Abstract
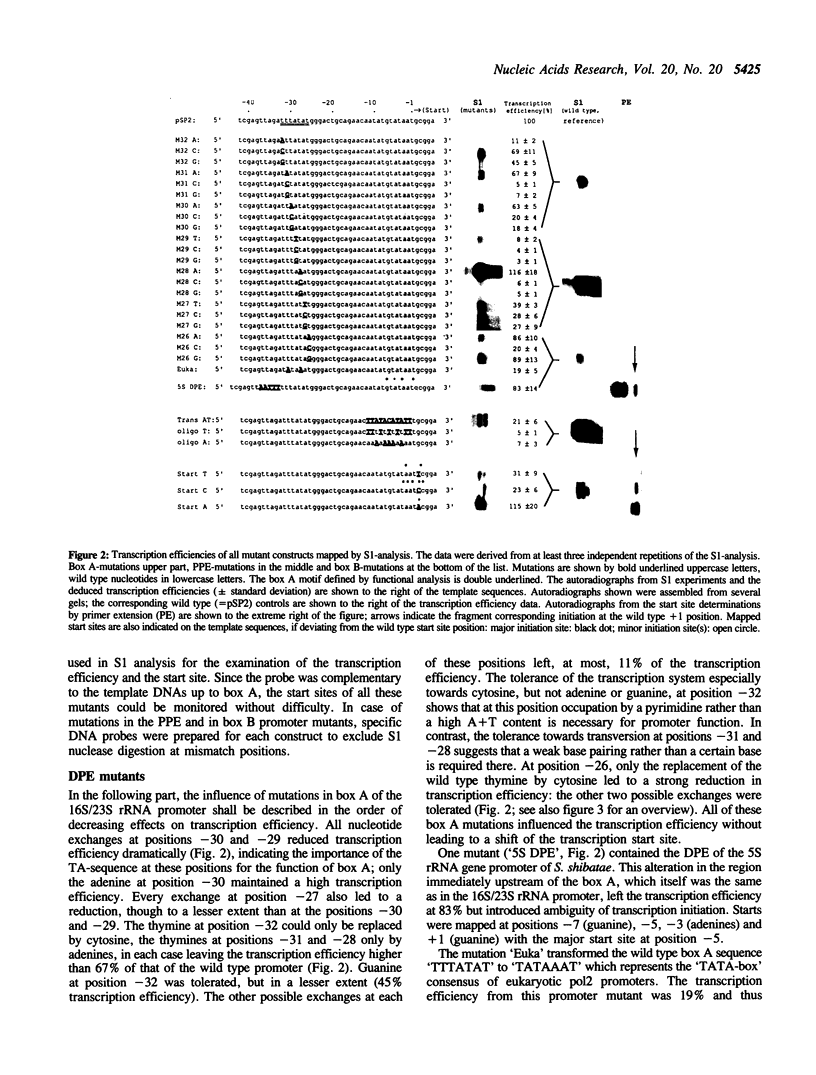
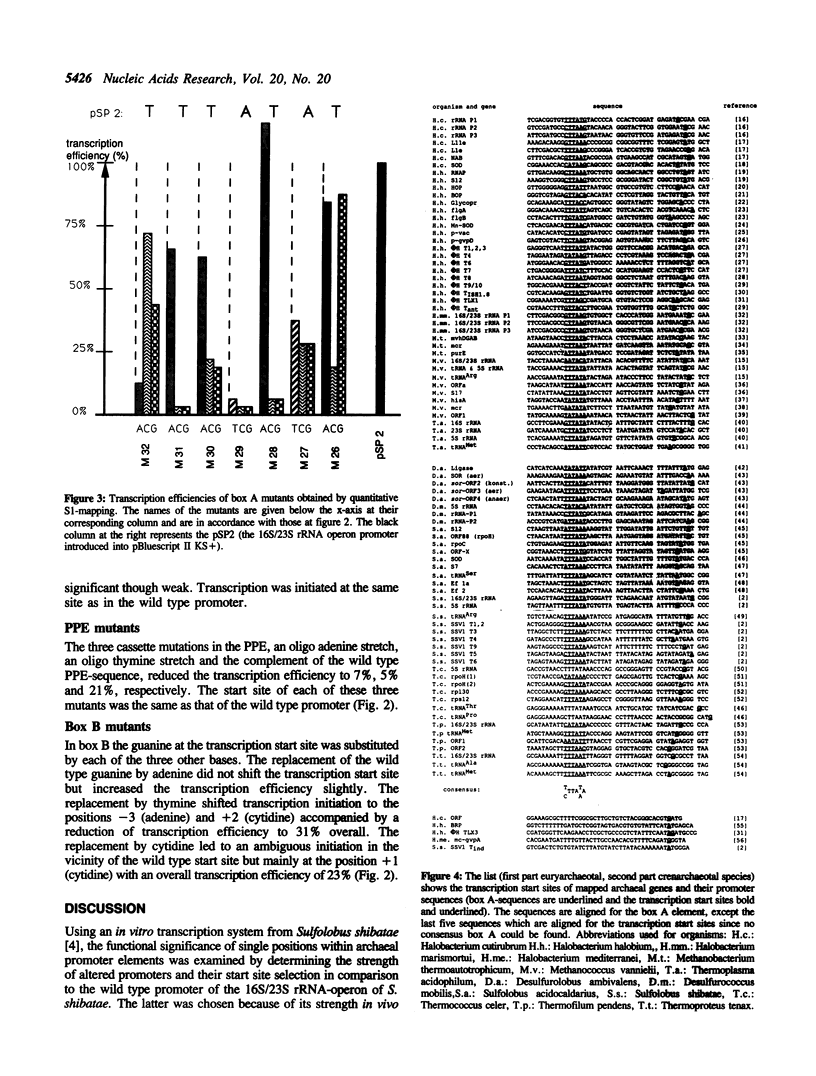
The sequence requirements for specific and efficient transcription from the 16S/23S rRNA promoter of Sulfolobus shibatae were analysed by point mutations and by cassette mutations using an in vitro transcription system. The examination of the box A-containing distal promoter element (DPE) showed the great importance of the TA sequence in the center of box A for transcription efficiency and the influence of the sequence upstream of box A on determining the distance between the DPE and the start site. In most positions of box A, replacement of the wild type bases by adenines or thymines are less detrimental than replacements by cytosines or guanines. The effectiveness of the proximal promoter element (PPE) was not merely determined by its high A + T content but appeared to be directly related to its nucleotide sequence. At the start site a pyrimidine/purine (py/pu) sequence was necessary for unambiguous initiation as shown by analysis of mutants where the wild type start base was replaced. The sequence of box A optimal for promoter function in vitro is identical to the consensus of 84 mapped archaeal promoter sequences.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldea M., Claverie-Martín F., Díaz-Torres M. R., Kushner S. R. Transcript mapping using [35S]DNA probes, trichloroacetate solvent and dideoxy sequencing ladders: a rapid method for identification of transcriptional start points. Gene. 1988 May 15;65(1):101–110. doi: 10.1016/0378-1119(88)90421-0. [DOI] [PubMed] [Google Scholar]
- Auer J., Spicker G., Böck A. Organization and structure of the Methanococcus transcriptional unit homologous to the Escherichia coli "spectinomycin operon". Implications for the evolutionary relationship of 70 S and 80 S ribosomes. J Mol Biol. 1989 Sep 5;209(1):21–36. doi: 10.1016/0022-2836(89)90167-8. [DOI] [PubMed] [Google Scholar]
- Betlach M., Friedman J., Boyer H. W., Pfeifer F. Characterization of a halobacterial gene affecting bacterio-opsin gene expression. Nucleic Acids Res. 1984 Oct 25;12(20):7949–7959. doi: 10.1093/nar/12.20.7949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanck A., Oesterhelt D. The halo-opsin gene. II. Sequence, primary structure of halorhodopsin and comparison with bacteriorhodopsin. EMBO J. 1987 Jan;6(1):265–273. doi: 10.1002/j.1460-2075.1987.tb04749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokranz M., Bäumner G., Allmansberger R., Ankel-Fuchs D., Klein A. Cloning and characterization of the methyl coenzyme M reductase genes from Methanobacterium thermoautotrophicum. J Bacteriol. 1988 Feb;170(2):568–577. doi: 10.1128/jb.170.2.568-577.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. W., Reeve J. N. Transcription initiation and a RNA polymerase binding site upstream of the purE gene of the archaebacterium Methanobacterium thermoautotrophicum strain delta H. FEMS Microbiol Lett. 1989 Jul 15;51(1):131–136. doi: 10.1016/0378-1097(89)90495-3. [DOI] [PubMed] [Google Scholar]
- Brown J. W., Thomm M., Beckler G. S., Frey G., Stetter K. O., Reeve J. N. An archaebacterial RNA polymerase binding site and transcription initiation of the hisA gene in Methanococcus vannielii. Nucleic Acids Res. 1988 Jan 11;16(1):135–150. doi: 10.1093/nar/16.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung C. T., Niemela S. L., Miller R. H. One-step preparation of competent Escherichia coli: transformation and storage of bacterial cells in the same solution. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2172–2175. doi: 10.1073/pnas.86.7.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culham D. E., Nazar R. N. Multiple termination sites in an unlinked 5S rRNA operon in the archaebacterium, Thermococcus celer. Mol Gen Genet. 1989 Apr;216(2-3):412–416. doi: 10.1007/BF00334384. [DOI] [PubMed] [Google Scholar]
- Dassarma S., Rajbhandary U. L., Khorana H. G. Bacterio-opsin mRNA in wild-type and bacterio-opsin-deficient Halobacterium halobium strains. Proc Natl Acad Sci U S A. 1984 Jan;81(1):125–129. doi: 10.1073/pnas.81.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis P. P. Multiple promoters for the transcription of the ribosomal RNA gene cluster in Halobacterium cutirubrum. J Mol Biol. 1985 Nov 20;186(2):457–461. doi: 10.1016/0022-2836(85)90117-2. [DOI] [PubMed] [Google Scholar]
- Englert C., Horne M., Pfeifer F. Expression of the major gas vesicle protein gene in the halophilic archaebacterium Haloferax mediterranei is modulated by salt. Mol Gen Genet. 1990 Jul;222(2-3):225–232. doi: 10.1007/BF00633822. [DOI] [PubMed] [Google Scholar]
- Gerl L., Sumper M. Halobacterial flagellins are encoded by a multigene family. Characterization of five flagellin genes. J Biol Chem. 1988 Sep 15;263(26):13246–13251. [PubMed] [Google Scholar]
- Gropp F., Palm P., Zillig W. Expression and regulation of Halobacterium halobium phage phi H genes. Can J Microbiol. 1989 Jan;35(1):182–188. doi: 10.1139/m89-028. [DOI] [PubMed] [Google Scholar]
- Hausner W., Frey G., Thomm M. Control regions of an archaeal gene. A TATA box and an initiator element promote cell-free transcription of the tRNA(Val) gene of Methanococcus vannielii. J Mol Biol. 1991 Dec 5;222(3):495–508. doi: 10.1016/0022-2836(91)90492-o. [DOI] [PubMed] [Google Scholar]
- Horne M., Englert C., Pfeifer F. Two genes encoding gas vacuole proteins in Halobacterium halobium. Mol Gen Genet. 1988 Aug;213(2-3):459–464. doi: 10.1007/BF00339616. [DOI] [PubMed] [Google Scholar]
- Hüdepohl U., Gropp F., Horne M., Zillig W. Heterologous in vitro transcription from two archaebacterial promoters. FEBS Lett. 1991 Jul 22;285(2):257–259. doi: 10.1016/0014-5793(91)80811-g. [DOI] [PubMed] [Google Scholar]
- Hüdepohl U., Reiter W. D., Zillig W. In vitro transcription of two rRNA genes of the archaebacterium Sulfolobus sp. B12 indicates a factor requirement for specific initiation. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5851–5855. doi: 10.1073/pnas.87.15.5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J. G., Hackett N. R., Halladay J. T., Scothorn D. J., Yang C. F., Ng W. L., DasSarma S. Analysis of insertion mutants reveals two new genes in the pNRC100 gas vesicle gene cluster of Halobacterium halobium. Nucleic Acids Res. 1989 Oct 11;17(19):7785–7793. doi: 10.1093/nar/17.19.7785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjems J., Garrett R. A. Novel expression of the ribosomal RNA genes in the extreme thermophile and archaebacterium Desulfurococcus mobilis. EMBO J. 1987 Nov;6(11):3521–3530. doi: 10.1002/j.1460-2075.1987.tb02678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klenk H. P., Palm P., Lottspeich F., Zillig W. Component H of the DNA-dependent RNA polymerases of Archaea is homologous to a subunit shared by the three eucaryal nuclear RNA polymerases. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):407–410. doi: 10.1073/pnas.89.1.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klenk H. P., Schwass V., Zillig W. Nucleotide sequence of the genes encoding the L30, S12 and S7 equivalent ribosomal proteins from the archaeum Thermococcus celer. Nucleic Acids Res. 1991 Nov 11;19(21):6047–6047. doi: 10.1093/nar/19.21.6047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechner J., Sumper M. The primary structure of a procaryotic glycoprotein. Cloning and sequencing of the cell surface glycoprotein gene of halobacteria. J Biol Chem. 1987 Jul 15;262(20):9724–9729. [PubMed] [Google Scholar]
- Lechner K., Heller G., Böck A. Organization and nucleotide sequence of a transcriptional unit of Methanococcus vannielii comprising genes for protein synthesis elongation factors and ribosomal proteins. J Mol Evol. 1989 Jul;29(1):20–27. doi: 10.1007/BF02106178. [DOI] [PubMed] [Google Scholar]
- Leffers H., Gropp F., Lottspeich F., Zillig W., Garrett R. A. Sequence, organization, transcription and evolution of RNA polymerase subunit genes from the archaebacterial extreme halophiles Halobacterium halobium and Halococcus morrhuae. J Mol Biol. 1989 Mar 5;206(1):1–17. doi: 10.1016/0022-2836(89)90519-6. [DOI] [PubMed] [Google Scholar]
- May B. P., Dennis P. P. Unusual evolution of a superoxide dismutase-like gene from the extremely halophilic archaebacterium Halobacterium cutirubrum. J Bacteriol. 1990 Jul;172(7):3725–3729. doi: 10.1128/jb.172.7.3725-3729.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mevarech M., Hirsch-Twizer S., Goldman S., Yakobson E., Eisenberg H., Dennis P. P. Isolation and characterization of the rRNA gene clusters of Halobacterium marismortui. J Bacteriol. 1989 Jun;171(6):3479–3485. doi: 10.1128/jb.171.6.3479-3485.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray M. G. Use of sodium trichloroacetate and mung bean nuclease to increase sensitivity and precision during transcript mapping. Anal Biochem. 1986 Oct;158(1):165–170. doi: 10.1016/0003-2697(86)90605-6. [DOI] [PubMed] [Google Scholar]
- Pühler G., Leffers H., Gropp F., Palm P., Klenk H. P., Lottspeich F., Garrett R. A., Zillig W. Archaebacterial DNA-dependent RNA polymerases testify to the evolution of the eukaryotic nuclear genome. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4569–4573. doi: 10.1073/pnas.86.12.4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ree H. K., Zimmermann R. A. Organization and expression of the 16S, 23S and 5S ribosomal RNA genes from the archaebacterium Thermoplasma acidophilum. Nucleic Acids Res. 1990 Aug 11;18(15):4471–4478. doi: 10.1093/nar/18.15.4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeve J. N., Beckler G. S., Cram D. S., Hamilton P. T., Brown J. W., Krzycki J. A., Kolodziej A. F., Alex L., Orme-Johnson W. H., Walsh C. T. A hydrogenase-linked gene in Methanobacterium thermoautotrophicum strain delta H encodes a polyferredoxin. Proc Natl Acad Sci U S A. 1989 May;86(9):3031–3035. doi: 10.1073/pnas.86.9.3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter W. D., Hüdepohl U., Zillig W. Mutational analysis of an archaebacterial promoter: essential role of a TATA box for transcription efficiency and start-site selection in vitro. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9509–9513. doi: 10.1073/pnas.87.24.9509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter W. D., Palm P. Identification and characterization of a defective SSV1 genome integrated into a tRNA gene in the archaebacterium Sulfolobus sp. B12. Mol Gen Genet. 1990 Mar;221(1):65–71. doi: 10.1007/BF00280369. [DOI] [PubMed] [Google Scholar]
- Reiter W. D., Palm P., Voos W., Kaniecki J., Grampp B., Schulz W., Zillig W. Putative promoter elements for the ribosomal RNA genes of the thermoacidophilic archaebacterium Sulfolobus sp. strain B12. Nucleic Acids Res. 1987 Jul 24;15(14):5581–5595. doi: 10.1093/nar/15.14.5581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter W. D., Palm P., Zillig W. Analysis of transcription in the archaebacterium Sulfolobus indicates that archaebacterial promoters are homologous to eukaryotic pol II promoters. Nucleic Acids Res. 1988 Jan 11;16(1):1–19. doi: 10.1093/nar/16.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnabel H., Palm P., Dick K., Grampp B. Sequence analysis of the insertion element ISH1.8 and of associated structural changes in the genome of phage PhiH of the archaebacterium Halobacterium halobium. EMBO J. 1984 Aug;3(8):1717–1722. doi: 10.1002/j.1460-2075.1984.tb02037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimmin L. C., Dennis P. P. Characterization of the L11, L1, L10 and L12 equivalent ribosomal protein gene cluster of the halophilic archaebacterium Halobacterium cutirubrum. EMBO J. 1989 Apr;8(4):1225–1235. doi: 10.1002/j.1460-2075.1989.tb03496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomm M., Sherf B. A., Reeve J. N. RNA polymerase-binding and transcription initiation sites upstream of the methyl reductase operon of Methanococcus vannielii. J Bacteriol. 1988 Apr;170(4):1958–1961. doi: 10.1128/jb.170.4.1958-1961.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomm M., Wich G. An archaebacterial promoter element for stable RNA genes with homology to the TATA box of higher eukaryotes. Nucleic Acids Res. 1988 Jan 11;16(1):151–163. doi: 10.1093/nar/16.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wich G., Hummel H., Jarsch M., Bär U., Böck A. Transcription signals for stable RNA genes in Methanococcus. Nucleic Acids Res. 1986 Mar 25;14(6):2459–2479. doi: 10.1093/nar/14.6.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wich G., Leinfelder W., Böck A. Genes for stable RNA in the extreme thermophile Thermoproteus tenax: introns and transcription signals. EMBO J. 1987 Feb;6(2):523–528. doi: 10.1002/j.1460-2075.1987.tb04784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C. R., Kandler O., Wheelis M. L. Towards a natural system of organisms: proposal for the domains Archaea, Bacteria, and Eucarya. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4576–4579. doi: 10.1073/pnas.87.12.4576. [DOI] [PMC free article] [PubMed] [Google Scholar]




