Abstract
The methyltransferase DOT1L methylates histone H3 at K79 to facilitate specific biological events. H3K79 dimethylation (H3K79-2Me) by DOT1L influences the DNA damage response by promoting 53BP1 recruitment to DNA damage sites; however, it is unclear if this methylation is required as 53BP1 interacts with dimethylated H4 (H4K20-2Me) with a much higher affinity. We demonstrate that H3K79-2Me, while negligible during S-phase, is required for ionizing radiation (IR)-induced 53BP1 foci formation during G1/G2-phases when H4K20-2Me levels are low. Further, we describe an essential role for HLA-B-associated transcript 3 (Bat3) in regulating this process in U2OS cells. Bat3 co-localizes with DOT1L at histone H3, and Bat3 knockdown results in decreased DOT1L–H3 interaction and H3K79-2Me, leading to a reduction in IR-induced 53BP1 foci formation, defects in DNA repair and increased sensitivity to IR. We demonstrate that a conserved Bat3 ubiquitin-like motif and a conserved DOT1L ubiquitin-interacting motif promote DOT1L–Bat3 interaction to facilitate efficient H3K79-2Me and IR-induced 53BP1 foci formation during G1/G2-phases. Taken together, our findings identify a novel role for Bat3 in regulating DOT1L function, which plays a critical role in DNA damage response.
Keywords: cell cycle, DNA damage response, histone methylation, 53BP1 foci formation
Introduction
Histone modifications, including acetylation, ubiquitination, phosphorylation, and methylation, have been shown to influence, either directly or indirectly, the regulation of DNA damage response processes such as cell cycle checkpoint activation, apoptosis, and DNA repair (Vidanes et al, 2005). Histone methylation in particular plays an important and direct role in the DNA damage response as it has been demonstrated to modulate the recruitment and foci formation of 53BP1, a critical regulator of DNA damage signalling and repair, to sites of ionizing radiation (IR)-induced DNA damage (Huyen et al, 2004; Botuyan et al, 2006). Initial studies in yeast revealed that the 53BP1 homologues Rad9 (Saccharomyces cerevisiae) and Crb2 (Schizosaccharomyces pombe) are recruited to sites of DNA damage through a direct interaction with dimethylated K79 of histone H3 (H3K79-2Me) or dimethylated K20 of histone H4 (H4K20-2Me), respectively (Sanders et al, 2004; Giannattasio et al, 2005; Nakamura et al, 2005; Wysocki et al, 2005; Du et al, 2006; Lazzaro et al, 2008). These interactions are achieved due to a highly conserved Tudor domain possessed by both Rad9 and Crb2 that binds preferentially to the dimethylated residues described above (Sanders et al, 2004; Grenon et al, 2007). Interestingly, human 53BP1 also contains a conserved tandem Tudor domain that bears a striking similarity to those of its yeast homologues. This domain has been shown to interact specifically and directly with both H3K79-2Me and H4K20-2Me, and these interactions, as well as both of these modified residues have been shown to be required for efficient 53BP1 foci formation in response to IR (Huyen et al, 2004; Botuyan et al, 2006). In vitro studies demonstrate that 53BP1 binds most efficiently to H4K20-2Me and that this residue may be the major regulator of IR-induced 53BP1 foci formation; however, dimethylation of this residue is regulated in a cell-cycle-specific manner with levels peaking during S-phase and then becoming significantly reduced during G1- and G2/M-phase (Botuyan et al, 2006; Karachentsev et al, 2007). This suggests that an alternate means of 53BP1 recruitment may be required during these phases of the cell cycle. Interestingly, in contrast to what has been observed in yeast, H3K79-2Me levels in humans remain consistently high and do not appear to fluctuate throughout the cell cycle (van Leeuwen et al, 2002; Schulze et al, 2009) and thus may provide a means for 53BP1 recruitment in response to IR stress encountered during G1- and G2/M-phases.
The exact mechanism by which IR stress causes 53BP1 to interact with these dimethylated histone residues remains unknown; however, the prevailing hypothesis is that aberrant exposure of these residues may result in 53BP1 recruitment and foci formation (Huyen et al, 2004; Botuyan et al, 2006). A recent study also indicates that H4K20-2Me becomes enriched at sites of DNA damage through the action of the histone methyltransferase MMSET resulting in 53BP1 recruitment (Pei et al, 2011). In euchromatin during normal cellular metabolism, dimethylation of H3K79 and H4K20 is involved in various mechanisms of transcriptional regulation (Steger et al, 2008). In heterochromatin, these dimethylated residues have been hypothesized to map to the histone core and would be inaccessible to 53BP1 if higher-order chromatin structure involves nucleosome stacking (Huyen et al, 2004). Following DNA damage, heterochromatin and nucleosome structure can be disrupted allowing these dimethylated lysines to become aberrantly exposed. 53BP1 then binds specifically to these residues by way of its conserved tandem Tudor domain, ultimately forming distinct foci and facilitating the activation of downstream checkpoint and DNA repair pathways (Huyen et al, 2004; Botuyan et al, 2006).
The only recognized protein responsible for H3K79 methylation is the highly conserved histone methyltransferase DOT1L (van Leeuwen et al, 2002; Jones et al, 2008). Multiple groups have demonstrated in yeast and human cell systems that DOT1L methylates H3 at K79 both in vivo and in vitro (Feng et al, 2002; Min et al, 2003; Jones et al, 2008; Steger et al, 2008). In addition to promoting DNA damage response mechanisms after IR exposure, context-specific H3K79 methylation by DOT1L regulates a number of cellular mechanisms including gene transcription, embryonic development, embryonic stem cell division, and cardiac function (Zhang et al, 2004; Okada et al, 2005; Jones et al, 2008; Barry et al, 2009; Nguyen et al, 2011). Given its diverse roles in facilitating numerous cellular functions, it is not surprising that misregulation of DOT1L has been linked to disruption of several intracellular processes and the initiation and maintenance of disease (Barry et al, 2010). The importance of proper DOT1L regulation is further underscored by the observation that loss of DOT1L function can lead to mitotic misregulation, loss of cell cycle control, and apoptotic failure (Nguyen et al, 2011). In addition, CALM–AF10 fusion leukaemias and many of the MLL-rearranged leukaemias are dependent upon DOT1L for initiation and viability (Okada et al, 2006; Barry et al, 2010). Specifically, these fusion proteins can interact with and recruit DOT1L to the promoter regions of the HoxA cluster where aberrant methylation patterns catalysed by DOT1L cause upregulation of HoxA cluster gene expression which in turn drives leukaemogenesis (Ayton and Cleary, 2003; Okada et al, 2005, 2006).
While it is clearly established that DOT1L is responsible for H3K79 methylation, little is known regarding the mechanisms regulating DOT1L function in the context of both normal cellular metabolism and disease states. Several recent studies indicated a correlation between ubiquitination of histone H2B at K120 in humans and K123 in yeast with enhanced H3K79 methylation (Jeltsch and Rathert, 2008; McGinty et al, 2008). A subsequent study revealed that this increase in methylation is likely due to enhanced allosteric accessibility of H3K79 caused by the ubiquitination of H2B at K120 (McGinty et al, 2009). These studies provide valuable insight into mechanisms facilitating DOT1L activity, but they leave open the question of what factors are required for recruitment or stabilization of DOT1L to histone H3.
Because of the numerous functions of DOT1L in development, transcriptional regulation, and the DNA damage response, it is likely that DOT1L can interact with multiple proteins in a context-specific manner. For example, in yeast, DOT1 can interact directly with H3, but complexes such as Paf1 have been demonstrated to be required for DOT1 function (Krogan et al, 2003). In humans, studies indicate that DOT1L activity can be regulated under normal conditions by proteins such as AF10 and in disease states such as leukaemia by CALM–AF10 and MLL–AF10 fusion proteins, although these mechanisms remain poorly understood (Jones et al, 2008; Steger et al, 2008; Jacinto et al, 2009). Recently, HLA-B-associated transcript 3 (Bat3 or Scythe) was shown to be involved in regulating histone H3K4 dimethylation (H3K4-2Me) and subsequent gene transcription (Nguyen et al, 2008). In addition to its role in regulating H3K4-2Me, Bat3 is also known to be a regulator of apoptosis in response to numerous stresses including IR (Thress et al, 1998; Sasaki et al, 2007; Desmots et al, 2008; Tsukahara et al, 2009). Interestingly, similar to DOT1L, Bat3 is also known to function in embryonic development, cell division, and cell cycle checkpoint activation (Huyen et al, 2004; Mu et al, 2007). Given the functional similarities between Bat3 and DOT1L, the interaction of Bat3 with histone H3, and the observation that Bat3 has a conserved ubiquitin-like domain and DOT1L contains a putative ubiquitin-interacting motif, we hypothesized that Bat3 may interact directly with DOT1L to recruit or stabilize this protein to at least a subset of histone H3 to facilitate H3K79 methylation.
Here, we demonstrate that Bat3 co-localizes with DOT1L at histone H3, and knockdown of Bat3 results in decreased DOT1L–H3 interaction and a reduction in H3K79-2Me in multiple cell types. Bat3-depleted cells display a marked reduction in the number of IR-induced 53BP1 foci, specifically during the G1- and G2/M-phases of the cell cycle. These cells also exhibit defects in DNA repair and increased sensitivity to IR. Finally, we demonstrate that a conserved ubiquitin-like motif (UBL) at the N-terminal region of Bat3 (Banerji et al, 1990) and a conserved DOT1L ubiquitin-interacting motif (UIM) consensus sequence are required for DOT1L–Bat3 interaction and are necessary for efficient H3K79-2Me and IR-induced 53BP1 foci formation.
Results
Bat3 is required for efficient IR-induced 53BP1 foci formation and H3K79-2Me
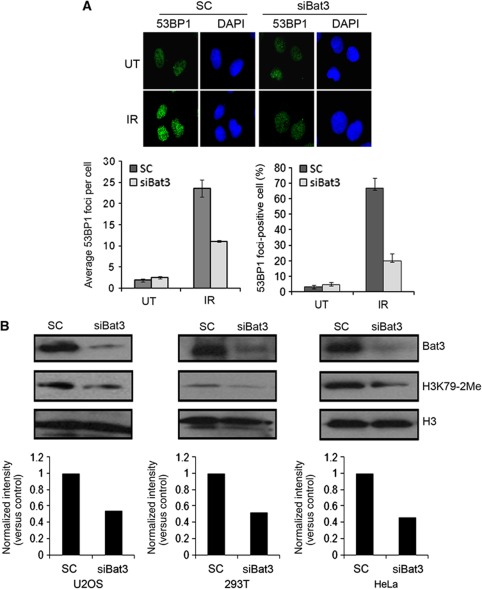
It was previously reported that Bat3 knockdown results in deficient G1 and G2/M checkpoint activation following exposure to IR; however, the underlying mechanism was not studied (Mu et al, 2007). Since 53BP1 foci formation is required for efficient cell cycle checkpoint function following IR exposure, we decided to test the role of Bat3 in regulating this event. Human U2OS cells were transfected twice with scrambled control (SC) siRNA or siRNA targeting Bat3 (siBat3) and allowed to grow for 48 h. The cells were then either left untreated or exposed to 5 Gy IR followed with fixation 1 h after. Both experimental groups showed a similar basal level of 53BP1 foci, but after irradiation the Bat3 knockdown cells exhibited a marked reduction in the formation of IR-induced 53BP1 foci as measured by average foci per cell and the percentage of foci-positive cells (Figure 1A).
Figure 1.
Bat3 is required for efficient IR-induced 53BP1 foci formation and H3K79-2Me. (A) 53BP1 foci formation was negatively affected by downregulation of Bat3. U2OS cells were transfected twice with SC siRNA or a siBat3, and then allowed to grow for 48 h. Cells were left untreated (UT) or exposed to 5 Gy of IR. After 1 h, cells were fixed and stained with 53BP1 antibody, and the IR-induced 53BP1 foci were visualized with IF and the average foci per cell and the percentage of foci-positive cells were quantified by counting at least 200 cells. A cell detected with five or more discrete foci was regarded as foci positive. The error bars represent the standard deviation from at least three independent experiments. (B) H3K79-2Me was decreased with reduction in Bat3. U2OS, 293T and HeLa cells were transfected twice with scramble control (SC) or a siBat3 and then allowed to grow for 48 h, respectively. The cells were harvested and then cellular extracts were immunoblotted with specific antibody against H3K79-2Me and histone H3. Histone H3 blot was used as loading control. Bat3 knockdown resulted in noticeable reduction (∼50%) of H3K79-2Me. Image J software was used to measure the intensity of H3K79-2Me and H3 band, and with the ratio of H3K79-2Me and H3 set as 1.0 for scramble control. Then the ratio of these two bands for Bat3 knockdown samples was presented as normalized intensity versus the ratio of the scramble control in the y axis.
To determine how Bat3 regulates 53BP1 foci formation, we tested the effects of Bat3 knockdown on histone H3K79-2Me, as it has been demonstrated that H3K79-2Me is required for 53BP1 targeting to sites of DNA double-stranded break (DSB) (Huyen et al, 2004). U2OS cells were transfected twice with SC or siBat3 and then allowed to grow for 48 h. The cells were then probed by western blot for H3K79-2Me and total histone H3. Bat3 knockdown resulted in noticeable reduction (∼50%) of H3K79-2Me compared with SC in multiple cell lines (U2OS, 293T, and HeLa), while total H3 levels remained unchanged (Figure 1B). These results were confirmed using a separate siRNA sequence targeting Bat3 to rule out any off-target effect (Supplementary Figure S1A). Moreover, we noticed a reduction in H3K79-1Me and 3Me in Bat3 knockdown cells compared with control cells, further implicating the role for Bat3 in regulation of DOT1L function (Supplementary Figure S1B).
H3K79-2Me is required for efficient 53BP1 foci formation when H4K20-2Me levels are low
In vitro data indicate that the interaction of 53BP1 with H4K20-2Me occurs with a much higher affinity than the interaction between 53BP1 and H3K79-2Me, and consequently it is thought that H4K20-2Me is the major histone modification responsible for IR-induced 53BP1 foci formation (Botuyan et al, 2006). Interestingly, it has also been shown that H4K20-2Me levels are cell cycle regulated, with this modification peaking during S-phase and becoming significantly reduced during G1- and G2/M-phases (Karachentsev et al, 2007). Depending on the degree of H4K20-2Me reduction, an alternate mechanism of 53BP1 recruitment to sites of DNA damage during these phases of the cell cycle may be required. Since H3K79-2Me has previously been demonstrated to contribute to 53BP1 foci formation (Huyen et al, 2004) and in humans this modification is known to exist at high levels and does not fluctuate throughout the cell cycle, we decided to test if H3K79-2Me is required for IR-induced 53BP1 foci formation specifically during G1- and G2/M-phases. Using U2OS cells with a tet-on system, we generated stable, doxicyline (Dox)-inducible Bat3 knockdown (shBat3) and SC cell lines. The SC or shBat3 containing clones selected for experimental use displayed similar morphology and growth patterns, and knockdown of Bat3 in the shBat3 cell line is 80–90% following treatment with 1 μg/ml Dox for 48 h (data not shown). These cells were treated with Dox for 96 h and then synchronized at the G1/S boundary by thymidine-mimosine block, with normally cycling cells used as a control. Cells were then released back into the cell cycle, irradiated, and harvested 10 min later for western blot or fixed for immunofluorescence (IF) at specific time points. Efficient synchronization was confirmed by PI staining and flow cytometry (Supplementary Figure S2A). As in our previous experiments, Bat3 knockdown cells showed significantly reduced levels of IR-induced 53BP1 foci formation and H3K79-2Me relative to control cells (Figure 2A and B). In the synchronized cell population, similar to previously reported data (Karachentsev et al, 2007), H4K20-2Me levels were high during S-phase but became nearly undetectable during the remainder of the cell cycle (Figure 2C). This was in contrast to H3K79-2Me levels, which remained constant throughout the cell cycle (Figure 2C). Since Bat3 is also involved in other processes, we further performed similar experiments using DOT1L siRNA knockdown cells to determine if the cell-cycle-dependent pattern of H3K79-2Me is associated with DOT1L. Consistent with previously reported findings that depletion of DOT1L resulted in a remarkable decrease in H3K79-2Me throughout the cell cycle (Supplementary Figure S2B), the observed effect of Bat3 (Figure 2C) on H3K79-2Me is most likely relevant to the function of DOT1L as both Bat3 knockdown and DOT1L knockdown display a similar phenotype in terms of H3K79-2me. Analysis of IR-induced 53BP1 foci formation indicated that this modification remained largely unchanged in control cells throughout the cell cycle; however, in Bat3 knockdown cells, 53BP1 foci formation was slightly reduced during S-phase and became significantly reduced during G1- and G2/M-phases (Figure 2D), suggesting that 53BP1 foci formation is relevant to Bat3-mediated changes in H3K79-2Me. The apparent synchrony of H4K20-2Me levels with 53BP1 foci formation in cells with reduced H3K79-2Me levels (Bat3 knockdown cells) supports the notion that H4K20-2Me is the main histone modification for IR-induced 53BP1 foci formation during S-phase when this modification is high, and the deficient 53BP1 foci formation observed in Bat3 depleted, H3K79-2Me low cells during G1- and G2/M-phases demonstrates that H3K79-2Me is required for efficient 53BP1 foci formation during these cell-cycle phases when H4K20-2Me is low.
Figure 2.
H3K79-2Me is required for efficient 53BP1 foci formation when H4K20-2Me levels are low. (A) Inducible reduction in Bat3 led to decrease in 53BP1 foci formation. Stable inducible scramble control cells (SC) or Bat3 knockdown cells (Bat3 shRNA) were treated with 1 μg/ml Dox for 48 h. Cells were then either left untreated (UT) or exposed to 5 Gy IR. In all, 10 min after IR, cells were fixed and stained with 53BP1 antibody and IR-induced 53BP1 foci were examined under immunofluorescent microscope. The percentage of 53BP1 foci-positive cells was calculated by accounting at least 200 cells. Cell with five or more discrete focus was considered as foci positive. The error bars represent standard deviation from three independent experiments. (B) Inducible reduction in Bat3 led to decrease in H3K79-2Me without affecting H4K20-2Me. The cells were treated in the same way as (A) and western blot analysis was performed using indicated antibodies. (C) H3K79-2Me and H4K20-2Me displayed different patterns during the cell cycle. The procedure of inducible knockdown of Bat3 was the same as (A). These cells were then synchronized at the G1/S boundary by thymidine-mimosine block. Cells were then released back into the cell cycle and harvested at indicated time points. Various methylated or unmethylated proteins as indicated were determined by western blot analysis.
Figure 2.
(D) 53BP1 foci formation was reduced with Bat3 knockdown correlated with reduction in H3K79-2Me but not H4K20-2Me. Cells were treated with Dox, synchronized at G1/S and released back into cell cycle as performed in (C). At indicated time points, cells were exposed to 5 Gy IR and then fixed 10 min later. IR-induced 53BP1 foci were visualized with IF and the average foci per cell and the percentage of foci-positive cells were quantified by counting at least 200 cells. Cell detected with five or more discrete foci were regarded as foci positive.
It should be noted that H3K79-2Me does not appear to be required for 53BP1 foci formation in all cell types, as a previous study analysing HeLa cells revealed that H3K79-2Me was not required for IR-induced 53BP1 foci formation (Botuyan et al, 2006). Interestingly, HeLa cells display a high level of H4K20-2Me, and while this modification does fluctuate in a cell-cycle-dependent manner, it is still readily detectable at all phases (Karachentsev et al, 2007). This is not the case in U2OS cells, where H3K79-2Me appears to play a more important role in 53BP1 foci formation (Figure 2C and 2D). To confirm the results of previous studies, we synchronized U2OS and HeLa cells transfected with SC or siBat3 constructs and tested these cells for IR-induced 53BP1 foci formation at all phases of the cell cycle. We also tested normally cycling HeLa cells that had been transfected with SC or siBat3 constructs. Consistent with previous reports, we found that HeLa cells displayed only a slight reduction in IR-induced 53BP1 foci formation when Bat3 levels were suppressed; however, 53BP1 foci formation was noticeably reduced in U2OS cells, specifically during G1- and G2-phases, following Bat3 knockdown (Supplementary Figure S3).
Bat3 knockdown results in defective repair of IR-induced DNA damage
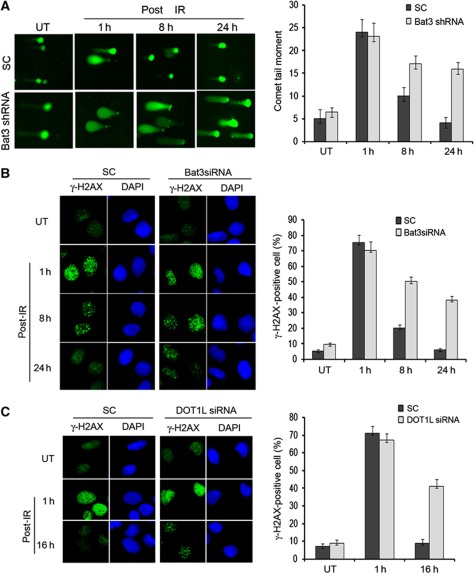
Since 53BP1 foci are required for efficient DNA repair following IR and 53BP1 foci formation is reduced in Bat3 knockdown cells, we next tested for repair deficiencies following Bat3 knockdown using the single-cell gel electrophoresis assay (comet assay). This assay is sensitive enough to detect the small amounts of stress induced during transfection, so we again used our stable inducible Bat3 knockdown cell lines to minimize stress under basal assaying conditions. For the comet assay, SC or shBat3 cells were treated with Dox for 96 h and then either left untreated or exposed to 5 Gy IR. Cells were then harvested at 1, 8, and 24 h time points and subjected to comet analysis. Both cell lines exhibited similar amounts of DNA damage when untreated or at 1 h post-IR, but they displayed a dramatic difference during recovery. In SC cells, the amounts of DNA damage were significantly reduced at 8 h and returned essentially to basal levels at 24 h, while by contrast the shBat3 cells exhibited persistent DNA damage at 8 and 24 h post-IR (Figure 3A). Consistent with this result, the shBat3 cells exhibited a marked decrease in their ability to survive IR stress compared with the SC cells (Supplementary Figure S4A).
Figure 3.
Bat3 knockdown results in DNA repair defects following IR. (A) Reduction in Bat3 led to prolonged DNA repair. Single-cell gel electrophoresis assay was performed on stable inducible Bat3 knockdown versus SC cells treated with 5 Gy IR after indicated time points. The level of DNA break repair was visualized with the length of comet tail. Images were analysed by using CometScore software (Tritek) to quantify the comet tail movement of at least 75 cells for each sample. The experiments were repeated at least three times independently. (B) Reduction in Bat3 led to prolonged presence of γ-H2AX. U2OS cells were transfected twice with SC siRNA or Bat3siRNA and allowed to grow for 48 h. Cells were then exposed to 5 Gy IR and harvested at indicated time points following IR. Cells were fixed and stained with γ-H2AX antibody, and γ-H2AX foci were visualized with IF. The percentage of foci-positive cells were quantified by counting at least 100 cells and plotted with cells detected with 10 or more discrete foci regarded as foci positive. The experiments were repeated three times independently. (C) Reduction in DOT1L led to prolonged presence of γ-H2AX. The experiment was done in the same manner as in (B) except using DOT1L siRNA instead of Bat3 siRNA.
Exposure to IR results in numerous types of intracellular damage, with the most deleterious being the DNA DSBs. To assess the importance of Bat3 and H3K79-2Me in the repair of DSBs and to confirm the results of our comet assay, we used IF analysis to observe the reduction of γ-H2AX foci, a biomarker of individual DSBs, in SC or Bat3 knockdown cells following exposure to IR. Cells were transfected twice with SC or siBat3 constructs and after 48 h were exposed to 5 Gy IR or left untreated. The cells were then fixed at 1, 8, or 24 h and γ-H2AX foci analysis was performed by IF microscopy. Similar to the results obtained from our comet assay, initial γ-H2AX foci levels were nearly identical in both cell types after 1 h; however, at 8 and 24 h, SC cells exhibited a marked reduction in γ-H2AX foci while their levels remained high in the Bat3 knockdown cells (Figure 3B).
To also confirm the importance of DOT1L in the DNA repair process, we repeated the γ-H2AX foci staining assay by treating U2OS cells twice with siRNA specific for DOT1L and then leaving the cells untreated or exposing them to 5 Gy IR before harvesting at 1 or 16 h to measure the percentage of H2AX foci-positive cells. Similar to the data found in Bat3 knockdown cells, our results clearly showed that downregulation of DOT1L leads to a significant defect in DNA repair indicated by a much higher percentage of γ-H2AX foci-positive cells compared with those containing normal level of DOT1L (Figure 3C).
Bat3 is required for efficient binding of DOT1L to H3
To determine the underlying mechanism that allows Bat3 to regulate H3K79-2Me, we examined if Bat3 could interact with DOT1L, the methyltransferase responsible for H3K79-2Me, and regulate its ability to interact with histone H3. Under highly stringent conditions that should rule out non-specific protein–protein interactions, we performed immunoprecipitation (IP) assays for these proteins. IP of Bat3, DOT1L, or H3 brought down each of the other proteins including H3K79-2Me (Figure 4A), indicating that they interact with each other at endogenous levels. To determine if Bat3 is critical for the ability of DOT1L to bind to histone H3, we performed H3 IP experiments with reduced expression of Bat3 in U2OS cells and found a significant decrease (at least 50%) in the interaction between DOT1L and histone H3 (Figure 4B), whereas the cellular level of DOT1L remained unaffected (Figure 4C). Furthermore, we performed IF staining to examine if Bat3 co-localizes with DOT1L at endogenous conditions. As shown in Figure 4D, it is clear that Bat3 constitutively co-localizes with DOT1L in the nucleus and that downregulation of Bat3 or DOT1L results in a significant reduction of the co-localization. Together, these data indicate that Bat3 is required for efficient binding of DOT1L to histone H3.
Figure 4.
Bat3, DOT1L, and H3 interact at endogenous levels. (A) Co-IP followed by western blots using the indicated antibodies was performed to examine the interaction among Bat3, DOT1L, histone H3, and dimethylated histone H3 at Lys79. The input shown represents 4% of the lysates. (B) Reduction in Bat3 led to decrease in the association of DOT1L and H3. U2OS cells were transfected twice with SC siRNA or Bat3 siRNA and then allowed to grow for 48 h. Co-IPs were performed using H3 antibody in the presence and absence of siBat3. (C) Reduction in Bat3 did not affect total level of DOT1L. Cellular lysates of SC siRNA and Bat3 siRNA transfected cells were immunoblotted with Bat3 and DOT1L antibody as indicated. (D) Bat3 is constitutively co-localized with DOT1L. U2OS cells were either left untreated or transfected by Bat3 siRNA or DOT1L siRNA and then fixed and stained with Bat3 and DOT1L antibodies as well as DAPI, and the co-localization of Bat3 and DOT1L was examined using immunofluorescent microscopy.
The N-terminal ubiquitin homology domain of Bat3 is required for DOT1L–Bat3 interaction, H3K79-2Me, and efficient 53BP1 foci formation following IR
To further define the mechanism by which Bat3 regulates DOT1L function, we tested the importance of a conserved 71 amino acids long UBL domain of Bat3 (Banerji et al, 1990) in facilitating DOT1L–Bat3 interaction. We chose to study this domain because analysis of the DOT1L protein sequence revealed a previously undefined putative UIM consensus sequence that is well conserved in mammals (depicted in Figure 6A). Endogenous Bat3 levels were reduced using siRNA specific for Bat3 and then RNAi-resistant wild-type (WT) or UBL mutant (ΔUBL) Bat3 or empty vector were added back into U2OS cells via transient transfection. After 72 h, these cells were lysed and probed for H3K79-2Me. Consistent with our previous results, Bat3 knockdown led to a marked decrease in H3K79-2Me, and reintroduction of WTBat3 was able to restore H3K79-2Me, whereas empty vector and Bat3 ΔUBL were not (Figure 5A). To test if the failure of Bat3 ΔUBL to restore H3K79-2Me was due to a reduction in DOT1L–Bat3 interaction, we performed IP analysis on these lysates using anti-Bat3 antibody. As shown in Figure 5B, add-back of WTBat3, but not empty vector or Bat3 ΔUBL, was able to restore DOT1L–Bat3 interaction. It should also be noted that WTBat3 and Bat3 ΔUBL exhibited no difference in their ability to interact with histone H3 and that the Bat3 ΔUBL mutant could localize normally to the nucleus (Supplementary Figure S5A and B). To further explore the biological significance of the Bat3 UBL domain, we performed add-back experiments to determine if we could restore IR-induced 53BP1 foci formation in Bat3 knockdown cells. U2OS cells were transfected with siBat3 and then with empty vector, WTBat3, or Bat3 ΔUBL and allowed to grow for 72 h. The cells were then treated with 5 Gy IR and fixed after 1 h. Add-back of WTBat3 fully restored 53BP1 foci formation, whereas cells with add-back of empty vector or Bat3 ΔUBL still exhibited a significant reduction in the percentage of 53BP1 foci-positive cells (Figure 5C). Additionally, add-back of WTBat3 was able to restore radioresistance in U2OS cells, whereas expression of the Bat3 ΔUBL mutant was not (Supplementary Figure S4B). Taken together, these data indicate that the Bat3 N-terminal UBL domain is required for the interaction between Bat3 and DOT1L, for DOT1L-mediated H3 dimethylation at K79, and for the efficient formation of 53BP1 foci and cellular survival following IR.
Figure 5.
The N-terminal ubiquitin homology domain of Bat3 is required for interaction between H3K79-2Me and DOT1L–Bat3, as well as efficient 53BP1 foci formation following IR. (A) WT but not ΔUBL Bat3 restored H3K79-2Me level. U2OS cells were transfected twice with SC siRNA or Bat3 siRNA and followed by the add-back of RNAi-resistant WT or UBL mutant (ΔUBL) Bat3 or empty vector as indicated. After 72 h, cellular extracts were immunoblotted with indicated specific antibodies. (B) The UBL motif is critical for interaction between Bat3 and DOT1L. The same cell lysates described in (A) were used to perform co-IP using Bat3 antibody. Western blot analysis was utilized to examine the interaction changes between Bats and DOT1L. In all, 4% of cell lysate was used as input control. (C) The UBL motif is required for the formation of 53BP1 foci induced by IR. U2OS cells were treated as in (A) and then 5 Gy IR with fixation 1 h after irradiation. 53BP1 foci were visualized with IF and the average foci per cell and the percentage of foci-positive cells were quantified by counting at least 200 cells. Cell detected with five or more discrete foci were regarded as foci positive. The experiments were repeated three times independently.
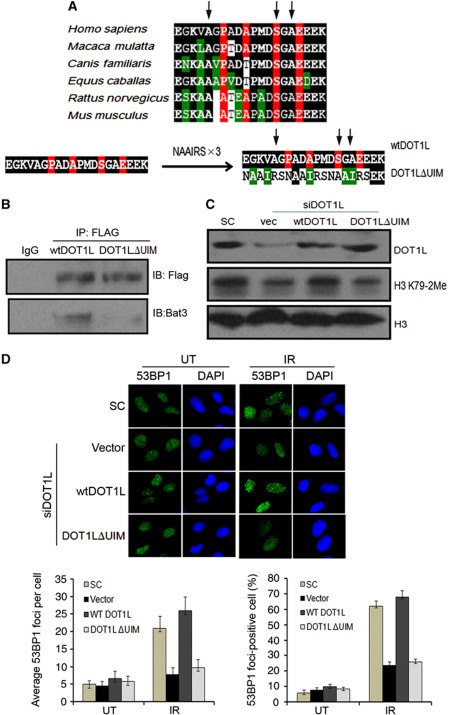
A novel DOT1L UIM is required for DOT1L–Bat3 interaction, efficient H3K79-2Me and 53BP1 recruitment following IR
To further characterize the interaction between DOT1L and Bat3, we analysed the DOT1L amino-acid sequence for putative protein–protein interaction motifs that would be likely to bind to the Bat3 UBL domain. Interestingly, our analysis revealed a well-conserved UIM consensus sequence located just C-terminal to the catalytic domain of DOT1L (Figure 6A), consistent with a previous report on Xenopus Bat3/Scythe (Kikukawa et al, 2005). This UIM has been shown to be present in a large number of proteins and is thought to function in a variety of processes including protein degradation, receptor endocytosis, and protein–protein interaction (Hofmann and Falquet, 2001). In order to examine how the UIM domain affects interaction between DOT1L and Bat3 and H3K79 methylation, we first replaced the UIM sequence of a Flag-tagged DOT1L construct with a triple NAAIRS repeat (DOT1LΔUIM) to alter the local amino-acid sequence to effectively abolish the UIM consensus site (Wilson et al, 1985) with the substitution of three key residues that are directly involved in the interaction with ubiquitin (Lim et al, 2011). U2OS cells were then transfected with wtDOT1L or DOT1LΔUIM and after 48 h interaction with Bat3 was determined by co-IP. IP with anti-Flag antibody indicated that wtDOT1L binds strongly to Bat3 whereas DOT1LΔUIM is unable to interact efficiently (Figure 6B). It should also be noted that deletion of the UIM domain does not affect the binding of DOT1L to H3 (Supplementary Figure S5C). We next tested the functional significance of this interaction by using siRNA to knockdown endogenous DOT1L in U2OS cells and subsequently expressing siRNA-resistant wtDOT1L or DOT1LΔUIM. After 48 h, cell lysates were collected and probed for H3K79-2Me. Cells expressing wtDOT1L exhibited normal levels of H3K79-2Me whereas cells transfected with DOT1LΔUIM showed reduced H3K79 dimethylation similar to vector control (Figure 6C), suggesting that this UIM domain is important for efficient H3K79-2Me through promoting interaction between DOT1L and Bat3. To further test the functional significance of DOT1L UIM domain, we performed add-back experiments to examine the necessity of this domain for the formation of IR-induced 53BP1 foci. Following siRNA-mediated knockdown of endogenous DOT1L, we added back wtDOT1L, DOT1LΔUIM, or empty vector into cells and then subject them to 5 Gy IR or left untreated. After 1 h, immunostaining was performed to examine 53BP1 foci formation. As shown in Figure 6D, add-back of wtDOT1L was able to restore 53BP1 foci formation, but empty vector and DOT1LΔUIM were not. Taken together, these results suggest that H3K79-2Me mediated by DOT1L is required for 53BP1 foci formation and that UIM is a critical motif in DOT1L for the DOT1L–Bat3 interaction and functional regulation of DOT1L by Bat3.
Figure 6.
A novel DOT1L UIM is required for DOT1L–Bat3 interaction and efficient H3K79-2Me and 53BP1 foci formation. (A) Diagram showing conserved DOT1L UIM consensus sequence and a representation of the DOT1L UIM mutagenesis strategy. UIM consensus sequence is highlighted in red, and residues invariant or conservatively substituted are highlighted in black or green, respectively. The arrows point to the three residues, Ala365, Ser374, and Ala376, that have been shown to be involved in direct interactions with ubiquitin (Lim et al, 2011). (B) UIM is required for interaction between DOT1L and Bat3. U2OS cells were transfected with Flag-tagged wtDOT1L or DOT1LΔUIM construct. After 48 h, co-IP was performed using Flag antibody and western blot analysis conducted to examine the interaction between DOT1L and Bat3. (C) UIM is important for H3K79-2Me mediated by DOT1L. U2OS cells were subjected to two rounds of DOT1L knockdown and then transfected with the siRNA-resistant wtDOT1L or DOT1LΔUIM constructs. After 48 h, cell lysates were immunoblotted with DOT1L, H3K79-2Me, or Histone H3 antibodies. (D) UIM is required for DOT1L-mediated 53BP1 foci formation in response to IR. Cells were treated as in (C) and exposed to 5 Gy IR followed by fixation 1 h after. 53BP1 foci were visualized with IF and the average foci per cell and the percentage of foci-positive cells were quantified by counting at least 200 cells. Cell detected with five or more discrete foci were regarded as foci positive. The experiments were repeated three times independently.
Discussion
In this study, we investigated the role of Bat3 in regulating DOT1L function in the context of DNA damage response. We chose this approach in order to clarify the function of DOT1L in facilitating IR-induced 53BP1 foci formation, as this protein has been demonstrated to be required for this process in some systems, while unimportant in others (Huyen et al, 2004; Giannattasio et al, 2005; Botuyan et al, 2006; FitzGerald et al, 2011). In support of a role for DOT1L in facilitating 53BP1 foci formation, it has been suggested that in budding yeast, the 53BP1 homologue Rad9 binds to H3 at Lys79 methylated by Dot1, the yeast homologue of DOT1L (Wysocki et al, 2005). Interestingly, in fission yeast, the 53BP1 homologue Crb2 instead binds to H4K20-2Me that occurs independently of Dot1 (Sanders et al, 2004; Du et al, 2006). In humans, studies have implicated both of these residues as necessary for IR-induced 53BP1 foci formation; however, it has been demonstrated that of the two residues, 53BP1 binds much more tightly to H4K20-2Me, leading to the suggestion that this residue is more important for this process (Huyen et al, 2004; Botuyan et al, 2006). Interestingly, H4K20-2Me has been demonstrated to be cell cycle regulated with levels peaking during S-phase and declining during G1- and G2/M-phases (Karachentsev et al, 2007). Given this observation, it is likely that an alternate mechanism for 53BP1 foci formation may be required during these cell-cycle phases. Since in humans, H3K79-2Me occurs at high levels and does not fluctuate throughout the cell cycle (Figure 2C), we postulated that this modification may be important for IR-induced 53BP1 foci formation exclusively during G1 and G2/M.
Further support for this hypothesis came from a study that demonstrated that Bat3 is required for G1 and G2/M, but not S-phase checkpoint response upon IR-induced DSB damage (Mu et al, 2007). Another clue came from a separate study that Bat3 can interact with complexes at histone H3 to promote H3K4 methylation (Nguyen et al, 2008). Here, we demonstrate that Bat3 can also promote the interaction of DOT1L with histone H3, as knockdown of Bat3 results in a marked reduction in the amount of DOT1L that co-immunoprecipitated with H3 compared with control cells. This reduction in H3–DOT1L interaction results in an evident decrease in H3K79-2Me. To test the importance of this modification in the process of 53BP1 foci formation, we synchronized Bat3-proficient or -depleted cell lines and exposed them to IR every 2.5 h for 20 h. The Bat3 knockdown cells exhibited the expected reduction in H3K79-2Me and significant decrease in 53BP1 foci formation during G1- and G2-phase when H4K20-2Me is low (Figure 2C and 2D), indicating that H3K79-2Me regulated by Bat3 is critical for IR-induced 53BP1 foci formation in a cell-cycle-specific manner.
To further delineate the mechanism by which Bat3 regulates DOT1L function, we explored in more detail the nature of the Bat3–DOT1L interaction. Bat3 contains numerous conserved protein interacting domains including an N-terminal UBL domain, central poly-proline and poly-glycine tracts, and a C-terminal BAG domain (Banerji et al, 1990). While the BAG domain has been studied extensively (Kwak et al, 2008; Sasaki et al, 2008; Tsukahara et al, 2009) and the function of the poly-proline/glycine tract is partially understood (Nguyen et al, 2008), very little is known concerning the highly conserved Bat3 UBL motif. We chose to focus our studies on the Bat3 UBL domain because of the common role of histone ubiquitination in promoting the formation of various complexes at the histone (Jason et al, 2002). Interestingly, we observed that the UBL domain of Bat3 was not required for Bat3–H3 interaction; however, this domain was critical for efficient binding of DOT1L to H3. Analysis of the DOT1L sequence revealed a highly conserved UIM consensus motif located just N-terminal to the catalytic and DNA-binding domains. The proximity of this putative UIM to the functional domains of DOT1L indicated that this sequence is likely exposed to the cytosol instead of being buried within the protein, making it a good candidate to interact with the UBL domain of Bat3. To study the importance of this domain, we chose the strategy of replacing it with a triple NAAIRS repeat because this sequence has been postulated to not disrupt overall protein structure (Wilson et al, 1985). Our data revealed that the DOT1L mutant with its UIM motif substituted with new sequences to remove key residues known to be involved directly in ubiquitin-binding failed to interact with Bat3, could not efficiently promote H3K79-2Me, and was unable to restore IR-induced 53BP1 foci formation. Together, these findings identify the DOT1L UIM as a novel domain required for DOT1L function. Since DOT1L is the only methyltransferase for H3K79-2Me, the results from DOT1L knockdown and add-back experiments indicate that H3K79-2Me is indeed required for 53BP1 foci formation, which is consistent with the previous finding of Huyen et al. Based on the fact that Bat3 can interact with DOT1L and is required for the effective binding of DOT1L to H3, we propose that Bat3-associated DNA damage responses are mediated through DOT1L.
Our findings, coupled with the previously published data showing the ability of Bat3 to modulate H3K4 methylation, point to Bat3 as being a critical regulator of multiple types of histone methylation, and raise the distinct possibility that, in addition to its pro-apoptotic and cell cycle checkpoint functions, Bat3 also serves as a major regulator of mammalian gene expression (Nguyen et al, 2008). Additionally, the regulation of DOT1L by Bat3 suggests that Bat3 may also be implicated in DOT1L-driven leukaemogenesis. Indeed, preliminary data from our laboratory indicate that Bat3 knockdown in the CALM–AF10-positive U937 leukaemia cells results in decreased growth and viability and increased sensitivity to IR. It will be interesting to further assess the role of DOT1L–Bat3 interaction in the context of these leukaemias, as the DOT1L–Bat3 interacting region may potentially serve as a clinically relevant therapeutic target.
In conclusion, our results not only clarify the roles of Bat3 and DOT1L in cell cycle checkpoint activation and DNA damage repair, but reveal a far broader and much more significant role for Bat3 in regulating DOT1L function. It will be of interest to further explore the nature and context of how Bat3 and DOT1L interact, including specific gene promoters, phase of the cell cycle, disease states, and post-translational modifications that could regulate that interaction to achieve a more detailed grasp of the underlying mechanism. Further understanding of how Bat3 regulates DOT1L function will provide valuable insight into the role of histone methylation in normal development and disease processes such as tumourigenesis, and could potentially lead to identification of novel therapeutic targets in the treatment of certain cancers.
Materials and methods
Cell culture and irradiation
U2OS cells (ATCC) were cultured in McCoy's 5A media (Mediatech, Inc.) supplemented with 10% FBS (Atlanta Biologicals, Inc.) and antibiotics (Mediatech, Inc.), and HeLa and 293T cells (ATCC) were cultured in DMEM (Mediatech, Inc.) supplemented with 10% FBS and antibiotics. Irradiation was carried out using a 137Cs source delivered at a dose rate of 120 cGy/min.
Antibodies, plasmid constructs, and siRNA
The antibodies used for immunoblotting, IP, or IF were purchased from Cell Signaling (rabbit polyclonal Histone H3, and H3K79-2Me), Bethyl Laboratories (rabbit polyclonal Dot1L, 53BP1, and 53BP1-pS25), Sigma (mouse monoclonal tubulin), Abcam (H3K79 mono- and tri-methyl) or obtained as kind gifts from Dr S Kornbluth (rabbit polyclonal Bat3). The Bat3 WT and ΔUBL constructs were obtained from Dr S Kornbluth (the Bat3 ΔUBL mutant was made by site-directed mutagenesis to remove the first 80 amino-acid sequence representing the conserved UBL motif). Bat3 and DOT1L constructs resistant to previously described siRNAs (Huyen et al, 2004; Sasaki et al, 2007), and the DOT1LΔUIM mutant were generated using the Stratagene Quickchange II XL site-directed mutagenesis kit according to the manufacturer's protocol. Tet-inducible Bat3 shRNA and SC pTRIPZ plasmids were obtained from Open Biosystems.
Generation of stable cell lines
U2OS cells were transfected with Tet-inducible Bat3 shRNA or SC pTRIPZ plasmids using lipofectamine 2000 reagent (Invitrogen) and allowed to grow for 48 h. Stable clones were then selected for by culturing in media containing puromycin (1 μg/ml) for 10 days.
Immunoblotting and IP
For immunoblotting, cells were lysed in RIPA buffer (Tris 50 mM, NaCl 150 mM, SDS 0.1%, Na.Deoxycholate 0.5%, and NP40 1%) supplemented with protease inhibitors (20 μg/ml leupeptin, 10 μg/ml pepstatin A, and 10 μg/ml aprotonin) and phosphatase inhibitors (20 mM β-glycerophosphate and 0.5 μM OA). Cell lysates were subsequently resolved by sodium dodecyl sulphate–polyacrylamide gel electrophoresis and immunoblotted with appropriate antibodies. For examination of the interaction between Bat3, DOT1L, and histone H3, U2OS cells were harvested with the previously described RIPA buffer containing protease and phosphatase inhibitors. Lysates were precleared and incubated with the indicated antibodies and protein A beads (Calbiochem). After extensive washing with lysis buffer, the immunoprecipitates were analysed by immunoblotting with the indicated antibodies. The control mouse immunoglobulin G was from Santa Cruz.
Cell synchronization assay
Cells were synchronized at the G1/S boundry by thymidine-mimosine block as previously described (Karachentsev et al, 2007). The cells were exposed to 2 mM thymidine (Sigma) for 16 h, released into fresh media for 8 h, and then treated with 0.4 mM mimosine (Sigma) for an additional 12 h. The cells were then washed with warm PBS, released into fresh media, and irradiated and then harvested 10 min later every 2.5 h for 20 h.
IF microscopy
U2OS cells were plated onto glass cover slips prior to IF analysis. In brief, cells were fixed with 2% paraformaldehyde for 15 min and permeabilized in PBS containing 0.1% triton-X100 for 5 min. After washing with PBS and incubation with blocking buffer (1% BSA in PBS) for 30 min, the cells were stained with primary antibodies in blocking buffer for 1 h at room temperature, followed by three PBS washes and incubation with the appropriate secondary antibodies (Jackson Immunoresearch) in blocking buffer for 30 min at 37 °C. After the cells were washed with PBS, they were mounted with fluorescence mounting medium containing 4′,6′-diamidino-2-phenylindole (DAPI) (Santa Cruz) and analysed by fluorescence microscopy.
Clonogenic survival assay
Stable Bat3-inducible knockdown or SC cell lines were plated at 500 cells per dish in 60 mm dishes and exposed to indicated doses of IR at 12 h post-seeding. After treatment, cells were rinsed twice with PBS and allowed to recover in fresh media for 14 days with the medium being changed every 3 days. Colonies were stained with crystal violet (Sigma), counted, and normalized to untreated control. All experiments were done in triplicate, and only colonies containing ⩾50 cells were scored.
Single-cell gel electrophoresis assay (comet assay)
The repair kinetics of IR-induced DNA damage was evaluated by the alkaline comet assay according to the manufacturer's protocol (Trevigen). Briefly, cells were exposed to the indicated doses of IR and harvested at various recovery time points for single-cell gel electrophoresis. Nuclei were stained with Sybr green, and comets were visualized by epifluorescence on a Zeiss microscope. Images were analysed using the public domain software program ImageJ, and comets were evaluated by quantifying the tail moment of 75 cells using the comet-analysing program CometScore (Tritek).
Supplementary Material
Acknowledgments
We thank Dr S Kornbluth and Dr M Thomenius for various Bat3 constructs and antibodies, Dr Y Wan and members of the Wang laboratory for helpful suggestions and discussions during execution of the experiments and preparation of the manuscript. This research was funded by NIH Grant R01 CA123250 and an internal grant from the Duke Cancer Institute (XF Wang).
Author contributions: TPW and QW contributed equally to the design and execution of the experiments, data analysis, and writing of the manuscript. JF made specific contributions to the initial phase of the project. XFW contributed to the writing and data analysis of this manuscript.
Footnotes
The authors declare that they have no conflict of interest.
References
- Ayton PM, Cleary ML (2003) Transformation of myeloid progenitors by MLL oncoproteins is dependent on Hoxa7 and Hoxa9. Genes Dev 17: 2298–2307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerji J, Sands J, Strominger J, Spies T (1990) A gene pair from the human major histocompatibility complex encodes large prolin-rich proteins with multiple repeated motifs and a single ubiquitin-like doman. Proc Natl Acad Sci USA 87: 2324–2378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry ER, Corry GN, Rasmussen TP (2010) Targeting DOT1L action and interactions in leukemia: the role of DOT1L in transformation and development. Expert Opin Ther Targets 14: 405–418 [DOI] [PubMed] [Google Scholar]
- Barry ER, Krueger W, Jakuba CM, Veilleux E, Ambrosi DJ, Nelson CE, Rasmussen TP (2009) ES cell cycle progression and differentiation require the action of the histone methyltransferase Dot1L. Stem Cells 27: 1538–1547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botuyan MV, Lee J, Ward IM, Kim JE, Thompson JR, Chen J, Mer G (2006) Structural basis for the methylation state-specific recognition of histone H4-K20 by 53BP1 and Crb2 in DNA repair. Cell 127: 1361–1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmots F, Russell HR, Michel D, McKinnon PJ (2008) Scythe regulates apoptosis-inducing factor stability during endoplasmic reticulum stress-induced apoptosis. J Biol Chem 283: 3264–3271 [DOI] [PubMed] [Google Scholar]
- Du LL, Nakamura TM, Russell P (2006) Histone modification-dependent and -independent pathways for recruitment of checkpoint protein Crb2 to double-strand breaks. Genes Dev 20: 1583–1596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Q, Wang H, Ng HH, Erdjument-Bromage H, Tempst P, Struhl K, Zhang Y (2002) Methylation of H3-lysine 79 is mediated by a new family of HMTases without a SET domain. Curr Biol 12: 1052–1058 [DOI] [PubMed] [Google Scholar]
- Fitzgerald J, Moureau S, Drogaris P, O’Connell E, Abshiru N, Verreault A, Thibault P, Grenon M, Lowndes NF (2011) Regulation of the DNA damage response and gene expression by the Dot1L histone methyltransferase and the 53Bp1 tumour suppressor. PLoS One 6: e14714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannattasio M, Lazzaro F, Plevani P, Muzi-Falconi M (2005) The DNA damage checkpoint response requires histone H2B ubiquitination by Rad6-Bre1 and H3 methylation by Dot1. J Biol Chem 280: 9879–9886 [DOI] [PubMed] [Google Scholar]
- Grenon M, Costelloe T, Jimeno S, O’Shaughnessy A, Fitzgerald J, Zgheib O, Degerth L, Lowndes NF (2007) Docking onto chromatin via the Saccharomyces cerevisiae Rad9 Tudor domain. Yeast 24: 105–119 [DOI] [PubMed] [Google Scholar]
- Hofmann K, Falquet L (2001) A ubiquitin-interacting motif conserved in components of the proteasomal and lysosomal protein degradation systems. Trends Biochem Sci 26: 347–350 [DOI] [PubMed] [Google Scholar]
- Huyen Y, Zgheib O, Ditullio RA Jr, Gorgoulis VG, Zacharatos P, Petty TJ, Sheston EA, Mellert HS, Stavridi ES, Halazonetis TD (2004) Methylated lysine 79 of histone H3 targets 53BP1 to DNA double-strand breaks. Nature 432: 406–411 [DOI] [PubMed] [Google Scholar]
- Jacinto FV, Ballestar E, Esteller M (2009) Impaired recruitment of the histone methyltransferase DOT1L contributes to the incomplete reactivation of tumor suppressor genes upon DNA demethylation. Oncogene 28: 4212–4224 [DOI] [PubMed] [Google Scholar]
- Jason LJ, Moore SC, Lewis JD, Lindsey G, Ausio J (2002) Histone ubiquitination: a tagging tail unfolds? Bioessays 24: 166–174 [DOI] [PubMed] [Google Scholar]
- Jeltsch A, Rathert P (2008) Putting the pieces together: histone H2B ubiquitylation directly stimulates histone H3K79 methylation. Chembiochem 9: 2193–2195 [DOI] [PubMed] [Google Scholar]
- Jones B, Su H, Bhat A, Lei H, Bajko J, Hevi S, Baltus GA, Kadam S, Zhai H, Valdez R, Gonzalo S, Zhang Y, Li E, Chen T (2008) The histone H3K79 methyltransferase Dot1L is essential for mammalian development and heterochromatin structure. PLoS Genet 4: e1000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karachentsev D, Druzhinina M, Steward R (2007) Free and chromatin-associated mono-, di-, and trimethylation of histone H4-lysine 20 during development and cell cycle progression. Dev Biol 304: 46–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikukawa Y, Minami R, Kobayashi M, Tanaka K, Yokosawa H, Kawahara H (2005) Unique proteasome subunit Xrpn10c is a specific receptor for the antiapoptotic ubiquitin-like protein Scythe. FEBS J 272: 6373–6386 [DOI] [PubMed] [Google Scholar]
- Krogan NJ, Dover J, Wood A, Schneider J, Heidt J, Boateng MA, Dean K, Ryan OW, Golshani A, Johnston M, Greenblatt JF, Shilatifard A (2003) The Paf1 complex is required for histone H3 methylation by COMPASS and Dot1p: linking transcriptional elongation to histone methylation. Mol Cell 11: 721–729 [DOI] [PubMed] [Google Scholar]
- Kwak JH, Kim SI, Kim JK, Choi ME (2008) BAT3 interacts with transforming growth factor-beta (TGF-beta) receptors and enhances TGF-beta1-induced type I collagen expression in mesangial cells. J Biol Chem 283: 19816–19825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazzaro F, Sapountzi V, Granata M, Pellicioli A, Vaze M, Haber JE, Plevani P, Lydall D, Muzi-Falconi M (2008) Histone methyltransferase Dot1 and Rad9 inhibit single stranded DNA accumulation at DSBs and uncapped telomeres. EMBO J 27: 1502–1512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J, Son W-S, Park JK, Kim EE, Lee B-J, Ahn H-C (2011) Solution structure of UIM and interaction of tandem ubiquitin binding domains in STAM1 with ubiquitin. Biochem Biophys Res Commun 405: 24–30 [DOI] [PubMed] [Google Scholar]
- McGinty RK, Kim J, Chatterjee C, Roeder RG, Muir TW (2008) Chemically ubiquitylated histone H2B stimulates hDot1L-mediated intranucleosomal methylation. Nature 453: 812–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinty RK, Kohn M, Chatterjee C, Chiang KP, Pratt MR, Muir TW (2009) Structure-activity analysis of semisynthetic nucleosomes: mechanistic insights into the stimulation of Dot1L by ubiquitylated histone H2B. ACS Chem Biol 4: 958–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min J, Feng Q, Li Z, Zhang Y, Xu RM (2003) Structure of the catalytic domain of human DOT1L, a non-SET domain nucleosomal histone methyltransferase. Cell 112: 711–723 [DOI] [PubMed] [Google Scholar]
- Mu JJ, Wang Y, Luo H, Leng M, Zhang J, Yang T, Besusso D, Jung SY, Qin J (2007) A proteomic analysis of ataxia telangiectasia-mutated (ATM)/ATM-Rad3-related (ATR) substrates identifies the ubiquitin-proteasome system as a regulator for DNA damage checkpoints. J Biol Chem 282: 17330–17334 [DOI] [PubMed] [Google Scholar]
- Nakamura TM, Moser BA, Du LL, Russell P (2005) Cooperative control of Crb2 by ATM family and Cdc2 kinases is essential for the DNA damage checkpoint in fission yeast. Mol Cell Biol 25: 10721–10730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen AT, Xiao B, Neppl RL, Kallin EM, Li J, Chen T, Wang DZ, Xiao X, Zhang Y (2011) DOT1L regulates dystrophin expression and is critical for cardiac function. Genes Dev 25: 263–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen P, Bar-Sela G, Sun L, Bisht KS, Cui H, Kohn E, Feinberg AP, Gius D (2008) BAT3 and SET1A form a complex with CTCFL/BORIS to modulate H3K4 histone dimethylation and gene expression. Mol Cell Biol 28: 6720–6729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y, Feng Q, Lin Y, Jiang Q, Li Y, Coffield VM, Su L, Xu G, Zhang Y (2005) hDOT1L links histone methylation to leukemogenesis. Cell 121: 167–178 [DOI] [PubMed] [Google Scholar]
- Okada Y, Jiang Q, Lemieux M, Jeannotte L, Su L, Zhang Y (2006) Leukaemic transformation by CALM-AF10 involves upregulation of Hoxa5 by hDOT1L. Nat Cell Biol 8: 1017–1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei H, Zhang L, Luo K, Qin Y, Chesi M, Fei F, Bergsagel PL, Wang L, You Z, Lou Z (2011) MMSET regulates histone H4K20 methylation and 53BP1 accumulation at DNA damage sites. Nature 470: 124–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders SL, Portoso M, Mata J, Bahler J, Allshire RC, Kouzarides T (2004) Methylation of histone H4 lysine 20 controls recruitment of Crb2 to sites of DNA damage. Cell 119: 603–614 [DOI] [PubMed] [Google Scholar]
- Sasaki T, Gan EC, Wakeham A, Kornbluth S, Mak TW, Okada H (2007) HLA-B-associated transcript 3 (Bat3)/Scythe is essential for p300-mediated acetylation of p53. Genes Dev 21: 848–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki T, Marcon E, McQuire T, Arai Y, Moens PB, Okada H (2008) Bat3 deficiency accelerates the degradation of Hsp70-2/HspA2 during spermatogenesis. J Cell Biol 182: 449–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze JM, Jackson J, Nakanishi S, Gardner JM, Hentrich T, Haug J, Johnston M, Jaspersen SL, Kobor MS, Shilatifard A (2009) Linking cell cycle to histone modifications: SBF and H2B monoubiquitination machinery and cell-cycle regulation of H3K79 dimethylation. Mol Cell 35: 626–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steger DJ, Lefterova MI, Ying L, Stonestrom AJ, Schupp M, Zhuo D, Vakoc AL, Kim JE, Chen J, Lazar MA, Blobel GA, Vakoc CR (2008) DOT1L/KMT4 recruitment and H3K79 methylation are ubiquitously coupled with gene transcription in mammalian cells. Mol Cell Biol 28: 2825–2839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thress K, Henzel W, Shillinglaw W, Kornbluth S (1998) Scythe: a novel reaperbinding apoptotic regulator. EMBO J 17: 6135–6143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukahara T, Kimura S, Ichimiya S, Torigoe T, Kawaguchi S, Wada T, Yamashita T, Sato N (2009) Scythe/BAT3 regulates apoptotic cell death induced by papillomavirus binding factor in human osteosarcoma. Cancer Sci 100: 47–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Leeuwen F, Gafken PR, Gottschling DE (2002) Dot1p modulates silencing in yeast by methylation of the nucleosome core. Cell 109: 745–756 [DOI] [PubMed] [Google Scholar]
- Vidanes GM, Bonilla CY, Toczyski DP (2005) Complicated tails: histone modifications and the DNA damage response. Cell 121: 973–976 [DOI] [PubMed] [Google Scholar]
- Wilson IA, Haft DH, Getzoff ED, Tainer JA, Lerner RA, Brenner S (1985) Identical short peptide sequences in unrelated proteins can have different conformations: a testing ground for theories of immune recognition. Proc Natl Acad Sci USA 82: 5255–5259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysocki R, Javaheri A, Allard S, Sha F, Cote J, Kron SJ (2005) Role of Dot1-dependent histone H3 methylation in G1 and S phase DNA damage checkpoint functions of Rad9. Mol Cell Biol 25: 8430–8443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Hayashizaki Y, Kone BC (2004) Structure and regulation of the mDot1 gene, a mouse histone H3 methyltransferase. Biochem J 377: 641–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.