Abstract
There currently is an unmet clinical need for novel immunosuppressants for the long-term prevention of kidney transplant rejection as alternatives to the nephrotoxic calcineurin inhibitor cyclosporine A (CsA). Recent studies show that K+-channels play a crucial role in T-lymphocyte activities. In the present study we investigated whether combined blockade of the T-cell K+-channels KCa3.1 and Kv1.3, both of which regulate calcium signaling during lymphocyte activation, is effective in preventing rejection of kidney allografts from Fisher to Lewis rats. All recipients were treated for an initial period of 7 days with CsA (5mg/kg/d). In rats with intact allograft function, treatment was continued for another 10 days either with CsA (5mg/kg/d), or a combination of TRAM-34 (KCa3.1 inhibitor; 120mg/kg/d) plus ShK (Kv1.3 inhibitor; 80μg/kg thrice daily), or vehicle alone. Kidney sections were stained with PAS/H&E as well as immunohistochemically for markers of macrophages (CD68), T-lymphocytes (CD43) or cytotoxic T-cells (CD8) and evaluated by standard scoring. Our results show that combined TRAM-34/ShK-treatment effectively reduced total interstitial mononuclear cell infiltration (−42%) as well as infiltration of CD43+ T-cells (−32%), cytotoxic CD8+ T-cells (−32%) and CD68+ macrophages (−26%) as compared to allografts from vehicle-treated controls. Notably, the efficacy of TRAM-34/ShK-treatment here was comparable to that of CsA. Besides, no visible organ damage or other discernible adverse effects were observed with this treatment. Thus, selective blockade of T-lymphocyte KCa3.1 and Kv1.3 channels may represent a novel alternative therapeutic approach for preventing kidney allograft rejection.
INTRODUCTION
Transplantation of solid organs has become the gold standard for the treatment of various irreversible organ failures such as end-stage renal disease. Induction of appropriate immunosuppression after kidney transplantation is usually essential, but also entails major clinical problems.1 Despite considerable advances in immunosuppressive therapy, there is still an unmet clinical need for new effective immunosuppressives that could be used for as alternatives to the classical calcineurin inhibitors such as cyclosporine A (CsA). Albeit being a standard component of current immunosuppressive regimens for transplant patients, CsA treatment is associated with a number of momentous negative side-effects including hypertension, hypertriglyceridemia, hypertrichosis, gingival hyperplasia, hepatotoxicity and nephrotoxicity, the latter of which manifests with tubular lesions, arteriolopathy, and HUS (hemolytic-uremic-syndrome)-like changes in glomeruli and vessels.2,3
T-lymphocytes constitute the primary target cells of most immunosuppressants because of their well-established role as main orchestrators of cell-mediated immune responses. Recently, a large body of evidence has emerged showing that K+-channels play a critical role in T-cell activities.4 T-cells express two types of K+-channels: the voltage-gated K+-channel Kv1.35 and the intermediate-conductance Ca2+-activated K+-channel KCa3.1.6 Both channels contribute to the regulation of Ca2+ signaling events via the control of membrane potential.7,8 Opening of Kv1.3 and KCa3.1 leads to efflux of K+ and cell hyperpolarization, thus providing the electrochemical force needed for sustained Ca2+ influx, which has been shown to be required for 75% of new gene expression via the phosphatase calcineurin.9,10 Conversely, selective blockade of these K+-channels results in membrane depolarization which reduces Ca2+ influx, thus inhibiting cytokine production and proliferation of T-cells.11,12 Moreover, activation of T -lymphocytes is associated with an up-regulation of Kv1.3 and/or KCa3.1 functions.13,14 Strikingly, in animal models of autoimmune diseases such as multiple sclerosis,15 rheumatoid arthritis,16 and type-1 diabetes mellitus,16 selective pharmacological blockade of T-lymphocyte K+-channels has been shown to effectively attenuate the course of disease by damping T-cell activity. However, whether inhibition of T-lymphocyte K+-channels could also be useful for suppressing T-cell mediated responses after solid organ transplantation has not yet been investigated. Here, we hypothesized that combined inhibition of Kv1.3 and KCa3.1 channels is capable of attenuating kidney allograft rejection in the Fisher to Lewis model.
MATERIALS AND METHODS
Animals
Adult male Fisher (F344) and Lewis (LEW) rats weighing 140–200 g were purchased from River Charles. Rats were housed in standard rodent cages and provided chow and water ad libitum. LEW rats served as recipients of F344 kidney allografts and LEW kidney isografts, respectively. All experimental procedures were performed in accordance to the German animal care and ethics legislation and were approved by the local governmental authorities.
T-cells, Patch-Clamp Studies, and MLR
LEW rat splenocytes were incubated for 4 days with irradiated F344 rat splenocytes. For identification of T-cells, cells were stained with a FITC-conjugated mouse anti-rat anti-CD3 Ab (clone G4.18; BD Pharmingen, USA) and then patch-clamped. Whole-cell patch-clamp studies and calculations of Kv1.3- and KCa3.1-channel numbers were performed as described previously.14 Currents conducted by KCa3.1 and Kv1.3 were identified by their distinct biophysical and pharmacological properties (sensitivity to TRAM-34 (KCa3.1) and ShK (Kv1.3), respectively).
For mixed leukocyte reaction (MLR) in the presence or absence of blockers, spleens from 3 male LEW rats were minced, the pieces mixed, passed through a 100 mm cell strainer and erythrocytes lysed with ACK lysing buffer (150 mM NH4Cl, 10 mM KHCO3, 0.1 mM EDTA). The resulting mononuclear cells (MNC) were washed and then seeded at 5x104 cells per well into round bottom 96-well plates in RPMI-1640 culture medium supplemented with 10% fetal calf serum, 2 mM L-glutamine, 1 mM Na+ pyruvate, 1% non-essential amino acids, 100 units/ml penicillin, and 100 mg/ml streptomycin. TRAM-34, ShK and/or CsA (Sigma, USA) were added at different concentrations and the cells stimulated with 5x104 irradiated F344 MNC (2500 rad; pooled MNC from three F344 spleens). The cells were incubated for 5 days and proliferation measured through [3H]-thymidine incorporation (1 mCi per well for the last 16 h). Control counts were 12.000 cpm.
Kidney Allograft Model and Treatment Protocol
Left kidneys were transplanted orthothopically into LEW recipients by standard microvascular techniques, as described in detail elsewhere.17 Briefly, after recipient and donor rats had been ether-anesthetized, the abdomen was opened by median incision and the left kidney exposed. The donor kidney was flushed with 4°C cold Euro-Collins® solution (Fresenius, Bad Homburg, Germany) in situ and then harvested preserving patches of aorta and vena cava. After the native left kidney had been removed, the vessels of the donor kidney were anastomosed to the recipient’s abdominal aorta and vena cava inferior by end-to-side anastomoses using fine suture material andthe graft thenallowed to be reperfused. Finally, the graft ureter was inserted into the cranial part of the recipient’s bladder and the abdomen closed by sutures.
To allow exclusion of animals with non-functional transplants due to e.g. inadequate graft perfusion or ureteral obstruction, all rats with allografts were given CsA (5mg/kg /d; s.c.) for an initial period of 7 days followed by a second-look procedure for graft reassessment. Rats with intact allografts were divided into three groups and treated for another 10 days either with CsA (5 mg/kg/d; s.c.), or a combination of specific KCa3.1 inhibitor TRAM-34 (1-[(2-chlorophenyl)diphenylmethyl]-1H-pyrazole; 120 mg/kg/d; i.p.) plus specific Kv1.3 inhibitor ShK (sea anemone Stichodactyla helianthus toxin; 80 μg/kg thrice daily; s.c.), or vehicle (peanut oil; i.p./saline; s.c.) alone. A non-treated fourth group consisted of LEW rats, which had received kidney isografts. The small molecule KCa3.1 blocker TRAM-34 was synthesized as reported earlier.18 ShK was obtained from Bachem Bioscience (King of Prussia, USA).
Histological Analysis
After sacrifice, transplant kidneys were rapidly excised, fixed at room temperature in 4% neutral buffered formalin for 24h and then embedded in para ffin. Light microscopy was performed on 3–5 μm sections stained with haematoxylin and eosin (H&E) or periodic acid-Schiff (PAS). Mononuclear cell infiltration was evaluated semiquantitatively by standard scoring criteria (infiltration score (% stained area): 0- none (<0.1%), 1- mild (0.1–0.5%), 2- moderate (0.5–3%), 3- severe (3–10%), 4- massive (>10%)) in a blinded fashion. To study the leukocyte infiltration in more detail we conducted immunohistochemical analyses using three primary mouse anti-rat monoclonal antibodies: W3/13 (Serotec MCA54R, CD43) identifying T-lymphocytes, MRCOX -8 (Serotec MCA48R, CD8) as a marker of cytotoxic T-cells, and ED1 (Serotec MCA341R, CD68) as a marker of macrophages. Bound primary antibodies were detected with a biotinylated donkey anti-mouse IgG secondary antibody followed by a horseradish peroxidase-conjugated avidin complex (Vectastain Elite ABC Kit, Vector Laboratories, Burlingame, CA). Peroxidase activity was visualized with 3,3’-diaminobenzidine (DAB Substrate Kit for Peroxidase, Vector Laboratories, Burlingame, CA). Sections were counterstained with hematoxylin (Fisher, Pittsburg, PA), dehydrated and mounted with Permount (Fisher, Pittsburg, PA). For quantification of staining, ten photographs (magnification ×400) were taken per slide in a semi-random fashion. Photographs were centered on a glomerulus in the outer cortex region and analyzed according to the method reported by Lehr et al.19 with slight modifications.
Statistics
Data are given as mean ± SEM. The Student’s t-test was used for statistical comparison of groups. P-values of less than 0.05 were considered statistically significant.
RESULTS
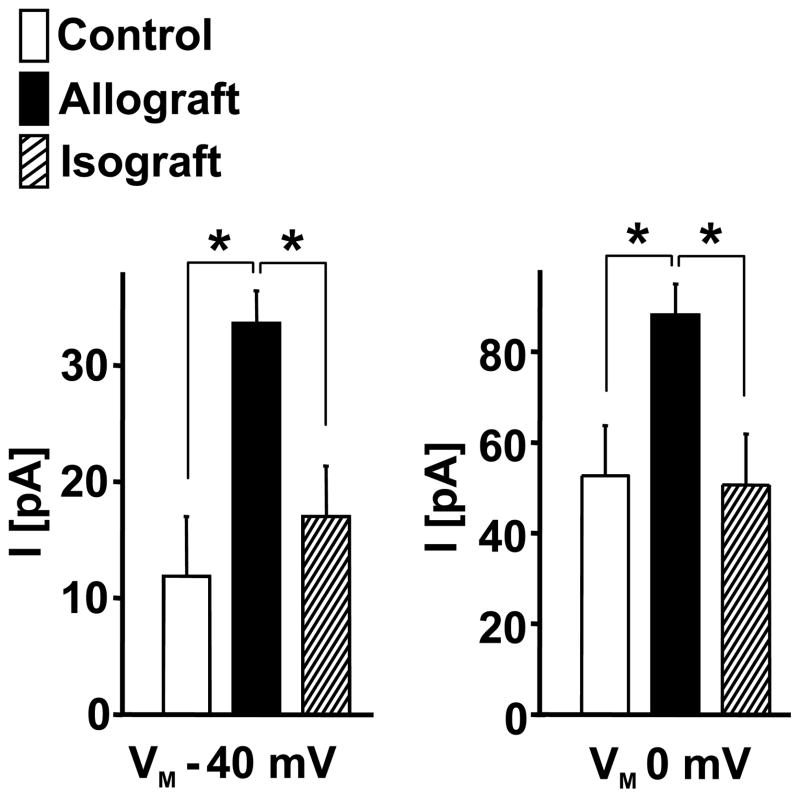
As a fist step in our study we investigated whether allogeneic stimulation would lead to a similar increase in Kv1.3 and KCa3.1 expression as previously reported for mitogenic or antigenic stimulation of human and rat T cells.13–15 Our electrophysiological data show that the number of Kv1.3 and KCa3.1 channels per cells strongly increased from ≈ 10 K v1.3 channels and ≈ 9 KCa3.1 in the unstimulated T-cells to ≈ 230 K v1.3 and ≈ 225 KCa3.1 channels in the allogeneically stimulated LEW T-cells (for representative traces of ShK-sensitive Kv1.3 and TRAM-34-sensitive KCa3.1 currents see Fig.1A, for statistics Fig. 1B). This increase in Kv1.3 and KCa3.1 expression is very similar to what has been previously reported for concanavalinA (Con A)- or myelin-basic protein (MBP)-stimulated LEW rat lymph node or spleen T-cells.15
Figure 1.
Increased expression of KCa3.1 and Kv1.3 channels in LEW T-lymphocytes after MLR (stimulated for 4 days with irradiated F344 splenocytes). (A) Representative traces of Kv1.3 and KCa3.1 currents in unstimulated LEW rat T-cells (upper panel) and after allogeneic stimulation (lower panel). Voltage-dependent Kv1.3 currents were sensitive to ShK and the voltage-independent KCa3.1 current component was sensitive to the selective KCa3.1 blocker TRAM-34. (B) Quantification of KCa3.1 and Kv1.3 channel numbers per cell in stimulated and unstimulated T-cells. Data are given as mean ± SEM.
Based on this strong increase in functional expression of Kv1.3 and KCa3.1 channels in the allogeneically stimulated LEW T-cell, we next tested whether pharmacological inhibition of the channels reduces T-cell proliferation in MLR. As shown in Figure 2, LEW T-cell proliferation as measured by [3H]-thymidine incorporation after 5 days of stimulation with irradiated F344 splenocytes was significantly and dose-dependently reduced by the Kv1.3-blocker ShK (at ≥10 nM) or the KCa3.1 blocker TRAM-34 (IC50 ≈ 250 nM). Moreover, ShK (10 nM) had additive inhibitory effects on proliferation in the presence of TRAM-34.
Figure 2.
Inhibition of KCa3.1 and Kv1.3 channels suppresses LEW/F344 MLR. The plot shows dose-dependent effects of TRAM-34 and ShK and of the combination of both on [3H]-thymidine incorporation after 5 days of MLR. Data are given as mean ± SD, n = 3 for each data point.
Encouraged by the inhibitory effects of TRAM-34 and ShK on MLR, we next investigated whether inhibition of KCa3.1 and Kv1.3 channels prevents allograft rejection after transplantation in vivo. For that purpose we conducted a comparative treatment study and treated allograft recipients either with a combination of the KCa3.1 and Kv1.3 blockers TRAM-34 and ShK, or the calcineurin inhibitor CsA at standard dose, or vehicle alone. To ensure that channel blocker doses that were used yielded plasma concentrations to effectively block KCa3.1 and Kv1.3 in vivo, we obtained blood from the animals at the end of the last treatment interval and determined plasma levels of TRAM-34 and ShK by using whole-cell patch-clamp on cells stably expressing Kv1.3 or KCa3.1 as a “bioassay” as previously described.15 Plasma concentrations were found to average 168 ± 58 nM (n=7) for TRAM-34 (24 h after injection) and 71 ± 10 pM (n=7) for ShK (8h after injection), indicating that the treatment yielded significant amounts of TRAM-34 and ShK in vivo.
Our histological analyses show that the combination of TRAM-34 and ShK significantly reduced total interstitial mononuclear cell infiltration into transplanted kidneys by ≈ - 42% as compared to vehicle-treated controls (Fig.3A). Interestingly, this reduction was similar to that observed with CsA treatment. To dissect whether TRAM-34/ShK treatment suppressed the infiltration of a specific mononuclear cell population, allograft and isograft cross sections were stained for T-lymphocytes (CD43), cytotoxic T-cells (CD8), or macrophages (CD68). The TRAM-34/ShK treatment substantially reduced allograft infiltration by CD43+ T-lymphocytes by ≈ - 32% (Fig. 3B), which was comparable to findings in recipients with continued CsA treatment (≈ - 39%). Likewise, the presence of cytotoxic CD8+ T-cells was reduced by ≈ - 32% in allografts from TRAM-34/ShK-treated recipients when compared to vehicle-treated controls, again resembling the effect accomplished with standard CsA treatment (≈ - 40%; Fig. 3C). Albeit not reaching statistical significance, there was further a tendency towards a decrease in numbers of infiltrating macrophages in kidney transplants of animals, which had received TRAM-34/ShK (P=0.07; Fig.3D). A similar tendency towards lower macrophage infiltration was also observed with CsA treatment (P=0.16).
Figure 3. In vivo blockade of KCa3.1 and Kv1.3 inhibits mononuclear cell infiltration into renal allografts in vivo.
(A) Left panel: Photographs display representative areas of PAS-stained cross sections from rat kidney grafts seventeen days after transplantation (magnification ×400). Note reduced tubulointerstitial infiltrates in allografts from TRAM-34/ShK- and CsA-treated animals compared to vehicle-treated controls. Right panel: Histopathological evaluation of cross sections from kidney allografts with TRAM-34/ShK (n=7), CsA (n=5), or vehicle (Ve; n=7) treatment. Non-treated animals with isografts (Iso; n=3) served as negative controls. (B) Left panel: Photographs display representative areas of kidney graft cross sections stained for T-cell marker CD43 (W3/13 Ab) seventeen days after transplantation (magnification ×400). Right panel: Histopathological evaluation of cross sections. (C) Histopathological evaluation of sections stained for cytotoxic T-cell marker CD8 (MRC OX-8Ab). Note that CD8+ T-cells were not detectable in isografts. (D) Histopathological evaluation of CD68+ macrophage infiltration (ED1Ab) . Data are given as mean ± SEM; *P<0.05, **P<0.01.
Of note, combined TRAM-34 and ShK treatment at therapeutic concentrations did not result in visible organ damage, increased susceptibility to infections or other discernible adverse effects such as neurological abnormalities in the animals.
Taken together, these findings demonstrate that combined blockade of KCa3.1 and Kv1.3 channels is equally capable of preventing renal allograft infiltration by mononuclear cells and in particular T-lymphocytes as the calcineurin inhibitor CsA in the LEW-F344 combination.
DISCUSSION
Activation of T-cells has been reported to lead to an up-regulation of Kv1.3 and/or KCa3.1 expression as compared to resting T-cells.15 These potassium channels appear critical for regulating Ca2+ signals, which dictate T-lymphocyte activation and proliferation.7,9,13,20 Moreover, observations from several animal and human in vivo-studies of T-cell-mediated immune diseases such as autoimmune encephalomyelitis,14,15 rheumatoid arthritis,16 inflammatory bone resorption21 and allergic contact dermatitis22 corroborate the notion that elevated potassium channel functions play an important pathophysiological role in activated T-cells. In the present study we tested whether pharmacological blockade of Kv1.3 and KCa3.1 channels suppresses kidney allograft rejection in the Fisher-to-Lewis model. Here, we demonstrate that allogeneic activation of LEW T-cells resulted in a strong increase in functional Kv1.3/KCa3.1 expression as reflected by the ≈20 -fold increase in channel numbers in allogeneically stimulated LEW T-cells. In addition, LEW/F344 MLR was inhibited by pharmacological blockade of each individual channel and more effectively by simultaneous blockade of both. Finally, in our pilot in vivo-trial, we here show that treatment of LEW recipients of kidney allografts with a combination of TRAM-34 and ShK significantly reduced T-cell infiltration (mainly of CD8-positive T-cells) and to some extent also macrophage infiltration into the transplant. Strikingly, these immunosuppressive effects were comparable to the impact of standard CsA-treatment in this model.
In conclusion, the present study provides some first promising evidence that pharmacological inhibition of T-cell KCa3.1 and Kv1.3 channels has immunomodulatory effects after kidney transplantation in the LEW/F344 model. Targeting T-cell KCa3.1 and Kv1.3 channels might thus constitute a novel treatment strategy and safe alternative to standard CsA-therapy for the prevention of kidney allograft rejection. This hypothesis will in future need to be evaluated in more detail in longer-term animal trials.
Acknowledgments
This work was supported by grants from the Deutsche Forschungsgemeinschaft (SFB593/A11), the German Kidney Foundation, the University Medical Center Giessen and Marburg, and by NIH grant RO1 GM076063.
References
- 1.Leichtman AB. Balancing efficacy and toxicity in kidney-transplant immunosuppression. N Engl J Med. 2007;357:2625. doi: 10.1056/NEJMe078181. [DOI] [PubMed] [Google Scholar]
- 2.Kahan BD. Cyclosporine. N Engl J Med. 1989;321:1725. doi: 10.1056/NEJM198912213212507. [DOI] [PubMed] [Google Scholar]
- 3.Mihatsch MJ, Kyo M, Morozumi K, et al. The side-effects of ciclosporine-A and tacrolimus. Clin Nephrol. 1998;49:356. [PubMed] [Google Scholar]
- 4.Chandy KG, Wulff H, Beeton C, et al. K+ channels as targets for specific immunomodulation. Trends Pharmacol Sci. 2004;25:280. doi: 10.1016/j.tips.2004.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grissmer S, Dethlefs B, Wasmuth JJ, et al. Expression and chromosomal localization of a lymphocyte K+ channel gene. Proc Natl Acad Sci U S A. 1990;87:9411. doi: 10.1073/pnas.87.23.9411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Logsdon NJ, Kang J, Togo JA, et al. A novel gene, hKCa4, encodes the calcium-activated potassium channel in human T lymphocytes. J Biol Chem. 1997;272:32723. doi: 10.1074/jbc.272.52.32723. [DOI] [PubMed] [Google Scholar]
- 7.Cahalan MD, Wulff H, Chandy KG. Molecular properties and physiological roles of ion channels in the immune system. J Clin Immunol. 2001;21:235. doi: 10.1023/a:1010958907271. [DOI] [PubMed] [Google Scholar]
- 8.Wulff H, Beeton C, Chandy KG. Potassium channels as therapeutic targets for autoimmune disorders. Curr Opin Drug Discov Devel. 2003;6:640. [PubMed] [Google Scholar]
- 9.Feske S, Giltnane J, Dolmetsch R, et al. Gene regulation mediated by calcium signals in T lymphocytes. Nat Immunol. 2001;2:316. doi: 10.1038/86318. [DOI] [PubMed] [Google Scholar]
- 10.Lewis RS. Calcium signaling mechanisms in T lymphocytes. Annu Rev Immunol. 2001;19:497. doi: 10.1146/annurev.immunol.19.1.497. [DOI] [PubMed] [Google Scholar]
- 11.Price M, Lee SC, Deutsch C. Charybdotoxin inhibits proliferation and interleukin 2 production in human peripheral blood lymphocytes. Proc Natl Acad Sci U S A. 1989;86:10171. doi: 10.1073/pnas.86.24.10171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu L, Pennington M, Jiang Q, et al. Characterization of the functional properties of the voltage-gated potassium channel Kv1.3 in human CD4+ T lymphocytes. J Immunol. 2007;179:4563. doi: 10.4049/jimmunol.179.7.4563. [DOI] [PubMed] [Google Scholar]
- 13.Ghanshani S, Wulff H, Miller MJ, et al. Up-regulation of the IKCa1 potassium channel during T-cell activation. Molecular mechanism and functional consequences. J Biol Chem. 2000;275:37137. doi: 10.1074/jbc.M003941200. [DOI] [PubMed] [Google Scholar]
- 14.Wulff H, Calabresi PA, Allie R, et al. The voltage-gated Kv1.3 K(+) channel in effector memory T cells as new target for MS. J Clin Invest. 2003;111:1703. doi: 10.1172/JCI16921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beeton C, Wulff H, Barbaria J, et al. Selective blockade of T lymphocyte K(+) channels ameliorates experimental autoimmune encephalomyelitis, a model for multiple sclerosis. Proc Natl Acad Sci U S A. 2001;98:13942. doi: 10.1073/pnas.241497298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beeton C, Wulff H, Standifer NE, et al. Kv1.3 channels are a therapeutic target for T cell-mediated autoimmune diseases. Proc Natl Acad Sci US A. 2006;103:17414. doi: 10.1073/pnas.0605136103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fabre J, Lim SH, Morris PJ. Renal transplantation in the rat: details of a technique. Aust N Z J Surg. 1971;41:69. [PubMed] [Google Scholar]
- 18.Wulff H, Miller MJ, Hansel W, et al. Design of a potent and selective inhibitor of the intermediate-conductance Ca2+-activated K+ channel, IKCa1: a potential immunosuppressant. Proc Natl Acad Sci U S A. 2000;97:8151. doi: 10.1073/pnas.97.14.8151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lehr HA, Mankoff DA, Corwin D, et al. Application of photoshop-based image analysis to quantification of hormone receptor expression in breast cancer. J Histochem Cytochem. 1997;45:1559. doi: 10.1177/002215549704501112. [DOI] [PubMed] [Google Scholar]
- 20.DeCoursey TE, Chandy KG, Gupta S, et al. Voltage-gated K+ channels in human T lymphocytes: a role in mitogenesis? Nature. 1984;307:465. doi: 10.1038/307465a0. [DOI] [PubMed] [Google Scholar]
- 21.Valverde P, Kawai T, Taubman MA. Selective blockade of voltage-gated potassium channels reduces inflammatory bone resorption in experimental periodontal disease. J Bone Miner Res. 2004;19:155. doi: 10.1359/JBMR.0301213. [DOI] [PubMed] [Google Scholar]
- 22.Azam P, Sankaranarayanan A, Homerick D, et al. Targeting effector memory T cells with the small molecule Kv1.3 blocker PAP-1 suppresses allergic contact dermatitis. J Invest Dermatol. 2007;127:1419. doi: 10.1038/sj.jid.5700717. [DOI] [PMC free article] [PubMed] [Google Scholar]