Abstract
A repeated DNA sequence has been identified in the macronucleus of several Paramecium species. In P.tetraurelia the repeat was identified in the subtelomeric region of four randomly selected telomere clones, as well as downstream of the A type variable surface protein gene. The complete sequence of the A gene linked repeat consists of 15 tandem repeats of exactly 126 nucleotides that contain an open reading frame with significant similarity to the beta subunits of trimeric G proteins. The most striking consensus feature is the amino acid sequence DX omega WD where X is any amino acid and omega is I, L, or V spaced at precise 42 amino acids intervals. This sequence and spacing are found in G-protein beta subunits and other members of this protein motif family. Analysis of the five cloned telomeric restriction fragments showed the repeats can be found in either orientation with respect to the telomere. Poly(A) RNA transcripts containing this sequence have been identified in Paramecium tetraurelia. The conserved presence of this sequence in several species of Paramecium suggests an important physiological function, and the study of this repeat may reveal information about the evolution of this common protein motif.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blackburn E. H., Karrer K. M. Genomic reorganization in ciliated protozoans. Annu Rev Genet. 1986;20:501–521. doi: 10.1146/annurev.ge.20.120186.002441. [DOI] [PubMed] [Google Scholar]
- Caron F. A high degree of macronuclear chromosome polymorphism is generated by variable DNA rearrangements in Paramecium primaurelia during macronuclear differentiation. J Mol Biol. 1992 Jun 5;225(3):661–678. doi: 10.1016/0022-2836(92)90393-x. [DOI] [PubMed] [Google Scholar]
- Caron F., Meyer E. Molecular basis of surface antigen variation in paramecia. Annu Rev Microbiol. 1989;43:23–42. doi: 10.1146/annurev.mi.43.100189.000323. [DOI] [PubMed] [Google Scholar]
- Cherry J. M., Blackburn E. H. The internally located telomeric sequences in the germ-line chromosomes of Tetrahymena are at the ends of transposon-like elements. Cell. 1985 Dec;43(3 Pt 2):747–758. doi: 10.1016/0092-8674(85)90248-x. [DOI] [PubMed] [Google Scholar]
- Dalrymple M. A., Petersen-Bjorn S., Friesen J. D., Beggs J. D. The product of the PRP4 gene of S. cerevisiae shows homology to beta subunits of G proteins. Cell. 1989 Sep 8;58(5):811–812. doi: 10.1016/0092-8674(89)90930-6. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findly R. C., Gall J. G. Free ribosomal RNA genes in Paramecium are tandemly repeated. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3312–3316. doi: 10.1073/pnas.75.7.3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong H. K., Hurley J. B., Hopkins R. S., Miake-Lye R., Johnson M. S., Doolittle R. F., Simon M. I. Repetitive segmental structure of the transducin beta subunit: homology with the CDC4 gene and identification of related mRNAs. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2162–2166. doi: 10.1073/pnas.83.7.2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forney J. D., Blackburn E. H. Developmentally controlled telomere addition in wild-type and mutant paramecia. Mol Cell Biol. 1988 Jan;8(1):251–258. doi: 10.1128/mcb.8.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebl M., Yanagida M. The TPR snap helix: a novel protein repeat motif from mitosis to transcription. Trends Biochem Sci. 1991 May;16(5):173–177. doi: 10.1016/0968-0004(91)90070-c. [DOI] [PubMed] [Google Scholar]
- Hartley D. A., Preiss A., Artavanis-Tsakonas S. A deduced gene product from the Drosophila neurogenic locus, enhancer of split, shows homology to mammalian G-protein beta subunit. Cell. 1988 Dec 2;55(5):785–795. doi: 10.1016/0092-8674(88)90134-1. [DOI] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III in DNA sequence analysis. Methods Enzymol. 1987;155:156–165. doi: 10.1016/0076-6879(87)55014-5. [DOI] [PubMed] [Google Scholar]
- Herrick G., Cartinhour S., Dawson D., Ang D., Sheets R., Lee A., Williams K. Mobile elements bounded by C4A4 telomeric repeats in Oxytricha fallax. Cell. 1985 Dec;43(3 Pt 2):759–768. doi: 10.1016/0092-8674(85)90249-1. [DOI] [PubMed] [Google Scholar]
- Keleher C. A., Redd M. J., Schultz J., Carlson M., Johnson A. D. Ssn6-Tup1 is a general repressor of transcription in yeast. Cell. 1992 Feb 21;68(4):709–719. doi: 10.1016/0092-8674(92)90146-4. [DOI] [PubMed] [Google Scholar]
- Lauth M. R., Spear B. B., Heumann J., Prescott D. M. DNA of ciliated protozoa: DNA sequence diminution during macronuclear development of Oxytricha. Cell. 1976 Jan;7(1):67–74. doi: 10.1016/0092-8674(76)90256-7. [DOI] [PubMed] [Google Scholar]
- Levine M. A., Smallwood P. M., Moen P. T., Jr, Helman L. J., Ahn T. G. Molecular cloning of beta 3 subunit, a third form of the G protein beta-subunit polypeptide. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2329–2333. doi: 10.1073/pnas.87.6.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer E. Induction of specific macronuclear developmental mutations by microinjection of a cloned telomeric gene in Paramecium primaurelia. Genes Dev. 1992 Feb;6(2):211–222. doi: 10.1101/gad.6.2.211. [DOI] [PubMed] [Google Scholar]
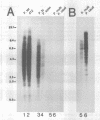
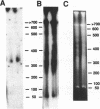
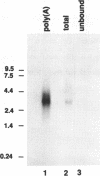
- Phan H. L., Forney J., Blackburn E. H. Analysis of Paramecium macronuclear DNA using pulsed field gel electrophoresis. J Protozool. 1989 Jul-Aug;36(4):402–408. doi: 10.1111/j.1550-7408.1989.tb05535.x. [DOI] [PubMed] [Google Scholar]
- Preer J. R., Preer L. B., Rudman B. M. mRNAs for the immobilization antigens of Paramecium. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6776–6778. doi: 10.1073/pnas.78.11.6776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudman B., Preer L. B., Polisky B., Preer J. R., Jr Mutants affecting processing of DNA in macronuclear development in paramecium. Genetics. 1991 Sep;129(1):47–56. doi: 10.1093/genetics/129.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi N., Monteagudo M. C., Koshland D., Hogan E., Burke D. J. The CDC20 gene product of Saccharomyces cerevisiae, a beta-transducin homolog, is required for a subset of microtubule-dependent cellular processes. Mol Cell Biol. 1991 Nov;11(11):5592–5602. doi: 10.1128/mcb.11.11.5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw D. R., Richter H., Giorda R., Ohmachi T., Ennis H. L. Nucleotide sequences of Dictyostelium discoideum developmentally regulated cDNAs rich in (AAC) imply proteins that contain clusters of asparagine, glutamine, or threonine. Mol Gen Genet. 1989 Sep;218(3):453–459. doi: 10.1007/BF00332409. [DOI] [PubMed] [Google Scholar]
- Simon M. I., Strathmann M. P., Gautam N. Diversity of G proteins in signal transduction. Science. 1991 May 10;252(5007):802–808. doi: 10.1126/science.1902986. [DOI] [PubMed] [Google Scholar]
- Soldo A. T., Godoy G. A. The kinetic complexity of Paramecium macronuclear deoxyribonucleic acid. J Protozool. 1972 Nov;19(4):673–678. doi: 10.1111/j.1550-7408.1972.tb03558.x. [DOI] [PubMed] [Google Scholar]
- Williams F. E., Trumbly R. J. Characterization of TUP1, a mediator of glucose repression in Saccharomyces cerevisiae. Mol Cell Biol. 1990 Dec;10(12):6500–6511. doi: 10.1128/mcb.10.12.6500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams F. E., Varanasi U., Trumbly R. J. The CYC8 and TUP1 proteins involved in glucose repression in Saccharomyces cerevisiae are associated in a protein complex. Mol Cell Biol. 1991 Jun;11(6):3307–3316. doi: 10.1128/mcb.11.6.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]