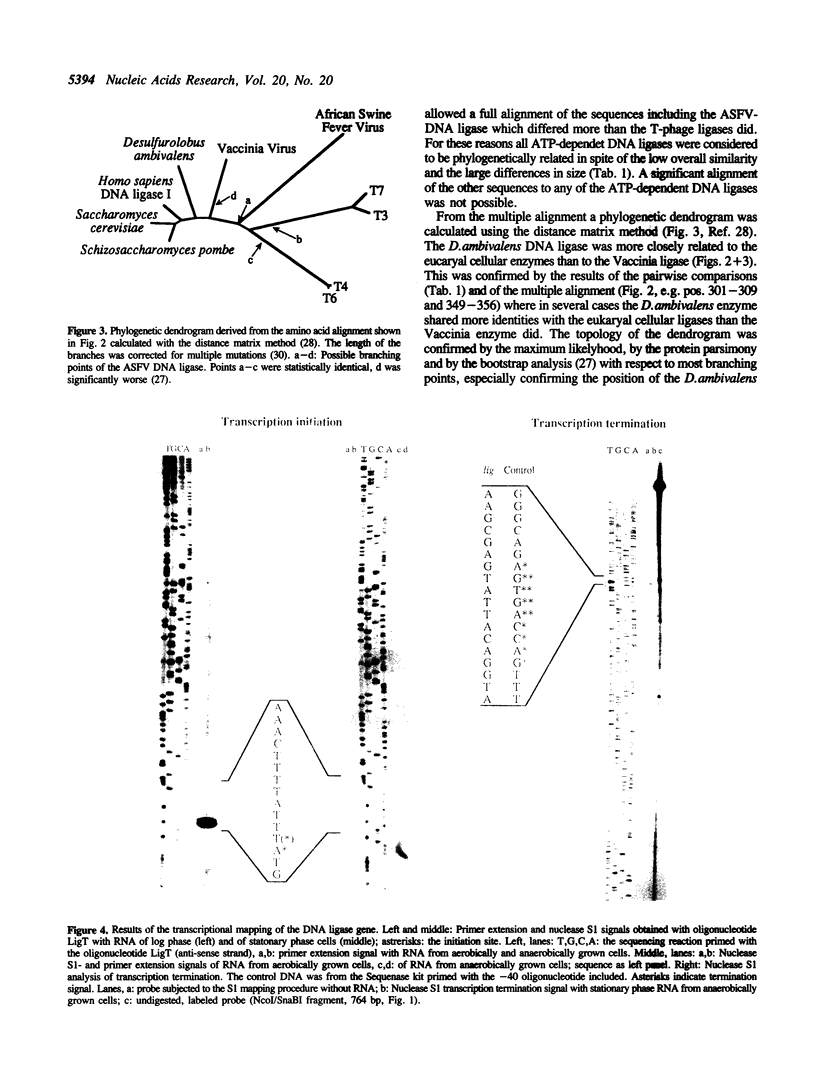
A 3382 bp fragment containing a gene for a DNA ligase from the extremely thermophilic, acidophilic, and facultatively anaerobic archaeon (archaebacterium) Desulfurolobus ambivalens was cloned and sequenced. The deduced amino acid sequence (600 amino acids, 67619 molecular weight) showed 30-34% sequence identity with the ATP-dependent eucaryal (eukaryotic) DNA ligases of Schizosaccharomyces pombe, Saccharomyces cerevisiae, the human DNA ligase I, and with the Vaccinia DNA ligase. Distant similarity to the DNA ligases from the bacteriophages T3, T4, T6, T7 and the African swine fever virus was found, whereas no similarities were detectable to the NAD-dependent DNA ligases from the bacteria (eubacteria) Escherichia coli and Thermus thermophilus, to the ATP-dependent RNA-ligase of bacteriophage T4, and to the tRNA-Ligase from S.cerevisiae. A detailed comparison of the phylogenetic relationship of the amino acid sequences of all known DNA and RNA ligases is presented including a complete alignment of the ATP-dependent DNA ligases. The in vivo-transcription initiation and termination sites of the D.ambivalens gene were mapped. The calculated transcript length was 1904-1911 nt.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong J., Brown R. S., Tsugita A. Primary structure and genetic organization of phage T4 DNA ligase. Nucleic Acids Res. 1983 Oct 25;11(20):7145–7156. doi: 10.1093/nar/11.20.7145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker D. G., Johnson A. L., Johnston L. H. An improved assay for DNA ligase reveals temperature-sensitive activity in cdc9 mutants of Saccharomyces cerevisiae. Mol Gen Genet. 1985;200(3):458–462. doi: 10.1007/BF00425731. [DOI] [PubMed] [Google Scholar]
- Barker D. G., White J. H., Johnston L. H. Molecular characterisation of the DNA ligase gene, CDC17, from the fission yeast Schizosaccharomyces pombe. Eur J Biochem. 1987 Feb 2;162(3):659–667. doi: 10.1111/j.1432-1033.1987.tb10688.x. [DOI] [PubMed] [Google Scholar]
- Barnes D. E., Johnston L. H., Kodama K., Tomkinson A. E., Lasko D. D., Lindahl T. Human DNA ligase I cDNA: cloning and functional expression in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6679–6683. doi: 10.1073/pnas.87.17.6679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. W., Daniels C. J., Reeve J. N. Gene structure, organization, and expression in archaebacteria. Crit Rev Microbiol. 1989;16(4):287–338. doi: 10.3109/10408418909105479. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn J. J., Studier F. W. Nucleotide sequence from the genetic left end of bacteriophage T7 DNA to the beginning of gene 4. J Mol Biol. 1981 Jun 5;148(4):303–330. doi: 10.1016/0022-2836(81)90178-9. [DOI] [PubMed] [Google Scholar]
- Feng D. F., Johnson M. S., Doolittle R. F. Aligning amino acid sequences: comparison of commonly used methods. J Mol Evol. 1984;21(2):112–125. doi: 10.1007/BF02100085. [DOI] [PubMed] [Google Scholar]
- Fitch W. M., Margoliash E. Construction of phylogenetic trees. Science. 1967 Jan 20;155(3760):279–284. doi: 10.1126/science.155.3760.279. [DOI] [PubMed] [Google Scholar]
- Hammond J. M., Kerr S. M., Smith G. L., Dixon L. K. An African swine fever virus gene with homology to DNA ligases. Nucleic Acids Res. 1992 Jun 11;20(11):2667–2671. doi: 10.1093/nar/20.11.2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hüdepohl U., Reiter W. D., Zillig W. In vitro transcription of two rRNA genes of the archaebacterium Sulfolobus sp. B12 indicates a factor requirement for specific initiation. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5851–5855. doi: 10.1073/pnas.87.15.5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishino Y., Shinagawa H., Makino K., Tsunasawa S., Sakiyama F., Nakata A. Nucleotide sequence of the lig gene and primary structure of DNA ligase of Escherichia coli. Mol Gen Genet. 1986 Jul;204(1):1–7. doi: 10.1007/BF00330179. [DOI] [PubMed] [Google Scholar]
- Ishino Y., Shinagawa H., Makino K., Tsunasawa S., Sakiyama F., Nakata A. Nucleotide sequence of the lig gene and primary structure of DNA ligase of Escherichia coli. Mol Gen Genet. 1986 Jul;204(1):1–7. doi: 10.1007/BF00330179. [DOI] [PubMed] [Google Scholar]
- Kaliman A. V., Zimin A. A., Nazipova N. N., Kriukov V. M., Taniashin V. I. Sravnitel'nyi analiz genov DNK-ligaz fagov T6 i T4. Dokl Akad Nauk SSSR. 1988;299(3):737–742. [PubMed] [Google Scholar]
- Lasko D. D., Tomkinson A. E., Lindahl T. Eukaryotic DNA ligases. Mutat Res. 1990 Sep-Nov;236(2-3):277–287. doi: 10.1016/0921-8777(90)90011-s. [DOI] [PubMed] [Google Scholar]
- Lauer G., Rudd E. A., McKay D. L., Ally A., Ally D., Backman K. C. Cloning, nucleotide sequence, and engineered expression of Thermus thermophilus DNA ligase, a homolog of Escherichia coli DNA ligase. J Bacteriol. 1991 Aug;173(16):5047–5053. doi: 10.1128/jb.173.16.5047-5053.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson W. R. Rapid and sensitive sequence comparison with FASTP and FASTA. Methods Enzymol. 1990;183:63–98. doi: 10.1016/0076-6879(90)83007-v. [DOI] [PubMed] [Google Scholar]
- Rand K. N., Gait M. J. Sequence and cloning of bacteriophage T4 gene 63 encoding RNA ligase and tail fibre attachment activities. EMBO J. 1984 Feb;3(2):397–402. doi: 10.1002/j.1460-2075.1984.tb01819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter W. D., Palm P., Zillig W. Analysis of transcription in the archaebacterium Sulfolobus indicates that archaebacterial promoters are homologous to eukaryotic pol II promoters. Nucleic Acids Res. 1988 Jan 11;16(1):1–19. doi: 10.1093/nar/16.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt M. P., Beck P. J., Kearney C. A., Spence J. L., DiGiovanni D., Condreay J. P., Molineux I. J. Sequence of a conditionally essential region of bacteriophage T3, including the primary origin of DNA replication. J Mol Biol. 1987 Feb 5;193(3):479–495. doi: 10.1016/0022-2836(87)90261-0. [DOI] [PubMed] [Google Scholar]
- Schmitt M. P., Beck P. J., Kearney C. A., Spence J. L., DiGiovanni D., Condreay J. P., Molineux I. J. Sequence of a conditionally essential region of bacteriophage T3, including the primary origin of DNA replication. J Mol Biol. 1987 Feb 5;193(3):479–495. doi: 10.1016/0022-2836(87)90261-0. [DOI] [PubMed] [Google Scholar]
- Smith G. L., Chan Y. S., Kerr S. M. Transcriptional mapping and nucleotide sequence of a vaccinia virus gene encoding a polypeptide with extensive homology to DNA ligases. Nucleic Acids Res. 1989 Nov 25;17(22):9051–9062. doi: 10.1093/nar/17.22.9051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thøgersen H. C., Morris H. R., Rand K. N., Gait M. J. Location of the adenylylation site in T4 RNA ligase. Eur J Biochem. 1985 Mar 1;147(2):325–329. doi: 10.1111/j.1432-1033.1985.tb08753.x. [DOI] [PubMed] [Google Scholar]
- Tomkinson A. E., Totty N. F., Ginsburg M., Lindahl T. Location of the active site for enzyme-adenylate formation in DNA ligases. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):400–404. doi: 10.1073/pnas.88.2.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westaway S. K., Phizicky E. M., Abelson J. Structure and function of the yeast tRNA ligase gene. J Biol Chem. 1988 Mar 5;263(7):3171–3176. [PubMed] [Google Scholar]
- Woese C. R., Kandler O., Wheelis M. L. Towards a natural system of organisms: proposal for the domains Archaea, Bacteria, and Eucarya. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4576–4579. doi: 10.1073/pnas.87.12.4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q., Teplow D., Lee T. D., Abelson J. Domain structure in yeast tRNA ligase. Biochemistry. 1990 Jul 3;29(26):6132–6138. doi: 10.1021/bi00478a004. [DOI] [PubMed] [Google Scholar]




