Abstract
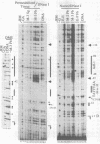
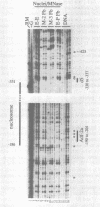
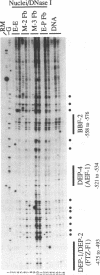
We performed a high resolution analysis of the chromatin structure within the regions required for distal transcription of the Drosophila melanogaster alcohol dehydrogenase gene (Adh). Using dimethyl sulfate, DNase I, and micrococcal nuclease as structural probes, and comparing chromatin structure in tissues isolated from several developmental stages, we have identified several sites of stage- and tissue-specific DNA-protein interactions that correlate with distal transcription initiation. Most were within previously identified cis-acting elements and/or in vitro protein binding sites of the adult enhancer (AAE) and distal promoter, including the TATA box. We also detected a novel stage-specific DNA-protein interaction at the Adf-2a binding site where a non-histone protein was bound to the DNA on the surface of a positioned nucleosome previously identified between the distal promoter and adult enhancer. In addition to footprints, we have also revealed stage- and tissue-specific DNA helix deformations between many of the non-histone protein binding sites. These helix distortions suggest there are interactions among the adjacently bound proteins that result in bending or kinking of the intervening DNA. The distal promoter and AAE have an accessible chromatin conformation in fat body prior to the third larval instar and many of the regulatory proteins that bind in these regions are also available before distal transcription begins. Nevertheless, the timing of DNA-protein interactions in the distal promoter and AAE suggest these proteins do not bind individually or assemble progressively as they and their binding sites become available. Instead, there appears to be a coordinated assembly of a large cooperative complex of proteins interacting with the distal promoter, the positioned nucleosome, the enhancer of the distal promoter (the AAE), and each other.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abel T., Bhatt R., Maniatis T. A Drosophila CREB/ATF transcriptional activator binds to both fat body- and liver-specific regulatory elements. Genes Dev. 1992 Mar;6(3):466–480. doi: 10.1101/gad.6.3.466. [DOI] [PubMed] [Google Scholar]
- Andres A. J., Thummel C. S. Hormones, puffs and flies: the molecular control of metamorphosis by ecdysone. Trends Genet. 1992 Apr;8(4):132–138. doi: 10.1016/0168-9525(92)90371-A. [DOI] [PubMed] [Google Scholar]
- Archer T. K., Cordingley M. G., Wolford R. G., Hager G. L. Transcription factor access is mediated by accurately positioned nucleosomes on the mouse mammary tumor virus promoter. Mol Cell Biol. 1991 Feb;11(2):688–698. doi: 10.1128/mcb.11.2.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayer S., Benyajati C. Conserved enhancer and silencer elements responsible for differential Adh transcription in Drosophila cell lines. Mol Cell Biol. 1990 Jul;10(7):3512–3523. doi: 10.1128/mcb.10.7.3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayer S., Benyajati C. The binding site of a steroid hormone receptor-like protein within the Drosophila Adh adult enhancer is required for high levels of tissue-specific alcohol dehydrogenase expression. Mol Cell Biol. 1992 Feb;12(2):661–673. doi: 10.1128/mcb.12.2.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benyajati C., Ayer S., McKeon J., Ewel A., Huang J. Roles of cis-acting elements and chromatin structure in Drosophila alcohol dehydrogenase gene expression. Nucleic Acids Res. 1987 Oct 12;15(19):7903–7920. doi: 10.1093/nar/15.19.7903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benyajati C., Spoerel N., Haymerle H., Ashburner M. The messenger RNA for alcohol dehydrogenase in Drosophila melanogaster differs in its 5' end in different developmental stages. Cell. 1983 May;33(1):125–133. doi: 10.1016/0092-8674(83)90341-0. [DOI] [PubMed] [Google Scholar]
- Bresnick E. H., Bustin M., Marsaud V., Richard-Foy H., Hager G. L. The transcriptionally-active MMTV promoter is depleted of histone H1. Nucleic Acids Res. 1992 Jan 25;20(2):273–278. doi: 10.1093/nar/20.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buratowski S., Hahn S., Guarente L., Sharp P. A. Five intermediate complexes in transcription initiation by RNA polymerase II. Cell. 1989 Feb 24;56(4):549–561. doi: 10.1016/0092-8674(89)90578-3. [DOI] [PubMed] [Google Scholar]
- Buratowski S., Sopta M., Greenblatt J., Sharp P. A. RNA polymerase II-associated proteins are required for a DNA conformation change in the transcription initiation complex. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7509–7513. doi: 10.1073/pnas.88.17.7509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright I. L. Developmental switch in chromatin structure associated with alternate promoter usage in the Drosophila melanogaster alcohol dehydrogenase gene. EMBO J. 1987 Oct;6(10):3097–3101. doi: 10.1002/j.1460-2075.1987.tb02618.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright I. L., Kelly S. E. Probing the nature of chromosomal DNA-protein contacts by in vivo footprinting. Biotechniques. 1991 Aug;11(2):188-90, 192-4, 196 passim. [PubMed] [Google Scholar]
- Cockell M., Rhodes D., Klug A. Location of the primary sites of micrococcal nuclease cleavage on the nucleosome core. J Mol Biol. 1983 Oct 25;170(2):423–446. doi: 10.1016/s0022-2836(83)80156-9. [DOI] [PubMed] [Google Scholar]
- Corbin V., Maniatis T. Role of transcriptional interference in the Drosophila melanogaster Adh promoter switch. Nature. 1989 Jan 19;337(6204):279–282. doi: 10.1038/337279a0. [DOI] [PubMed] [Google Scholar]
- Corbin V., Maniatis T. The role of specific enhancer-promoter interactions in the Drosophila Adh promoter switch. Genes Dev. 1989 Dec;3(12B):2191–2120. doi: 10.1101/gad.3.12b.2191. [DOI] [PubMed] [Google Scholar]
- Drew H. R. Structural specificities of five commonly used DNA nucleases. J Mol Biol. 1984 Jul 15;176(4):535–557. doi: 10.1016/0022-2836(84)90176-1. [DOI] [PubMed] [Google Scholar]
- Elgin S. C. The formation and function of DNase I hypersensitive sites in the process of gene activation. J Biol Chem. 1988 Dec 25;263(36):19259–19262. [PubMed] [Google Scholar]
- England B. P., Admon A., Tjian R. Cloning of Drosophila transcription factor Adf-1 reveals homology to Myb oncoproteins. Proc Natl Acad Sci U S A. 1992 Jan 15;89(2):683–687. doi: 10.1073/pnas.89.2.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- England B. P., Heberlein U., Tjian R. Purified Drosophila transcription factor, Adh distal factor-1 (Adf-1), binds to sites in several Drosophila promoters and activates transcription. J Biol Chem. 1990 Mar 25;265(9):5086–5094. [PubMed] [Google Scholar]
- Falb D., Maniatis T. A conserved regulatory unit implicated in tissue-specific gene expression in Drosophila and man. Genes Dev. 1992 Mar;6(3):454–465. doi: 10.1101/gad.6.3.454. [DOI] [PubMed] [Google Scholar]
- Gross D. S., Garrard W. T. Nuclease hypersensitive sites in chromatin. Annu Rev Biochem. 1988;57:159–197. doi: 10.1146/annurev.bi.57.070188.001111. [DOI] [PubMed] [Google Scholar]
- Heberlein U., England B., Tjian R. Characterization of Drosophila transcription factors that activate the tandem promoters of the alcohol dehydrogenase gene. Cell. 1985 Jul;41(3):965–977. doi: 10.1016/s0092-8674(85)80077-5. [DOI] [PubMed] [Google Scholar]
- Hogan M. E., Roberson M. W., Austin R. H. DNA flexibility variation may dominate DNase I cleavage. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9273–9277. doi: 10.1073/pnas.86.23.9273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horikoshi M., Bertuccioli C., Takada R., Wang J., Yamamoto T., Roeder R. G. Transcription factor TFIID induces DNA bending upon binding to the TATA element. Proc Natl Acad Sci U S A. 1992 Feb 1;89(3):1060–1064. doi: 10.1073/pnas.89.3.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnsrud L. Contacts between Escherichia coli RNA polymerase and a lac operon promoter. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5314–5318. doi: 10.1073/pnas.75.11.5314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavorgna G., Ueda H., Clos J., Wu C. FTZ-F1, a steroid hormone receptor-like protein implicated in the activation of fushi tarazu. Science. 1991 May 10;252(5007):848–851. doi: 10.1126/science.1709303. [DOI] [PubMed] [Google Scholar]
- Liu L. F., Wang J. C. Supercoiling of the DNA template during transcription. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7024–7027. doi: 10.1073/pnas.84.20.7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockett T. J., Ashburner M. Temporal and spatial utilization of the alcohol dehydrogenase gene promoters during the development of Drosophila melanogaster. Dev Biol. 1989 Aug;134(2):430–437. doi: 10.1016/0012-1606(89)90115-2. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Mendoza R., Markovits J., Jaffrezou J. P., Muzard G., Le Pecq J. B. DNase I susceptibility of bent DNA and its alteration by ditercalinium and distamycin. Biochemistry. 1990 May 29;29(21):5035–5043. doi: 10.1021/bi00473a006. [DOI] [PubMed] [Google Scholar]
- Moses K., Heberlein U., Ashburner M. The Adh gene promoters of Drosophila melanogaster and Drosophila orena are functionally conserved and share features of sequence structure and nuclease-protected sites. Mol Cell Biol. 1990 Feb;10(2):539–548. doi: 10.1128/mcb.10.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller P. R., Wold B. In vivo footprinting of a muscle specific enhancer by ligation mediated PCR. Science. 1989 Nov 10;246(4931):780–786. doi: 10.1126/science.2814500. [DOI] [PubMed] [Google Scholar]
- Ogata R. T., Gilbert W. An amino-terminal fragment of lac repressor binds specifically to lac operator. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5851–5854. doi: 10.1073/pnas.75.12.5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmann T., Wrange O. Specific glucocorticoid receptor binding to DNA reconstituted in a nucleosome. EMBO J. 1988 Oct;7(10):3073–3079. doi: 10.1002/j.1460-2075.1988.tb03172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piña B., Brüggemeier U., Beato M. Nucleosome positioning modulates accessibility of regulatory proteins to the mouse mammary tumor virus promoter. Cell. 1990 Mar 9;60(5):719–731. doi: 10.1016/0092-8674(90)90087-u. [DOI] [PubMed] [Google Scholar]
- Posakony J. W., Fischer J. A., Maniatis T. Identification of DNA sequences required for the regulation of Drosophila alcohol dehydrogenase gene expression. Cold Spring Harb Symp Quant Biol. 1985;50:515–520. doi: 10.1101/sqb.1985.050.01.063. [DOI] [PubMed] [Google Scholar]
- Roeder R. G. The complexities of eukaryotic transcription initiation: regulation of preinitiation complex assembly. Trends Biochem Sci. 1991 Nov;16(11):402–408. doi: 10.1016/0968-0004(91)90164-q. [DOI] [PubMed] [Google Scholar]
- Roth S. Y., Shimizu M., Johnson L., Grunstein M., Simpson R. T. Stable nucleosome positioning and complete repression by the yeast alpha 2 repressor are disrupted by amino-terminal mutations in histone H4. Genes Dev. 1992 Mar;6(3):411–425. doi: 10.1101/gad.6.3.411. [DOI] [PubMed] [Google Scholar]
- Suck D., Oefner C. Structure of DNase I at 2.0 A resolution suggests a mechanism for binding to and cutting DNA. Nature. 1986 Jun 5;321(6070):620–625. doi: 10.1038/321620a0. [DOI] [PubMed] [Google Scholar]
- Taylor I. C., Workman J. L., Schuetz T. J., Kingston R. E. Facilitated binding of GAL4 and heat shock factor to nucleosomal templates: differential function of DNA-binding domains. Genes Dev. 1991 Jul;5(7):1285–1298. doi: 10.1101/gad.5.7.1285. [DOI] [PubMed] [Google Scholar]
- Thomas G. H., Elgin S. C. Protein/DNA architecture of the DNase I hypersensitive region of the Drosophila hsp26 promoter. EMBO J. 1988 Jul;7(7):2191–2201. doi: 10.1002/j.1460-2075.1988.tb03058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeponteau B., Lundell M., Martinson H. Torsional stress promotes the DNAase I sensitivity of active genes. Cell. 1984 Dec;39(3 Pt 2):469–478. doi: 10.1016/0092-8674(84)90454-9. [DOI] [PubMed] [Google Scholar]
- Villeponteau B., Martinson H. G. Gamma rays and bleomycin nick DNA and reverse the DNase I sensitivity of beta-globin gene chromatin in vivo. Mol Cell Biol. 1987 May;7(5):1917–1924. doi: 10.1128/mcb.7.5.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westwood J. T., Clos J., Wu C. Stress-induced oligomerization and chromosomal relocalization of heat-shock factor. Nature. 1991 Oct 31;353(6347):822–827. doi: 10.1038/353822a0. [DOI] [PubMed] [Google Scholar]
- Zhang L., Gralla J. D. In situ nucleoprotein structure at the SV40 major late promoter: melted and wrapped DNA flank the start site. Genes Dev. 1989 Nov;3(11):1814–1822. doi: 10.1101/gad.3.11.1814. [DOI] [PubMed] [Google Scholar]
- Zhang L., Gralla J. D. In situ nucleoprotein structure involving origin-proximal SV40 DNA control elements. Nucleic Acids Res. 1990 Apr 11;18(7):1797–1803. doi: 10.1093/nar/18.7.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Gralla J. D. Micrococcal nuclease as a probe for bound and distorted DNA in lac transcription and repression complexes. Nucleic Acids Res. 1989 Jul 11;17(13):5017–5028. doi: 10.1093/nar/17.13.5017. [DOI] [PMC free article] [PubMed] [Google Scholar]