Abstract
The opening of ligand-gated ion channels in response to agonist binding is a fundamental process in biology. In ATP-gated P2X receptors, little is known about the molecular events that couple ATP binding to channel opening. In this paper, we identify structural changes of the ATP site accompanying the P2X2 receptor activation by engineering extracellular zinc bridges at putative mobile regions as revealed by normal mode analysis. We provide evidence that tightening of the ATP sites shaped like open ‘jaws’ induces opening of the P2X ion channel. We show that ATP binding favours jaw tightening, whereas binding of a competitive antagonist prevents gating induced by this movement. Our data reveal the inherent dynamic of the binding jaw, and provide new structural insights into the mechanism of P2X receptor activation.
Keywords: allosteric mechanism, ligand-gated ion channels, normal mode analysis, purinergic receptors, zinc-binding sites
Introduction
P2X receptors are membrane cation channels gated by extracellular ATP. They are involved in many physiological functions as diverse as synaptic transmission, presynaptic modulation, taste sensation, pain signalling, inflammation and intestinal motility (Khakh and North, 2006; Burnstock, 2008). Upon ATP binding, a large conformational change opens the transmembrane pore. This process, referred to as gating, allows Na+, K+ and Ca2+ ions to rapidly flow down their electrochemical gradients, leading to the depolarization of the cell and downstream signalling.
The crystal structure of the zebrafish P2X4 (zfP2X4) receptor has recently been solved in the absence of ATP by X-ray crystallography at 3.1 Å resolution (Kawate et al, 2009). It confirms previous biochemical evidence that the association of three subunits arranged around the three-fold axis of symmetry forms the ion channel (Nicke et al, 1998; Jiang et al, 2003). Each subunit (there are seven identified so far in mammals, termed P2X1 to P2X7) has intracellular amino and carboxyl termini, and two transmembrane α-helices, termed TM1 and TM2, joined by an ectodomain, which contains highly conserved amino-acid residues necessary for ATP function (Young, 2009; Browne et al, 2010; Evans, 2010). The ion-conducting pathway is defined by three TM2, each coming from a distinct subunit, surrounded by three peripheral TM1 (Kawate et al, 2009).
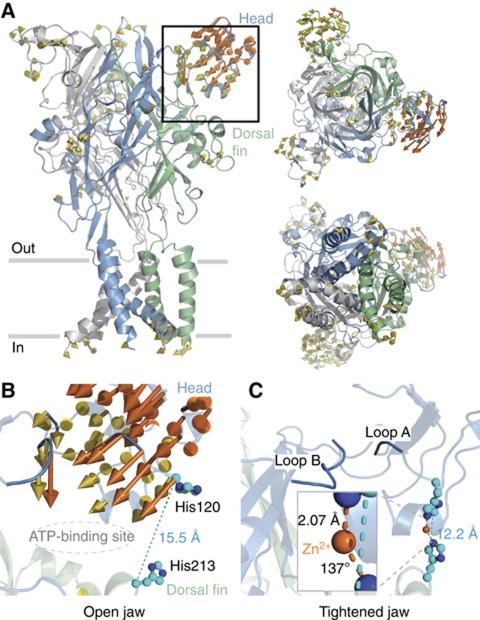
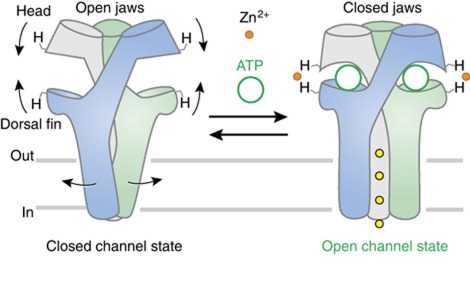
Very recently, we have localized ATP-binding sites in the rat P2X2 (rP2X2) receptor ectodomain through an engineered affinity labelling approach (Jiang et al, 2011). This consists in creating a covalent bond between a single-cysteine mutation introduced in the putative binding site and a sulfhydryl-reactive 8-thiocyano-ATP derivative (NCS-ATP). This reagent is a rP2X2 agonist that selectively cross-linked ATP sites at the level of positions Asn140 and Leu186. In the light of the crystal structure, we showed that these sites are located in large and deep intersubunit cavities, separated by ∼45 Å from the ion pore region (Jiang et al, 2011). Interestingly, the binding cavity is shaped like an open jaw, which is framed in its upper side by the cysteine-rich ‘head’ domain, which contains the identified residue Asn140, and in its bottom side by the ‘dorsal fin’ domain (Figure 1A). Whether or not the open jaw deforms upon ATP binding remains undetermined.
Figure 1.
Exploration of the rP2X2 receptor flexibility by NMA allows formation of the native potentiating zinc-binding site. (A) The rP2X2 receptor model is shown in cartoon representation and coloured by subunits. Three views are presented: lateral to the membrane plane (left), top view from the extracellular side (right-top) and bottom view from the intracellular side (right-bottom). Yellow arrows represent protein displacement captured by mode 10. Movements superior to 3 Å are coloured orange and those inferior to 1 Å are omitted for clarity. Close-up view of the binding site jaw shown in the previous panel before (B) and after (C) exploration of mode 10. The distance separating the Cα atoms from residues His120 to His213 known to be involved in zinc potentiation (Nagaya et al, 2005), as well as the location of the ATP site (Jiang et al, 2011), are also shown. Inset in C: Zoom-in view of zinc coordination showing the mean distance and angle measured between the divalent cation and coordinating atoms NE2 from the two histidines. Exploration of mode 10 also reveals that loops A and B, coloured in blue, get closer together.
Extracellular zinc modulates many different synaptic targets in the forebrain, including ligand-gated ion channels (LGICs; Paoletti et al, 2009), among which the P2X receptor is an emerging candidate (Huidobro-Toro et al, 2008; Lorca et al, 2011). Depending on P2X subunit subtypes (Jarvis and Khakh, 2009) and species (Tittle and Hume, 2008), zinc displays opposite effects, ranging from inhibition to potentiation of the ATP response. For instance, it is known that the extracellular divalent cation inhibits the human P2X2 receptor activity, while it strongly potentiates the ATP response in the rP2X2 receptor (Tittle and Hume, 2008). More detailed studies identified His120 and His213 from two adjacent subunits as contributing residues to potentiation in the rat receptor (Nagaya et al, 2005; Tittle et al, 2007). Interestingly, these two histidines are located, respectively, in the head and dorsal fin domains, and therefore are positioned on the two ‘lips’ of the open jaw. In the rP2X2 homology model based on the X-ray structure of zfP2X4, these two residues are too far apart to coordinate Zn2+ (Figure 1B). Because the structure was solved in the absence of ATP, and thus likely represents a closed channel state, this suggests that during ATP activation the head and dorsal fin domains must move closer to each other, allowing Zn2+ coordination. This hypothesis, although attractive, has no direct support.
In this paper, we studied protein conformational changes in the rP2X2 receptor accompanying ion channel activation by combining normal mode analysis (NMA) and experimental data. We successfully engineered receptors with histidines, in which extracellular Zn2+ was able to bridge specifically distant regions of the jaw that were predicted to come closer to each other during gating. By using a pore mutation background that displayed spontaneous openings (Cao et al, 2007), we showed that Zn2+ gated not only channel receptors in which the native Zn2+-potentiating site was present, but also receptors in which the native site was transferred to another part of the jaw. Finally, we provided evidence that binding of ATP favoured zinc activation through an allosteric conformational change, whereas binding of the competitive P2X antagonist 2′,3′-O-(2,4,6-trinitrophenyl)-ATP (TNP-ATP) prevented Zn2+ activation. Based on these results, we propose a model in which ATP gating requires tightening of the two lips of the binding jaw, whereas binding of TNP-ATP prevents jaw closure-induced gating. This original mechanism is reminiscent of those found in other LGICs.
Results
NMA suggests motions of the head domain coupled to ATP binding
NMA is a computational approach that can efficiently predict the collective motions and conformational flexibilities of biological macromolecules (Bahar, 1999). This method approximates the surface of the conformational landscape of the macromolecule and gives a decomposition of the movements into discrete modes. The elastic-network model, which is a simplified, but physically meaningful, representation of the interaction between atoms, is based on simple springs that connect close pairs of atoms in the structure (Tirion, 1996). At variance to molecular dynamics, this method leads to a time-independent equation that can be solved in closed form analytically, and therefore allows studying slow and collective conformational transitions that are biologically relevant (Krebs et al, 2002). More recently, NMA has been useful in studying gating transitions in other ion channels (Amiri et al, 2005; Taly et al, 2005; Cheng et al, 2006; Yang et al, 2009; Zhu and Hummer, 2009; Sukumaran et al, 2011). To speed up simulation, we represented the amino acids by Cα atoms (Hinsen, 1998). NMA using this approximation was shown to give a fair description of protein flexibility (Bahar, 1999; Tama and Brooks, 2002; Bahar and Rader, 2005). We used NMA to reveal inherent motions of the rP2X2 receptor homology model based on the closed-state zfP2X4 structure. We computed and selected the first 10 non-trivial normal modes, which revealed structural changes of the protein (Figure 1A and Supplementary Figure 1). Mild pore openings for modes 7, 9 and 11 were detected, but apparently not sufficient to allow ion flux (Supplementary Figure 2), suggesting that although these modes could, in principle, be involved in gating of the rP2X2 receptor, none described the transition in full.
Because no clear pore opening was observed in all tested modes, we decided to perturb the modes by the presence of ATP. This approach has already been successfully applied to another LGIC (Taly et al, 2006), and approximates changes that occur on protein dynamics upon ligand binding (Ming and Wall, 2005; Zheng et al, 2005; Mitternacht and Berezovsky, 2011). We docked ATP into the binding site, according to our recent affinity-labelling data (Jiang et al, 2011), and computed NMA in the presence of ATP. We found that changes in frequency of the non-trivial modes due to ATP ranged from 1 to 26%. Interestingly, mode 10, which corresponds to an asymmetric motion of the three heads out of the membrane plane, displayed the largest frequency difference, suggesting that the presence of ATP substantially modified the energy needed to explore this mode (Figure 1A and Supplementary Figure 3). These calculations thus suggest that the domains surrounding the ATP-binding site experience significant mobility that may be related to ATP binding, and potentially to P2X gating.
Interestingly, the distance separating Cα atoms from residues His120 to His213 that were initially found to be too far apart to create a Zn2+-coordinating site, shortened following mode 10 exploration from 15.5 to 12.2 Å (Figure 1B and C). More importantly, we succeeded in forming a Zn2+-binding site through these histidine residues, and the distance and angle measured between Zn2+ and the coordinating atom NE2 of the histidine residues were close to those obtained from the analysis of 111 crystal structures of Zn2+-binding proteins available in the Protein Data Bank (PDB; Alberts et al, 1998). Therefore, these results suggest that mode 10 is able to form the native Zn2+-potentiating site in the rP2X2 receptor, by getting residues His120 and His213 together.
Zinc activates the T339S mutant
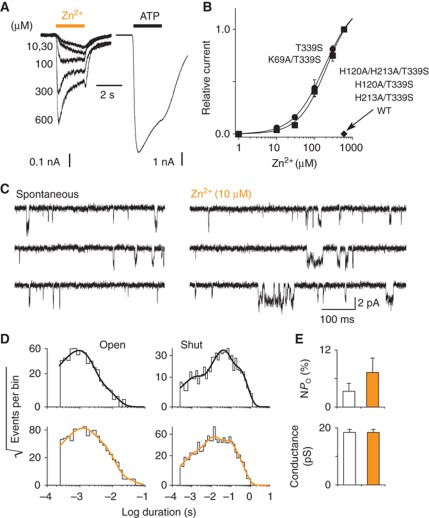
Although previous studies (Nagaya et al, 2005; Tittle et al, 2007; Tittle and Hume, 2008) have shown that zinc potentiates ATP currents, there is no direct evidence supporting the hypothesis that close apposition of residues His120 and His213 gates the ion channel. We thus asked whether zinc could open the ion channel, by its own, in the absence of ATP. In human embryonic kidney (HEK)-293 cells expressing the wild-type (WT) rP2X2 receptor, extracellular zinc failed to produce detectable currents as assayed by whole-cell (Figure 2B) and single-channel (Supplementary Figure 4) patch-clamp electrophysiology. We decided to introduce the T339S mutation, which is known to confer to the channel significant spontaneous openings in the absence of ATP (Cao et al, 2007), with the hope that the mutation would be able to reduce the energy barrier for the receptor to reach the open state. Outstandingly, Zn2+ produced significant currents that were concentration dependent with maximal current (58±22 pA/pF, n=5) representing about 4% of that evoked by ATP (Figure 2A and B). Currents were specific to Zn2+ binding, and not due to contamination by trace amounts of ATP because the double mutant K69A/T339S, in which the ATP-binding site was disrupted (Cao et al, 2007), still responded to Zn2+, with a concentration–response curve and maximal current that were very similar to those measured for the T339S mutant (33±12 pA/pF, n=5; Figure 2B). Furthermore, these currents were specific to the presence of both residues His120 and H213 because the triple mutant H120A/H213A/T339S and corresponding single-histidine mutants H120A/T339S and H213A/T339S, in which the native zinc-potentiating site was abolished (Nagaya et al, 2005), were not activated by Zn2+ anymore, while ATP current density remained robust (674±270 pA/pF, n=4; 432±106, n=6; 473±51, n=4 for H120A/H213A/T339S, H120A/T339S and H213A/T339S, respectively; Figure 2B). These results thus demonstrate that coordination of Zn2+ ions by the pair of histidines His120 and His213 gate the T339S mutant channel.
Figure 2.
Zn2+ ions gate the rP2X2 T339S mutant receptor through residues H120 and H213. (A) Representative traces of Zn2+- (with indicated concentrations) and ATP- (30 μM, saturating) evoked currents recorded from the same cell expressing the T339S mutant. (B) Zinc dose–response curves for T339S and K69A/T339S mutant receptors (EC50=221±47 μM, n H=1.2±0.2, n=5 for T339S; EC50=273±89 μM, n H=1.2±0.1, n=5 for K69A/T339S). No response was detected in cells expressing either the WT rP2X2, H120A/H213A/T339S, H120A/T339S or H213A/T339S mutant receptor. (C) Single-channel currents recorded from an outside-out patch excised from HEK cell expressing the T339S mutant (openings downward) showing the increase of spontaneous channel activities by Zn2+ ions. (D) Distributions of open- and shut-times (pooled data from seven patches). Data recorded in the absence (up) or presence (bottom) of Zn2+ were fitted by the sum of exponentials (for details see Supplementary Table 2). (E) Histograms (based on data from D) showing the increase of NP o and the unaffected conductance of channels activated by Zn2+ (indicated in orange). Pooled data in this and all other figures represent mean±s.e.m.
To further gain insights into the mechanism underlying zinc activation, we made single-channel recordings. In outside-out patches excised from HEK cells expressing the T339S mutant, a low concentration of Zn2+ (10 μM) further increased the nominal open probability (NPo) of single-channels that were spontaneously open (Figure 2C and E). However, there was no change of the unitary conductance that was, in fact, similar to that determined previously for the WT receptor (Cao et al, 2007; Jiang et al, 2011) in the presence of ATP (Figure 2E). Distributions of the spontaneous open times were best fitted with the sum of two exponential components, whereas those analysed in the presence of zinc showed both a large increase of the proportion of the slowest component—with no change of time constants—and the occurrence of a third, slower component (Figure 2D and Supplementary Table 2). This resulted in an increase of the mean open-time from 1.2 to 2.2 ms, suggesting that zinc further stabilizes the spontaneous open-channel state(s) in the T339S mutant. Analysis of the shut-time distributions revealed, in contrast, a decrease of the mean shut-time from 84.8 to 51.6 ms, suggesting that zinc increases pore opening frequency. Because some patches contained more than one active channel as evidenced by the presence of double openings that represented no more than 2% of total transitions (see Materials and Methods), absolute values of shut-time constants may not be meaningful. Taken together, these data clearly show that (i) the opening probability of the channel increases when zinc ions are bound to His120 and His213, (ii) the motion bringing these two residues together is coupled to pore opening, and (iii) this motion can occur without ATP binding.
Jaw tightening gates the ion channel
To further explore the dynamic motion of the ATP-binding site during gating, we decided to transfer the native zinc-potentiating site to another place of the jaw with the hope that the divalent cation would also bridge the engineered histidines in the activated state(s). To select all possible pairwise positions, we computed a matrix representing the relative movement of the protein between initial and final models explored in mode 10, and selected pairs of residues in which their relative movement measured from their respective Cα atoms was >2.5 Å, and the distance separating these Cα atoms was <13 Å in the final model, compatible with a putative zinc-coordinating site (Alberts et al, 1998). Interestingly, we found that two previously unidentified regions of the jaw, named loops A and B corresponding, respectively, to a segment of the β5-β6 loop from the head domain and the β7-α2 loop linking the head to the right flipper, satisfied these geometrical constrains, suggesting that these loops may get close enough together (Figure 1C).
To test experimentally this theoretical prediction, we first abolished the native zinc-potentiating site in the rP2X2 receptor channel as reported previously (Nagaya et al, 2005). We used 20 μM zinc, because higher concentrations inhibit the activity of the receptor in which the zinc-potentiation site was removed (Tittle and Hume, 2008). As expected, no potentiation by Zn2+ of a low concentration of ATP that elicited ∼10% of the maximal response (EC10) was observed in the double mutant H120A/H213A, while strong potentiation was detected in the WT rP2X2 receptor (Figure 3A). In addition, ATP sensitivity and Hill coefficient determined for the double mutant H120A/H213A remained similar to those found for the WT receptor (Supplementary Table 1). Second, we mutated in this background, referred to below as *, the selected residues from loops A and B into histidines. All mutants yielded robust ATP currents and EC50 values that did not exceed four-times the value obtained for the background * (Supplementary Table 1), suggesting that these mutations did not alter substantially the ability of ATP to bind to, and gate the receptor channel.
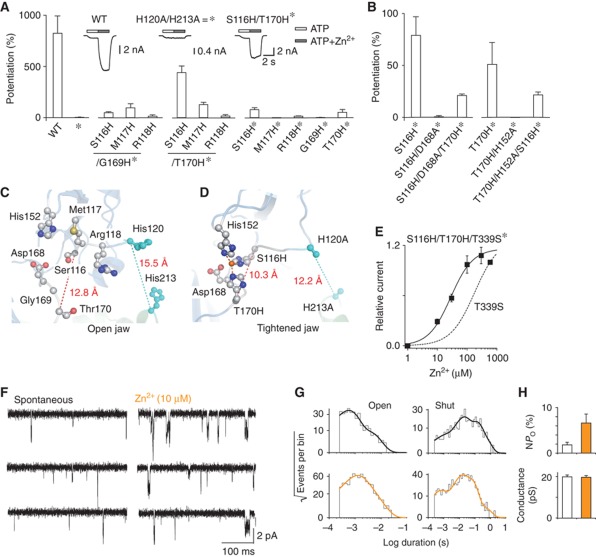
Figure 3.
Engineering of novel Zn2+-binding sites supports the tightening model of the binding site jaw. (A) Histogram showing ATP (EC∼10)-evoked responses potentiated by Zn2+ (20 μM) in cells expressing the indicated mutants (n=4–7). The H120A/H213A background is symbolized by *. Inset: representative traces showing the protocol used for establishing the histogram. (B) Histogram revealing the contribution of the endogenous residues Asp168 and His152 to the engineered Zn2+ potentiating site in the S116H/T170H* mutant. Data for S116H* and T170H* were taken from A. (C) Open jaw model of the WT rP2X2 receptor highlighting the identified residues from loops A and B establishing zinc bridges. (D) Tightened jaw model of the S116H/T170H* mutant following mode 10 exploration highlighting reconstitution of the engineered Zn2+-binding site. The distance and angle measured between zinc ion (orange sphere) and coordinating atoms NE2 from histidines are 2.3, 2.2, 2.3 Å and 95°, 137° and 128°. Note that the distance measured between Zn2+ and one oxygen of the carboxylate from Asp168 is 3.9 Å. (E) Zinc dose–response curves for the S116H/T170H/T339S* mutant (EC50=27±3 μM, n H=1.2±0.1, n=6). The dashed curve for T339S mutant was taken from Figure 2B. (F) Single-channel currents recorded from an outside-out patch excised from HEK expressing the S116H/T170H/T339S* mutant (openings downward) showing the increase of spontaneous channel activities by Zn2+ ions. (G) Distributions of open- and shut-times (pooled data from six patches). Data recorded in the absence (up) or presence (bottom) of Zn2+ were fitted by the sum of exponentials (for details see Supplementary Table 2). (H) Histograms (based on data from G) showing the increase of NP o and the unaffected conductance of channels activated by Zn2+ (indicated in orange).
Interestingly, when paired with the T170H* mutation, the histidine mutants in which loop A was investigated showed a gradual increase of zinc potentiation, resulting in a more than four-fold increase of the ATP response for the pair S116H/T170H*, whereas a weak or no potentiation was recorded for the pairs including the G169H* mutation (Figure 3A). In the control experiments, in which the single-histidine mutants were tested, virtually no potentiation was detected, except for S116H* and T170H*, for which a weak, but reproducible, zinc potentiation was recorded (Figure 3A). Because of these remaining potentiations, we considered the possibility that the engineered zinc-binding site may be formed exclusively from one side of the binding jaw, including unidentified endogenous residues. To address this issue, we sought to determine in our molecular model which endogenous residues carrying possible zinc-coordinating side chains were closely positioned to residues Ser116 and Thr170 (Figure 3C). We found that replacement of Asp168 with alanine from one side of the binding jaw (i.e., loop B) in the S116H* mutant abolished the remaining potentiation (Figure 3B and C). Similar results were obtained when H152A from the other side (i.e., the head domain) was introduced in the T170H* mutant (Figure 3B and C), suggesting that the remaining potentiation resulted only from a closure of the two lips of the jaw. Surprisingly, only small (∼0.2-fold) zinc potentiations were observed when D168A and H152A were individually introduced in the double-histidine mutant S116H/T170H* (Figure 3B), suggesting that both Asp168 and His152 side chains are necessary, but not sufficient to mediate the strong zinc potentiation observed in the S116H/T170H* mutant receptor (approximately four-fold). In support to this, the engineered zinc-binding site was reconstituted in the tightened jaw model, including the endogenous residue His152. Note, however, that Asp168 that was not constrained in modelling, did not directly contact zinc, but was located sufficiently close (∼4 Å) to the engineered zinc-binding site (Figure 3D). Altogether, these data argue against the possibility that potentiations observed in both single- and double-histidine mutants are due to zinc-binding sites formed exclusively from one side of the binding jaw; instead, they strongly suggest that both sides of the binding jaw are needed to form the engineered potentiation site.
Introduction of mutations S116H/T170H* in the T339S mutant enabled zinc to gate the ion channel in the absence of ATP, as revealed by both whole-cell (Figure 3E) and single-channel recordings (Figure 3F). Compared to the T339S mutant, S116H/T170H/T339S* mutant displayed a decrease of the Zn2+-gated current density (7±2 pA/pF, n=6), but a marked increase of about 10-fold in Zn2+ sensitivity (Figure 3E). Detailed analysis of the open-time distributions showed a similar trend to that observed for the T339S mutant. Indeed, distributions of the spontaneous open times were best fitted by the sum of two exponential components, whereas those analysed in the presence of zinc showed an increase of the proportion of the slowest component—with little change of time constants—and the occurrence of a third, slower component (Figure 3G and Supplementary Table 2). Similarly, there was an overall increase of both the mean open-time (from 1.1 to 1.9 ms) and NP o, and no change of the unitary conductance (Figure 3H). Analysis of the shut-time distribution revealed a decrease of the mean shut-time from 84.5 to 38.7 ms, suggesting that zinc increases pore opening frequency of the S116H/T170H/T339S* mutant channel, as observed for the T339S mutant. Taken together, these results indicate that (i) zinc gates the engineered channels by stabilizing the open-channel state(s), and (ii) the distance separating the Cα atoms from residues Ser116/His152 to Asp168/Thr170, along with that separating Cα atoms from residues His120 to His213, must shorten in the activated state(s) to be compatible with zinc-coordinating sites (Figure 3D). We conclude from these experiments that tightening of the two lips of the ATP-binding jaw favours pore opening.
Agonist binding favours jaw tightening, whereas binding of competitive antagonist inhibits gating induced by this movement
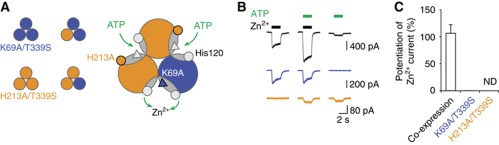
We have shown that in the absence of ATP, Zn2+-induced jaw tightening favours pore opening. To challenge the hypothesis that ATP also favours tightening, we tested if ATP was able to potentiate zinc currents through an allosteric mechanism. Validation of this hypothesis is important, because it remains possible that in the presence of ATP, Zn2+ may directly help the agonist to bind to the receptor and this, consequently, may produce longer residence time for ATP at its binding site. In that case, ATP by itself may induce another protein movement that is not related to jaw tightening. To address this issue, we engineered receptors, in which ATP and zinc were not able to mediate their effect on the same subunit interface. We thus co-expressed K69A/T339S mutant that is defective in ATP binding with H213A/T339S mutant that is not activated by zinc. The resulting trimeric heteromers contain K69A/T339S and H213A/T339S subunits with a stoichiometry of either 1:2 or 2:1 (Figure 4A). As shown in Figure 4A, in these heteromers, ATP gating and zinc-induced activation cannot be mediated simultaneously at a common binding jaw. We reasoned that if ATP favours zinc activation, this would mean that ATP binding in one interface bearing the H213A mutation must favour, through an allosteric conformational change, zinc-induced tightening of another interface, which carries the K69A mutation (Figure 4A). This is, in fact, what we observed (Figure 4B). First, evidence supporting the assembly of heteromeric channels came from the fact that both ATP sensitivity and Hill coefficient determined in cells expressing both K69A/T339S and H213A/T339S mutants decreased compared with those determined in cells expressing only the mutant H213A/T339S (Supplementary Figure 5). Second, we found that a very low concentration of ATP that produced currents that represented 19±4% of those evoked by zinc alone strongly potentiated zinc responses in cells expressing the heteromers. In contrast, no detectable potentiation of zinc currents by ATP was observed in cells expressing only the homomers K69A/T339S—no zinc-evoked inward current was detected in cells expressing the H213A/T339S mutant (Figure 4B and C). Overall, this suggests that ATP binding favours jaw tightening.
Figure 4.
ATP binding favours jaw tightening. (A) Schematic representation of the heteromeric expression of K69A/T339S and H213A/T339S mutants (see text for details). (B) Representative traces of Zn2+ (200 μM)-evoked currents potentiated by 0.1 μM ATP recorded from cells expressing the heteromer (black), the K69A/T339S homomer (blue) or the H213A/T339S homomer (orange). (C) Pooled data for each of the three constructions shown in B (n=4–7). Potentiation was defined as the ratio of currents recorded in the presence of both ATP and zinc to those recorded in the presence of zinc alone. ND, not determined, because no Zn2+ inward current was detected in cells expressing the mutant H213A/T339S.
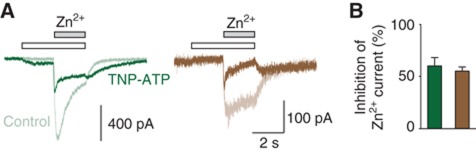
If this hypothesis is correct, then application of TNP-ATP, which is a known competitive P2X2 antagonist (Trujillo et al, 2006; Cao et al, 2007), should inhibit zinc activation that reported jaw tightening. Because it has been shown that TNP-ATP also acted as a partial agonist on the T339S mutant (Cao et al, 2007), we chose a concentration at which the antagonist effect is predominant over the agonist effect. In these conditions, we found indeed that TNP-ATP largely inhibited zinc currents in cells expressing either the T339S or the S116H/T170H/T339S* mutant (Figure 5), suggesting that TNP-ATP prevents zinc gating induced by jaw tightening.
Figure 5.
The competitive antagonist TNP-ATP inhibits Zn2+-gated currents through receptors containing either the native or the engineered Zn2+-binding site. (A) Representative traces showing Zn2+ (200 μM)-evoked currents recorded in the absence (indicated as control, light traces) or presence (heavy traces) of TNP-ATP (10 μM) in cells expressing either the T339S (green traces) or the S116H/T170H/T339S* mutant (brown traces). (B) Pooled data of TNP-ATP inhibition of zinc current for each construction shown in A (n=5–8).
Discussion
In this paper, we present a model of P2X receptor gating based on geometrical constrains established by zinc bridging at native and engineered binding sites in the ectodomain. We demonstrate that agonist binding tightens the open jaw forming the ATP-binding site and this motion is coupled to channel opening, whereas binding of the competitive antagonist TNP-ATP prevents gating induced by this movement.
Our data revealed the inherent molecular dynamic of the binding site jaw. This conclusion was reached essentially by the use of the T339S mutation, which produces channels displaying significant spontaneous openings (Cao et al, 2007). It should be noted that other spontaneously active mutants have been identified in LGICs (Changeux and Edelstein, 1998). These mutants, usually carrying mutations in the pore region such as L247T in the α7 nicotinic acetylcholine receptor, have provided valuable insights into the mechanism of receptor activation (Bertrand et al, 1992; Palma et al, 1999). In the present study, we propose that the T339S mutation reveals the inherent movement of the intact binding pocket for the following reasons. First, the conductance of mutated channels activated by zinc was not different from that of the WT receptor activated by ATP determined previously (Jiang et al, 2011; Figures 2E and 3H), suggesting that the open state(s) of the T339S ion channel stabilized by zinc is (are) similar to that (those) of the WT receptor normally induced by ATP. Second, it has been shown that the Thr339 residue, which is located in TM2, contributes to the gate of the channel (Migita et al, 2001; Li et al, 2008, 2010; Cao et al, 2009; Kracun et al, 2010) and, in agreement with this hypothesis, its homologous residue is positioned at the narrowest part of the closed channel in the zfP2X4 crystal structure (Kawate et al, 2009). Mutating this residue, which corresponds to a rather minor side chain alteration (Thr to Ser), may thus simply change locally the structure of the gate, leading to the occurrence of spontaneous openings. Third, because the Thr339 residue is separated by more than 50 Å from residues investigated in the present study, it is likely that T339S mutation has little impact on the structure of the binding pocket. Therefore, these arguments favour, as stated earlier (Cao et al, 2007) that the T339S mutation alters primarily the open–shut equilibrium by substantially destabilizing the closed channel, and thus, allows extracellular zinc to control gating of the ion channel without ATP.
We present evidence that our engineered zinc-binding site requires not only the presence of the engineered histidines S116H and T170H, but also of endogenous aspartate and histidine residues (Asp168 and His152). This is not surprising because most of zinc-binding sites in crystallized proteins are tetrahedral, in which three or four coordinating atoms are provided by the protein and the fourth site is usually occupied by water (Alberts et al, 1998). At present, we do not know the atomic details of zinc coordination spheres of both native and engineered sites, but analysis of 111 crystal structures of Zn2+-binding proteins revealed a nearly universal motif, in which one of the histidines provided by the first primary coordinating shell makes an H-bond to an oxygen atom from a protein residue (e.g., aspartate or glutamate) or a water molecule (Alberts et al, 1998). This interaction is believed to increase the basicity and ligand strength of the histidine and arrange it correctly for interaction with the metal. In this context, it is thus possible that Asp168 directly interacts with one of the engineered histidines, which in turn interacts more favourably with zinc. Alternatively, Asp168 may directly interact with zinc. In addition, it is possible that residue Asp136, which has recently been proposed to contribute to the native zinc-potentiating site (Friday and Hume, 2008), plays a role similar to Asp168, because this residue is located in the head domain, in relative close proximity to residue H120. Regardless of these details, our data clearly show that zinc activation cannot result from alternative zinc-binding sites provided by residues located exclusively in one lip of the binding jaw, but instead requires bridging of the two lips.
It should be noted that the theoretical molecular motions obtained by NMA presented in this study include some approximations that were due to the absence of water and membrane, and also to a simplified force field restricted to Cα atoms. These approximations imply that models may include deviations in the precise positioning of atoms. In the present study, NMA did not detect significant pore opening. The reason for this is unclear, but one explanation would be that the force field used in the modelling process overestimates the interaction of the three TM2 α-helices at the level of the gate, and this, in turn constitutes a barrier that locks the pore structure in a closed state. Another explanation is that gating results from a combination of different modes including mode 10. In this respect, mode 7 is of particular interest because it corresponds to a global twist of the receptor, which results in a decrease of the angle between TM2 α-helices and the membrane plane normal (Supplementary Figure 1), a hypothesis that is fully consistent with recent experimental studies (Kracun et al, 2010; Li et al, 2010), which showed through engineering metal bridges that pore opening is caused by the straightening of these helices.
We provide strong evidence that tightening of the ATP-binding jaw correlates with the opening of the ion channel. Considering that the rP2X2 homology model likely represents the resting closed state of the receptor, as previously proposed for the zfP2X4 crystal structure (Kawate et al, 2009), this indicates that the head and dorsal fin domains would need to move closer to each other to reach the open state(s), by a relative movement of more than 3 Å. Our data also show that movement of the jaw occurs in the absence of ATP, or even in receptors in which the ATP-binding sites have been disrupted by the K69A mutation. This suggests that binding of ATP is not an absolute prerequisite to the modification of the binding site during gating, supporting our hypothesis that the movement of the jaw is an inherent property of the rP2X2 receptor.
Does ATP actually induce tightening of the binding jaws in a way similar to that produced by zinc? We provide evidence that binding of ATP allosterically favours Zn2+ activation, essentially by tightening residues His120 and His213 around the metal (Figure 4). This prompts us to suggest that the mechanism by which ATP tightens these residues is similar to that involved in zinc activation (Figure 6). However, we do not eliminate the possibility that, in addition, a direct interaction between zinc and part of the ATP molecule may—at least partially—account for the potentiation mechanism of ATP response observed in the WT P2X2 receptor, because the position homologous to His120 in the P2X7 receptor has been shown to form a covalent bound with ADP-ribose (Adriouch et al, 2008) and therefore is potentially close to the ATP-binding pocket.
Figure 6.
Schematic representation of a plausible activation mechanism of P2X receptor. Agonist binding at the subunit interface tightens the open jaws forming ATP-binding sites, a movement that is mechanically coupled to channel opening. Zn2+ further stabilizes the conformation of closed jaws, through coordination of a pair of histidines that are located at opposite lips of the jaw.
We show that binding of the competitive antagonist TNP-ATP blocks pore opening upon zinc-induced jaw tightening. One straightforward interpretation would be that binding of TNP-ATP blocks movement of jaw tightening as a foot-in-the-door. Alternatively, binding of the antagonist may decouple jaw closure movement to pore opening, in which jaw closure still occurs but the subsequent conformation change cannot be spread to initiate pore opening. Additional experiments are needed to discriminate between these two mechanisms.
Taken together, these data pave the way for a plausible activation mechanism of P2X receptors. We propose that following ATP binding the head and dorsal fin domains move closer to each other, resulting in the tightening of the open jaw (Figure 6 and Supplementary Movie). This new conformational state, which binds zinc at native residues His120 and His213, favours the opening of the ion channel. Because recent studies have suggested that P2X gating requires a structural change of the three pore-forming TM2 α-helices (Li et al, 2008, 2010; Kracun et al, 2010; Browne et al, 2011), this indicates that there must exist a molecular link between jaws tightening and pore opening. The entire molecular pathway by which this allosteric conformational change is spread to the channel is not yet completely understood, but likely involves a structural change of the subunit interface, as previously proposed (Jiang et al, 2003, 2010; Nagaya et al, 2005; Marquez-Klaka et al, 2007).
In conclusion, we demonstrate that tightening of the ATP-binding sites correlates precisely with channel opening in the P2X2 receptor. This original mechanism that may likely be shared by other members of the mammalian P2X receptor family is reminiscent of those found in other LGICs, in which closing of lobes (Sobolevsky et al, 2009) or loops (Hibbs and Gouaux, 2011) around bound agonist has been reported to initiate pore opening.
Materials and methods
Homology models
The all-heavy-atom structure of the zfP2X4 receptor (PDB ID code 3H9V) was taken as the template to construct the initial rP2X2 model with the programme MODELLER (Sali and Blundell, 1993). The resulting model was energy-minimized by using the programme CHARMM (Brooks and Karplus, 1983) with the all-atom param22 parameter set; the solvent was represented implicitly with the GBSW model. For zinc-binding site reconstructions, a distance constrain (2.06 Å) was added between zinc and the NE2 atom of histidines.
Normal mode analysis
The gating mechanism was studied by NMA, as previously described (Taly et al, 2005), with a Cα atoms elastic-network model (Tirion, 1996), which represents the protein as a network of residues linked by springs (with a 8 Å cutoff). To examine the normal modes, the positions of the Cα atoms were modified along the normal mode vectors. The new positions of the Cα atoms were introduced, while retaining the positions of the other atoms. The position of those atoms were then adjusted by energy minimization using decreasing harmonic constraints with the programme CHARMM (Brooks and Karplus, 1983). The magnitude of the amplification of each mode is one of the variables of the study. It was set by modifying the energy applied for the exploration such as to attain the following objectives: first, for the initial analysis of all modes, the energy applied was adjusted such that the two structures would have an RMSD of ∼2 Å. This arbitrary RMSD value was chosen because it produced realistic structures with all modes and the changes were large enough to be analysed by inspection of the end points; second, for the reconstruction of the zinc-binding site, the structure along mode 10 with the lowest His120-Cβ/His213-Cβ distance was selected (Supplementary Figure 1).
Pore analysis
The size of the pore was measured by using the programme HOLE (Smart et al, 1996) with a radius of 4 Å for each Cα atom and the Connolly algorithm (Connolly, 1983).
ATP probe
To identify normal modes likely to participate in the gating mechanism, we studied the effect of the perturbation introduced by ATP. Given the simple model we were using, we added atoms to the protein Cα model so as to locally modify the elastic network (Ming and Wall, 2005; Zheng et al, 2005; Taly et al, 2006; Mitternacht and Berezovsky, 2011). The experiment-compatible position of ATP (Jiang et al, 2011) was projected on three interfaces. The probes were then modelled by retaining three atoms of ATP. This number was chosen to be similar to the coarse-graining of amino acids. The atoms were the gamma phosphate, the O from the furane and C8 from adenine. The resulting modification of the network mimics the effect of binding a ligand.
Complementary DNA construction and site-directed mutagenesis
The pcDNA-based expression plasmids, mutagenesis and sequencing procedure have been described previously (Jiang et al, 2010).
Cell culture and transfection
HEK-293 cells were cultured and transiently transfected with the rP2X2 constructs (0.01–2 μg) and a green fluorescent protein cDNA construct (0.3 μg), as described previously (Jiang et al, 2010). For the co-expression of K69A/T339S and H213A/T339S subunits described in Figure 4, HEK-293 cells were transfected with 1 μg cDNAs in a 1:1 ratio.
Chemicals
ZnCl2 solution and most of other drugs were purchased from Sigma (St Louis, MO, USA). TNP-ATP was purchased from Invitrogen (Eugen, OR, USA).
Whole-cell recordings
Data were acquired with a patch-clamp amplifier (HEKA EPC 10) using PATCHMASTER software (HEKA Co.). Patch pipettes (3–5 MΩ) contained (in mM): 140 KCl, 5 MgCl2, 5 EGTA, 10 HEPES, pH 7.3. External solution contained (in mM): 140 NaCl, 2.8 KCl, 2 CaCl2, 2 MgCl2, 10 glucose, 10 HEPES, pH 7.3. The holding potential was −60 mV. Dose–response relationship experiments and drug applications were carried out as described previously (Jiang et al, 2011).
Single-channel recordings
Single-channel recordings using outside-out configuration were made from HEK-293 cells at room temperature 24–72 h after transfection. Recording pipettes were coated with Sylgard 184 (Dow Corning Co.) and fire polished to yield resistances of 6–20 MΩ. The holding potential was −120 mV. The extracellular solution contained (in mM): 147 NaCl, 2 KCl, 1 CaCl2, 1 MgCl2, 10 HEPES and 13 glucose, pH 7.3. The intracellular solution contained (in mM): 147 NaF, 10 HEPES and 10 EGTA, pH 7.3. The osmolarity was ∼300 mOsmol kg−1 for all the solutions.
Data were sampled at 10 kHz and low-pass filtered at 2.9 kHz. For off-line analysis, data were refiltered to give a cascaded filter cutoff frequency of 2 kHz. Channel events were detected by using the 50% threshold criterion and idealized with TAC (Bruxton co.) as described previously (Ding and Sachs, 1999; Jiang et al, 2011). Nominal open probability (NP o) was determined with TACFit (Bruxton co.), and was defined as the fraction of time for which the channels are open, where N is the number of active channels in the patch and P o is the single-channel open probability. For obtaining dwell-time distributions of open- and shut-times, only patches in which individual channel openings could be clearly resolved (multiple-opening events represented <2% of total events) were subjected to further analysis as described previously (Jiang et al, 2011). The open- and shut-time histograms were fitted by the sum of exponentials with an imposed appropriated time resolution of 215 μs using TACFit.
Supplementary Material
Acknowledgments
We thank Professor Maurice Goeldner, Drs Alexandre Specht and Valérie Taly for critical reading of the manuscript. This work was supported by the ANR Grant 06–0050–01, the CNRS through the programme d’incitation à la mobilité d’équipe (PIME), the Ministère de la Recherche and has been funded in part by the ic-FRC (www.icfrc.fr). RJ is a recipient of a fellowship from the China Scholarship Council.
Author contributions: RJ, OC and DL performed cell culture, transfection and whole-cell patch-clamp electrophysiology and analysed data. RJ and DL preformed single-channel recordings. AT performed molecular modelling. AM and OC made mutations. TG designed research, supervised the project and wrote the manuscript with feedback from RJ and AT. All authors discussed the results and commented on the manuscript.
Footnotes
The authors declare that they have no conflict of interest.
References
- Adriouch S, Bannas P, Schwarz N, Fliegert R, Guse AH, Seman M, Haag F, Koch-Nolte F (2008) ADP-ribosylation at R125 gates the P2X7 ion channel by presenting a covalent ligand to its nucleotide binding site. FASEB J 22: 861–869 [DOI] [PubMed] [Google Scholar]
- Alberts IL, Nadassy K, Wodak SJ (1998) Analysis of zinc binding sites in protein crystal structures. Protein Sci 7: 1700–1716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiri S, Tai K, Beckstein O, Biggin PC, Sansom MS (2005) The alpha7 nicotinic acetylcholine receptor: molecular modelling, electrostatics, and energetics. Mol Membr Biol 22: 151–162 [DOI] [PubMed] [Google Scholar]
- Bahar I (1999) Dynamics of proteins and biomolecular complexes: inferring functional motions from structure. Rev Chem Eng 15: 319–347 [Google Scholar]
- Bahar I, Rader AJ (2005) Coarse-grained normal mode analysis in structural biology. Curr Opin Struct Biol 15: 586–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand D, Devillers-Thiery A, Revah F, Galzi JL, Hussy N, Mulle C, Bertrand S, Ballivet M, Changeux JP (1992) Unconventional pharmacology of a neuronal nicotinic receptor mutated in the channel domain. Proc Natl Acad Sci USA 89: 1261–1265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks B, Karplus M (1983) Harmonic dynamics of proteins: normal modes and fluctuations in bovine pancreatic trypsin inhibitor. Proc Natl Acad Sci USA 80: 6571–6575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne LE, Cao L, Broomhead HE, Bragg L, Wilkinson WJ, North RA (2011) P2X receptor channels show threefold symmetry in ionic charge selectivity and unitary conductance. Nat Neurosci 14: 17–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne LE, Jiang LH, North RA (2010) New structure enlivens interest in P2X receptors. Trends Pharmacol Sci 31: 229–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G (2008) Purinergic signalling and disorders of the central nervous system. Nat Rev Drug Discov 7: 575–590 [DOI] [PubMed] [Google Scholar]
- Cao L, Broomhead HE, Young MT, North RA (2009) Polar residues in the second transmembrane domain of the rat P2X2 receptor that affect spontaneous gating, unitary conductance, and rectification. J Neurosci 29: 14257–14264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L, Young MT, Broomhead HE, Fountain SJ, North RA (2007) Thr339-to-serine substitution in rat P2X2 receptor second transmembrane domain causes constitutive opening and indicates a gating role for Lys308. J Neurosci 27: 12916–12923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changeux JP, Edelstein SJ (1998) Allosteric receptors after 30 years. Neuron 21: 959–980 [DOI] [PubMed] [Google Scholar]
- Cheng X, Lu B, Grant B, Law RJ, McCammon JA (2006) Channel opening motion of alpha7 nicotinic acetylcholine receptor as suggested by normal mode analysis. J Mol Biol 355: 310–324 [DOI] [PubMed] [Google Scholar]
- Connolly ML (1983) Solvent-accessible surfaces of proteins and nucleic acids. Science 221: 709–713 [DOI] [PubMed] [Google Scholar]
- Ding S, Sachs F (1999) Single channel properties of P2X2 purinoceptors. J Gen Physiol 113: 695–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans RJ (2010) Structural interpretation of P2X receptor mutagenesis studies on drug action. Br J Pharmacol 161: 961–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friday SC, Hume RI (2008) Contribution of extracellular negatively charged residues to ATP action and zinc modulation of rat P2X2 receptors. J Neurochem 105: 1264–1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbs RE, Gouaux E (2011) Principles of activation and permeation in an anion-selective Cys-loop receptor. Nature 474: 54–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinsen K (1998) Analysis of domain motions by approximate normal mode calculations. Proteins 33: 417–429 [DOI] [PubMed] [Google Scholar]
- Huidobro-Toro JP, Lorca RA, Coddou C (2008) Trace metals in the brain: allosteric modulators of ligand-gated receptor channels, the case of ATP-gated P2X receptors. Eur Biophys J 37: 301–314 [DOI] [PubMed] [Google Scholar]
- Jarvis MF, Khakh BS (2009) ATP-gated P2X cation-channels. Neuropharmacology 56: 208–215 [DOI] [PubMed] [Google Scholar]
- Jiang LH, Kim M, Spelta V, Bo X, Surprenant A, North RA (2003) Subunit arrangement in P2X receptors. J Neurosci 23: 8903–8910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang R, Lemoine D, Martz A, Taly A, Gonin S, Prado de Carvalho L, Specht A, Grutter T (2011) Agonist trapped in ATP-binding sites of the P2X2 receptor. Proc Natl Acad Sci USA 108: 9066–9071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang R, Martz A, Gonin S, Taly A, Prado de Carvalho L, Grutter T (2010) A putative extracellular salt bridge at the subunit interface contributes to the ion channel function of the ATP-gated P2X2 receptor. J Biol Chem 285: 15805–15815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawate T, Michel JC, Birdsong WT, Gouaux E (2009) Crystal structure of the ATP-gated P2X(4) ion channel in the closed state. Nature 460: 592–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khakh BS, North RA (2006) P2X receptors as cell-surface ATP sensors in health and disease. Nature 442: 527–532 [DOI] [PubMed] [Google Scholar]
- Kracun S, Chaptal V, Abramson J, Khakh BS (2010) Gated access to the pore of a P2X receptor: structural implications for closed-open transitions. J Biol Chem 285: 10110–10121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs WG, Alexandrov V, Wilson CA, Echols N, Yu H, Gerstein M (2002) Normal mode analysis of macromolecular motions in a database framework: developing mode concentration as a useful classifying statistic. Proteins 48: 682–695 [DOI] [PubMed] [Google Scholar]
- Li M, Chang TH, Silberberg SD, Swartz KJ (2008) Gating the pore of P2X receptor channels. Nat Neurosci 11: 883–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Kawate T, Silberberg SD, Swartz KJ (2010) Pore-opening mechanism in trimeric P2X receptor channels. Nat Commun 1: 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorca RA, Rozas C, Loyola S, Moreira-Ramos S, Zeise ML, Kirkwood A, Huidobro-Toro JP, Morales B (2011) Zinc enhances long-term potentiation through P2X receptor modulation in the hippocampal CA1 region. Eur J Neurosci 33: 1175–1185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquez-Klaka B, Rettinger J, Bhargava Y, Eisele T, Nicke A (2007) Identification of an intersubunit cross-link between substituted cysteine residues located in the putative ATP binding site of the P2X1 receptor. J Neurosci 27: 1456–1466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migita K, Haines WR, Voigt MM, Egan TM (2001) Polar residues of the second transmembrane domain influence cation permeability of the ATP-gated P2X(2) receptor. J Biol Chem 276: 30934–30941 [DOI] [PubMed] [Google Scholar]
- Ming D, Wall ME (2005) Allostery in a coarse-grained model of protein dynamics. Phys Rev Lett 95: 198103. [DOI] [PubMed] [Google Scholar]
- Mitternacht S, Berezovsky IN (2011) Binding leverage as a molecular basis for allosteric regulation. PLoS Comput Biol 7: e1002148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaya N, Tittle RK, Saar N, Dellal SS, Hume RI (2005) An intersubunit zinc binding site in rat P2X2 receptors. J Biol Chem 280: 25982–25993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicke A, Baumert HG, Rettinger J, Eichele A, Lambrecht G, Mutschler E, Schmalzing G (1998) P2X1 and P2X3 receptors form stable trimers: a novel structural motif of ligand-gated ion channels. EMBO J 17: 3016–3028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palma E, Fucile S, Barabino B, Miledi R, Eusebi F (1999) Strychnine activates neuronal alpha7 nicotinic receptors after mutations in the leucine ring and transmitter binding site domains. Proc Natl Acad Sci USA 96: 13421–13426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti P, Vergnano AM, Barbour B, Casado M (2009) Zinc at glutamatergic synapses. Neuroscience 158: 126–136 [DOI] [PubMed] [Google Scholar]
- Sali A, Blundell TL (1993) Comparative protein modelling by satisfaction of spatial restraints. J Mol Biol 234: 779–815 [DOI] [PubMed] [Google Scholar]
- Smart OS, Neduvelil JG, Wang X, Wallace BA, Sansom MS (1996) HOLE: a program for the analysis of the pore dimensions of ion channel structural models. J Mol Graph 14: 354-360376. [DOI] [PubMed] [Google Scholar]
- Sobolevsky AI, Rosconi MP, Gouaux E (2009) X-ray structure, symmetry and mechanism of an AMPA-subtype glutamate receptor. Nature 462: 745–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukumaran M, Rossmann M, Shrivastava I, Dutta A, Bahar I, Greger IH (2011) Dynamics and allosteric potential of the AMPA receptor N-terminal domain. EMBO J 30: 972–982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taly A, Corringer PJ, Grutter T, Prado de Carvalho L, Karplus M, Changeux JP (2006) Implications of the quaternary twist allosteric model for the physiology and pathology of nicotinic acetylcholine receptors. Proc Natl Acad Sci USA 103: 16965–16970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taly A, Delarue M, Grutter T, Nilges M, Le Novere N, Corringer PJ, Changeux JP (2005) Normal mode analysis suggests a quaternary twist model for the nicotinic receptor gating mechanism. Biophys J 88: 3954–3965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tama F, Brooks CL III (2002) The mechanism and pathway of pH induced swelling in cowpea chlorotic mottle virus. J Mol Biol 318: 733–747 [DOI] [PubMed] [Google Scholar]
- Tirion MM (1996) Large amplitude elastic motions in proteins from a single-parameter, atomic analysis. Phys Rev Lett 77: 1905–1908 [DOI] [PubMed] [Google Scholar]
- Tittle RK, Hume RI (2008) Opposite effects of zinc on human and rat P2X2 receptors. J Neurosci 28: 11131–11140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tittle RK, Power JM, Hume RI (2007) A histidine scan to probe the flexibility of the rat P2X2 receptor zinc-binding site. J Biol Chem 282: 19526–19533 [DOI] [PubMed] [Google Scholar]
- Trujillo CA, Nery AA, Martins AH, Majumder P, Gonzalez FA, Ulrich H (2006) Inhibition mechanism of the recombinant rat P2X(2) receptor in glial cells by suramin and TNP-ATP. Biochemistry 45: 224–233 [DOI] [PubMed] [Google Scholar]
- Yang H, Yu Y, Li WG, Yu F, Cao H, Xu TL, Jiang H (2009) Inherent dynamics of the acid-sensing ion channel 1 correlates with the gating mechanism. PLoS Biol 7: e1000151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young MT (2009) P2X receptors: dawn of the post-structure era. Trends Biochem Sci 35: 83–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Brooks BR, Doniach S, Thirumalai D (2005) Network of dynamically important residues in the open/closed transition in polymerases is strongly conserved. Structure 13: 565–577 [DOI] [PubMed] [Google Scholar]
- Zhu F, Hummer G (2009) Gating transition of pentameric ligand-gated ion channels. Biophys J 97: 2456–2463 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.