Abstract
More than half of the cancer occurring today is preventable by applying knowledge that we already have. Tobacco, obesity, and physical inactivity are the modifiable causes of cancer that generate the most disease. Cancer burden can be reduced by alterations in individual and population behaviors and by public health efforts as long as these changes are driven by sound scientific knowledge and social commitment to change. The obstacles to these efforts are societal and arise from the organization of institutions, including academia, and in the habits of daily life. To achieve maximal possible cancer prevention, we will need better ways to implement what we know and improved infrastructure that will better incentivize and support transdisciplinary, multilevel research and successful intervention.
INTRODUCTION
For millennia, much of human cancer did not happen. In current terms, it was prevented by skin melanin, along with physical activity, diet, and other life-style habits of our ancestors. Humans did not live long, but they usually died of causes other than cancer. The sweeping transformation of daily life in the developed world and globally over the past 60 years, for both people and institutions, has driven up the burden of cancer. These changes, together with the increasing average age of our population, will double the number of cancer cases diagnosed annually by 2050 (1). The biggest single cause is tobacco, but there are many others.
Forty years ago, the United States declared war on cancer, promising a cure. In the years since, we have learned that there are many cancers, in different parts of the body, and we look almost entirely to medicine and its clinical and laboratory developments for relief from those cancers. Happily, the millions of Americans living after or with cancer testify to the many advances, but the ever-growing burden of cancer and other chronic illnesses is predicted to outstrip the national capacity to detect, treat, and follow up the millions affected (2).
Nevertheless, there are research-proven strategies and interventions that can prevent either a substantial proportion of cancer outright or a reoccurrence after diagnosis and treatment. These lie largely but not entirely in the realm of public health, with some participation from the medical, biotechnological, and nonmedical communities. But pursuing these prevention options currently requires navigating around obstacles, which takes time and reduces impact. These obstacles have obscured our view of how cancers form and progress and have led us to focus our attention and resources to less-than-optimal effect.
Here, we name obstacles that stand in the way of applying what we already know. We present examples of some successes, thereby offering glimpses of the power of these approaches. There is much more the United States and the world can be doing to prevent cancer. Right now.
OBSTACLE 1: SKEPTICISM THAT CANCER CAN BE PREVENTED
The causes of cancer may be classified as genetic or environmental (or both), and we understand much more about how cancer starts than we did 30 years ago. We have also learned effective strategies for applying this knowledge to reduce cancer risk, prevent disease, and advance population health. Moreover, we can now estimate how much cancer can be prevented through these approaches.
We no longer need to infer the magnitude of cancer prevention solely on the basis of international variation and migrant studies (3). Substantial longitudinal data, improved statistical methods, and evidence from interventions indicate that changes in behavior clearly reduce cancer incidence: Smoking cessation decreases lung cancer risk (4); selective estrogen receptor modulators (SERMs) like tamoxifen can prevent breast cancer (5); aspirin prevents colon cancer (6).
Estimates based on a broad range of scientific evidence indicate that more than 50% of cancers can be prevented (Table 1). Take for example the state of Utah, which at the population level has a low prevalence of current cigarette smoking, 9.8% overall in 2009. Contrast this with a smoking prevalence of more than 25% in Kentucky (7). On the basis of data from the National Program of Cancer Registries, the U.S. Centers for Disease Control (CDC) estimates that lung cancer mortality varied from 97.7 deaths per 100,000 in Kentucky to 26.4 per 100,000 in Utah, a 73% lower rate (even though almost 10% of Utah’s population continues to smoke cigarettes) (8). Thus, we conservatively estimate that 75% or more of lung cancer could be prevented through elimination of cigarette smoking in the United States.
Table 1.
Causes of cancer and potential reduction in cancer burden through preventive measures. N/A, not applicable.
| Cause* | Percentage of cancer caused |
Number of deaths in United States† |
Magnitude of possible reduction (%) |
Period of time (years) |
Evidence example |
|---|---|---|---|---|---|
| Smoking | 33 | 188,744 | 75 | 10–20 | Comparison of lung cancer mortality by state (Fig. 1) |
| Overweight and obesity | 20 | 114,390 | 50 | 2–20 | Bariatric surgery and sustained changes in weight and markers (62) |
| Diet | 5 | 28,600 | 50 | 5–20 | Folate and colorectal cancer (63) |
| Lack of exercise | 5 | 28,600 | 85 | 5–20 | Adolescent physical activity (18) |
| Occupation | 5 | 28,600 | 50 | 20–40 | Asbestos workplace regulation (10) |
| Viruses | 5 | 28,600 | 100 | 20–40 | Liver cancer reduction by vaccine (22) |
| Family history | 5 | 28,600 | 50 | 2–10 | Bilateral oophorectomy for BRCA1/2 (34); aspirin trial for Lynch syndrome (11) |
| Alcohol | 3 | 17,200 | 50 | 5–20 | Regulation (64) |
| UV and ionizing radiation | 2 | 11,400 | 50 | 5–40 | Reduced medical exposures (65) |
| Prescription drugs | 1 | 5,720 | 50 | 2–10 | Hormone therapy–related drop in breast cancer (66) |
| Reproductive factors | 3 | 17,200 | 0 | N/A | N/A |
| Pollution | 2 | 11,400 | 0 | N/A | N/A |
| Total potential reduction‡ = 54.5% | |||||
Studies of cancer in groups who change from one life-style to another upon immigration reveal large changes in cancer risk and a large potential for prevention. These studies, however, do not always address when in life the important behaviors or exposures (such as diet) are accrued, or even which life-style factors are important. Rapid changes in cancer incidence as populations experience industrialization, such as the soaring breast cancer rates in Korea (9), add further evidence that cancer rates can change in step with social changes and that, therefore, these changes can be reversed through behavioral preventive measures.
To estimate how much cancer could be avoided for each of the causes listed in Table 1, one must monitor the cancer incidence over time after the behavioral changes or intervention. The drop in incidence generally begins to appear relatively quickly but increases in magnitude over years. This long time course of beneficial outcomes requires data collection for years or decades, not at just one point soon after intervention. Benefits do not happen instantaneously but accrue over time. Humans are impatient, and that human trait itself is an obstacle to cancer prevention.
OBSTACLE 2: THE SHORT-TERM FOCUS OF CANCER RESEARCH
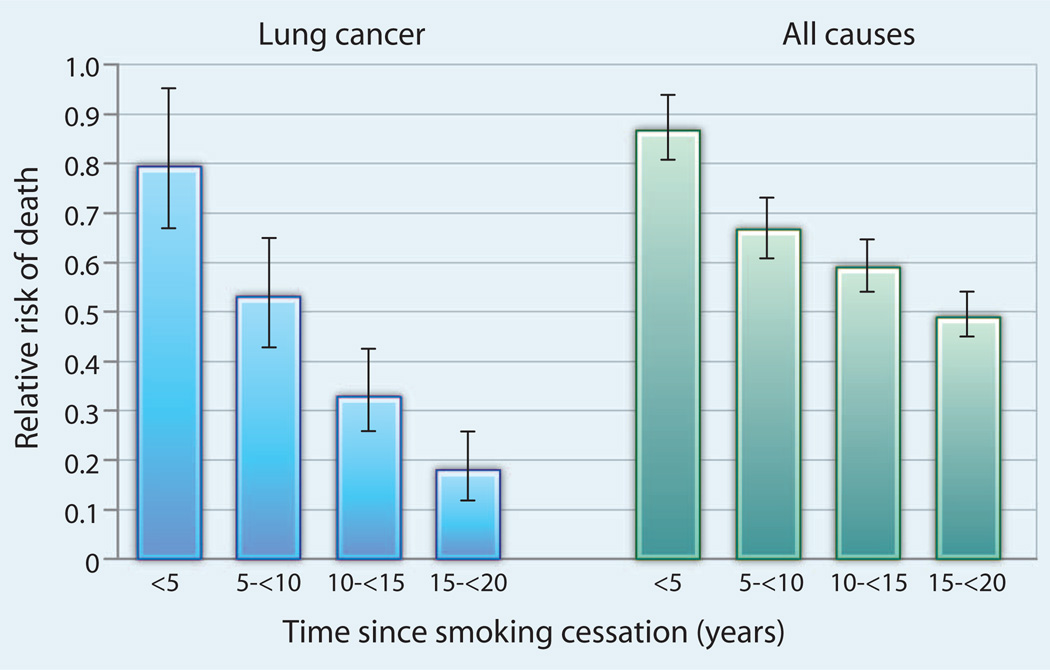
In Table 1, we provide estimates of the time required from a preventive action to achieve a decrease in cancer incidence or mortality. Many of the time periods are substantial. Smoking cessation powerfully reduces lung cancer and total mortality (Fig. 1). Compared to continuing smokers, those who successfully quit have only a 20% reduction in lung cancer mortality within 1 to 4 years of quitting, but this climbs to a 40% reduction within 5 to 9 years (4). Smoking cessation reduces total mortality by 13% in less than 5 years, and this increases to as much as 33% by 10 years, a result of the additional benefits of reduced risk of cardiovascular death and of death from other smoking-related cancers (4). The long-term benefits for smoking cessation are unarguable and substantial.
Fig. 1.
The risk of death decreases with time after smoking cessation. Reduction in mortality from lung cancer (left) and from all causes (right), according to time after cessation from smoking. Mortality reduction data (and 95% confidence intervals) are expressed as a fraction of the mortality risk of continuing smokers (set at 1.0). Adapted from (4).
For other cancers, it may also take decades to see the full benefit of preventative measures, in part because at the time of intervention some carcinogenic damage will already be in place for some people. Asbestos-related cancer in the United Kingdom is one such example. Regulation of workplace exposure was implemented in the 1970s, but most low-level exposure occurs in building trades that were not covered. Despite regulations, deaths will likely continue to rise until 2020 and only then will fall, a 40- to 50-year lag from policy implementation to reductions in population burden (10). Other examples are summarized in Table 1.
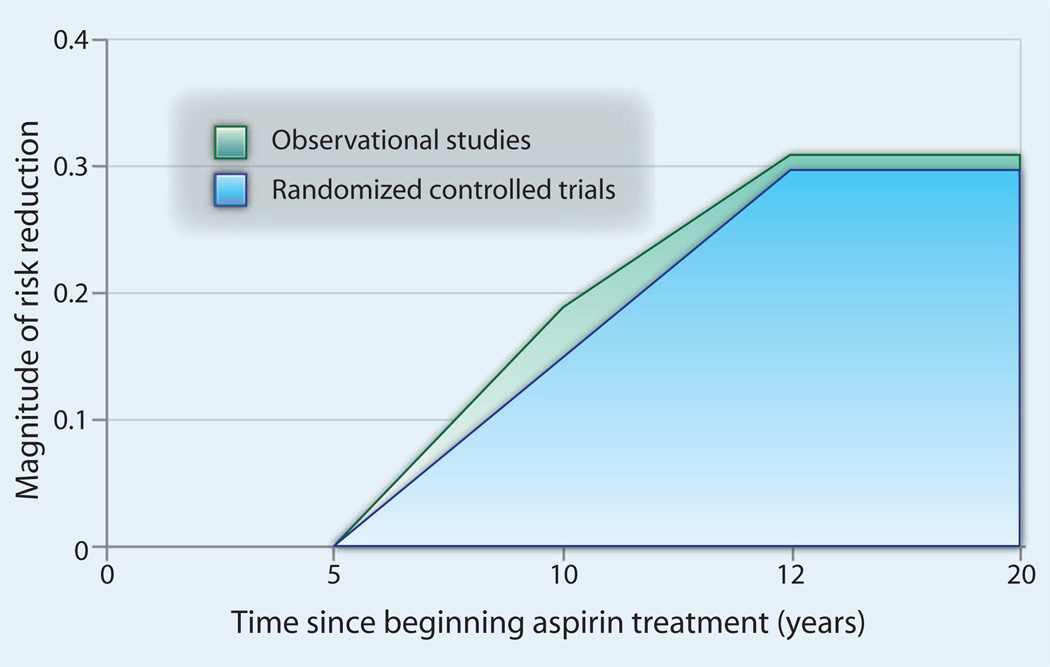
Given this reality, researchers looking for prevention effects may err by intervening too late in the disease process, expecting reductions in cancer over too short a follow-up time. The value of long-term follow-up is markedly illustrated by recent data from randomized clinical trials of aspirin. Using observational data techniques, investigators looked at cancer registry and death records to trace participants and noted that 5 years of aspirin use led to significant reductions in mortality from colon cancer 20 or more years later (6). These results are consistent with epidemiological data showing a 30% reduction in colorectal cancer at 20 years (Fig. 2). These data are confirmed in Lynch syndrome patients in whom no beneficial effect of aspirin was seen after 2 years but who experienced a reduced incidence of colorectal cancer after 5 years of follow-up (11). Thus, studies that focus on short-term exposures or short-term follow-up almost certainly will miss the true benefits of prevention. This highlights a major limitation of the current system of primary research funding, which typically lasts only a maximum of 5 years.
Fig. 2.
The beneficial effects of aspirin in reducing risk of colorectal cancer show a substantial lag. Data are expressed as a fraction of reduced risk compared to untreated individuals (set at 0). Adapted from randomized controlled trials (6) and observational studies (76).
Other interventions acting later in the process of cancer development (for example, screening) or through provision of medical services by health care providers provide other examples of prevention strategies. We can classify these as drugs (aspirin, SERMs), vaccines, and screening or surgical interventions. Although the time frame from intervention to benefit varies for different cancers, most of these medical interventions result in substantial benefits, and, typically, over the long run, the benefits outweigh the risks of exposing the population to medical procedures (Table 2). For example, we estimated that a large proportion of postmenopausal women would benefit from raloxifene (a SERM), and given its relative positive trade-off of benefits to risk, its widespread use would result in a substantial reduction in postmenopausal breast cancer (12). Likewise, for aspirin, which is recommended for men over 45 to reduce risk of cardiovascular disease and for women over 55 to reduce risk of cerebrovascular disease (13), the benefits for colon and other cancer risk reduction over 20 or more years of use are substantial (14). Perhaps the reduction in cancer is an unintended benefit of widespread use to reduce cardiovascular disease risk. Screening for colon cancer offers another population-wide benefit from a medical intervention—again with substantial delay from initiation of a program to observed benefit over a decade or more. More typically considered a population-wide intervention, vaccines require decades to reduce cancer incidence and need broad implementation to achieve their effects. Australia is among the countries that have embarked on such a traditional public health strategy for the human papillomavirus (HPV) vaccine to achieve reductions in the burden of cancer, and Taiwan has done the same with the hepatitis vaccine.
Table 2.
Medical interventions proven to prevent cancer. RCT, randomized controlled trial.
| Intervention | Target | Magnitude of reduction |
Period of time (years) |
Evidence |
|---|---|---|---|---|
| Aspirin | Total cancer mortality | 20% | 20 | Follow-up of eight RCTs (14) |
| Aspirin | Colon cancer mortality | 40% | 20 | Five RCTs (6) and RCT in Lynch syndrome (11) |
| SERMs (tamoxifen, raloxifene) | Breast cancer incidence | 40–50% | 5+ | RCT (5, 69) |
| Salpingo-oophorectomy | Familial breast cancer | 50% | 3+ | Synthesis of observational data (34) |
| Screening for colon cancer (sigmoidoscopy and colonoscopy) |
Colon cancer mortality | Sigmoidoscopy, 30–40% |
10 | UK RCT sigmoidoscopy (33) |
| Colonoscopy, 50% | Observational data and disease modeling (70, 71) |
|||
| Vaccines (HPV and hepatitis) | Cervical cancer incidence | 50–100% | 20+ | Modeling vaccination rates and persistence of protection (23) |
| Liver cancer incidence | 70–100% | Observational follow-up data from universal population vaccination program at birth (22, 72) |
||
| Mammography | Breast cancer mortality | 30% | 10–20 | RCT and modeling (73, 74) |
| Spiral computed tomography for lung cancer |
Lung cancer mortality | 20% | 6+ | RCT (75) |
The research on the time course of many cancers suggests that they develop over decades. Therefore, studies and interventions should be targeted at early stages of the human life span, but this rarely happens.
OBSTACLE 3: INTERVENTIONS DEPLOYED TOO LATE IN LIFE
Advances in genomics, long-term follow-up of atomic bomb survivors, and prevention studies all point to a long course for tumor development. We know that life-style habits are modifiable and that such change translates to reduced cancer risk, but a root question is whether we are intervening early enough in life to achieve benefits. For example, recent genomic studies of pancreatic cancer suggest that the timeline from the first cellular changes that initiate the tumor to the parental clone is 8 to 14 years, from the clone to subclones with metastatic potential 3 to 10 years, and a further 2 to 4 years to diagnosed metastatic disease (which is typically followed by death within a year) (15). Thus, pancreatic cancers form over 13 to 28 years.
The time course of pancreatic cancer development is similar to that of colon and cervical cancer, confirming that avoidance of early risk accumulation must occur 20 or more years before the projected onset of symptomatic disease. Retrospective case-control studies rarely assess data this long before diagnosis because subject recall is increasingly unreliable with longer times (16). Even the longest-running prospective studies have almost no data beyond 30 years after the original assessment of putative cancer-causing life-style and environmental factors. Indeed, there are few studies of adolescent and early-adult life-style and cancer risk, and they are lost in the preponderance of evaluations of adult diet, activity, and other risk factors. Thus, most of the available evidence on the causes of cancer is lacking data from the early stages of the relevant time course (17). Nevertheless, because some life-style behaviors are consistent over time, we can still glean some information about cancer prevention from the studies that have been done to date.
We know that behavioral changes and interventions early in the lives of young women reduce breast and cervical cancer, because these cancers develop over years and decades (Table 1). Physical activity, vaccination for viruses, and minimizing sun exposure are most effective when begun early in life, for young men as well as for young women.
More physical activity in women from the onset of menarche throughout the premenopausal years can reduce the risk of breast cancer by at least 25% (18). In contrast, alcohol intake (a known breast cancer carcinogen) during adolescence significantly increases the risk of benign breast disease, a precursor of breast cancer (19), while fiber intake during adolescence may substantially reduce breast cancer risk (20). These results mean that recent reductions to or elimination of school physical education and sport programs may increase breast cancer incidence, an effect that may not be evident for decades to come. This example illustrates why a younger, broader population than is typically considered to be at risk for cancer must be engaged to effect meaningful prevention.
Another environmental factor that can be modified early in life to prevent later cancers is infectious disease. Cancer resulting from infection accounts for ~5% of all cancer cases in the United States and in most established market economies; in low- and middle-income countries, these cancers account for a substantially larger percentage of the total. Cancer-causing infections with HPV (cervix), Helicobacter pylori (stomach), and hepatitis B and C (liver) together account for 18% of the global cancer burden (21). Already, vaccination against hepatitis has reduced liver cancer incidence by 70%, 20 years after initiation of the vaccination program, although protection into later adult life remains to be documented (22) (Table 2). Modeling indicates that HPV vaccination programs can reduce incidence of cervical cancer by 50 to 70%, depending on the level of vaccine coverage in the population (23).
Sun exposure also initiates cancer-promoting damage early in life. The sun, as the primary source of ultraviolet (UV) radiation, poses a significant risk of skin cancer, particularly but not exclusively in fair-skinned Caucasians. The U.S. National Cancer Institute (NCI) estimates that there will be 70,230 new cases in 2011 in the United States and, without prevention, this number will only increase as the protective ozone layer recedes. Prevention recommendations as simple as avoiding the sun in peak hours (10 a.m. to 3 p.m.), covering the skin whenever possible, protecting exposed skin with sunscreen, and avoiding tanning booths are effective in reducing skin cancer incidence, particularly if these life-style changes are adopted at an early age.
The effectiveness of prevention efforts such as those described above has been well documented. The CDC Task Force on Community Preventive Services concluded that school-based educational and policy interventions (for children) and recreational-based educational and policy interventions (for adults) were effective in reducing sun exposure (24). Community-scale urban design and land use policies, as well as street-scale design, may promote physical activity (25) Both types of intervention are exemplified by the Australian SunSmart (formerly Slip! Slop! Slap!) program, ongoing since 1982, which aims to reduce UV exposure through access to shade and consistent use of protective clothing, hats, sunglasses, and sunscreen (26). SunSmart efforts include formal accreditation of schools that adhere to its policy and practice requirements and collaboration with governmental agencies to protect outdoor workers.
SunSmart can be credited with successfully changing attitudes regarding sun tanning, increases in protective behavior, decreased costs of sun protection gear, societal acceptance of more protective attire (including hats, sunglasses, and neck-to-knee swimsuits for children), and, most important, decreasing incidence rates of skin cancer (26). The evidence from Australia indicates that active prevention efforts, including television advertising campaigns, can be highly effective in improving the population-wide sun-protective behaviors (27), resulting in falling age-specific incidence rates for melanoma in younger birth cohorts (28). In contrast, the United States fails even to enforce policies limiting preadolescent and adolescent tanning bed use (29).
Our now clear understanding of the years- to decades-long time frame of tumor development, which often starts early in life, should propel us to think anew about how we can organize and commit resources to markedly reduce the burden of cancer on individuals and society.
OBSTACLE 4: RESEARCH FOCUS ON TREATMENT, NOT PREVENTION
Only a small fraction of federal and nonfederal funding for cancer research goes to early detection and prevention; most is devoted to research on cancer treatment, cancer biology, and the causes of cancer (30). Translation from discovery to clinical application by commercial entities ensures the return of society’s collective investment in research. Less than 1.5% of total biomedical research funding (31) is devoted to health services and implementation of effective prevention programs. Accordingly, less attention is paid to the translation of scientific discovery to effective prevention and detection programs that will improve population health. In consequence, it is not surprising that most medical interventions for cancer—such as drugs, vaccines, and screening—only reduce mortality from cancer in a single organ. Interventions are tailored for a particular cancer in a particular part of the body, administered according to conventional medical specialties. The hunt for cancer drugs focuses on biologic processes in specific organ sites (32). In contrast, behavioral interventions such as smoking cessation or promotion of physical activity can diminish incidence and mortality of many types of cancer and of other chronic diseases while at the same time improving quality of life.
The U.S. health care system attends almost entirely to high-risk individuals. Much has been written on approaches to prevention through personalized medicine, particularly for those considered at risk as defined by pathways and markers, although seeking out individuals with a family history of a cancer for close monitoring predates molecular methods for cancer prevention. This approach has paid off through interventions delivered through the health care system such as SERMs that interrupt receptor signaling and significantly reduce breast cancer incidence (5), colon screening that removes premalignant polyps and reduces cancer incidence and mortality (33), and bilateral oophorectomy that reduces risk of breast cancer in high-risk women who carry a BRCA1 mutation (34) (Table 2). Yet, evidence is mounting that targeting built environment and life-style factors will even more significantly reduce cancer incidence, with the added benefit of reduced burden of other chronic diseases as well, expanding the benefit to society (35).
Rose, using heart disease as a model, emphasizes that the greatest benefit comes from wholesale shifting of population distributions of risk factors rather than focusing just on high-risk subsets of the population (36). This applies in breast cancer. For middle-aged women, only 25% of breast cancer arises from the 10% of the population most at risk, suggesting that only broadly applicable approaches will have a substantial impact on the other 75% of breast cancer, which is spread across the population among women with fewer risk factors (12). Evidence from more than 8500 breast cancer cases and 11,800 controls in the breast and prostate cancer cohort consortium shows that genetic changes in 17 germline single-nucleotide polymorphisms related to risk are independent of the life-style and reproductive factors that drive risk and that family history is not related to these genetic risk markers (37). Hence, the marginal benefit of identifying high-risk groups through genetics is trivial at a population level (38), and, therefore, we should promote population-wide changes, regardless of family patterns of inherited predisposition. A program that promotes physical activity for all women, particularly adolescent girls, would benefit all through exercise-induced modification of estrogen metabolism (18).
OBSTACLE 5: DEBATES AMONG SCIENTISTS
There is another source of inertia and delay that siphons off brainpower, time, and resources: the many disagreements among researchers over exactly how much of cancer is preventable, implying that we should wait to act until we are sure. However, such debates will never be resolved; they are the fiber of academic discourse and market claims. Different approaches come up with different numbers. For example, some have taken a rigorous quantitative approach that systematically reviews evidence (largely of exposures close to time of diagnosis) to estimate the magnitude of association of individual risk factors with cancer. Then, on the basis of the prevalence of exposure in a population, the proportion that might be prevented is derived, usually underestimating the contribution of causes of cancer (39, 40) due to misspecification of the magnitude of association between the cause and the cancer of interest. Arguments about the magnitude of attainable cancer prevention obscure the fact that we already know that more than half of cancers can be prevented. Each passing year leaves a substantially greater portion of the world population at risk for cancer, despite our current knowledge. We have a moral responsibility to act now and reduce that burden.
OBSTACLE 6: SOCIETAL FACTORS THAT AFFECT HEALTH OUTCOMES
Our discussion to this point has focused on life-style habits: what we eat, how we exercise, and which drugs, medications, and supplements we take. But life-style habits are not formed in a vacuum. Usually missing from discussions of life-style factors is a detailed breakdown of the social context and structural components of society that affect humans from birth. It is essential also to consider these societal factors—region of the country, economic class, occupation structure, education level, neighborhood configuration, and health care resources. These circumstances of living affect the choices that are available to individuals and can determine which choices they make (41). For example, these factors can make it difficult, if not impossible, to quit smoking and to practice sun safety (42). Poverty or stress during an individual’s developmental years influences cancer outcomes (43). Indeed, living in poverty—which is strongly related to nonnutritional diet, obesity, low educational attainment, and low health literacy—is associated with an increased risk of dying from mortality (44). Pollution and crime, poor public transportation, lack of parks for play and exercise, and absence of nearby supermarkets for fresh food hinder the adoption and sustained practice of a life-style that minimizes the risk of cancer and other diseases. As in other countries, social stratification in the United States exacerbates life-style differences such as access to health care, especially prevention and early detection services. Mammograms, colon screening, diet and nutrition support, smoking cessation resources, and sun protection mechanisms are simply less available to the poor (45). The substantial social imbalances of the United States promote these disparities (46, 47).
It does not matter how well a new treatment or drug works or how useful new health information is if people are unable to obtain them. Consider, for instance, that breast cancer diagnosis at later stages of disease, which is associated with poorer outcomes, is more common among women living in Chicago census tracts with a higher percentage of residents below the federal poverty line (48). Higher mortality among Chicago African-American women is associated with lower-quality mammography in medically underserved areas (49). Zeroing in on improving conditions and services in impoverished areas could quickly begin to reduce the disproportionate burden of cancer mortality among minority and lower socioeconomic status groups. A promising example is the 2009 Illinois Breast Cancer Act (PL95-1045), which eliminates co-payments for women on Medicaid who are diagnosed with breast cancer and incentivizes community clinics to improve the quality of their mammography by reimbursing them at Medicare rather than Medicaid rates (the former are three times higher in Illinois).
Government policies from local to federal levels also further skew the array of choices. For example, in school districts across the nation, physical education has been eliminated because of budget cuts. And agricultural and other food commodity lobbies ensure that salty, fat-filled cheese, high-fructose corn syrup, and corn-fed beef are prominent in food supplement programs and are subsidized in other ways (50). Fruits and vegetables receive no government subsidies and are therefore pricier for consumers. Yet, the nutrition-adding modifications to the Women, Infants and Children (WIC) program and the recent disappearance of trans fats from the national diet show that change is possible (51).
OBSTACLE 7: LACK OF TRANSDISCIPLINARY APPROACHES
As they mushroomed over the last 40 years, cancer treatment and research are divided into myriad specialties, multiple public and private funding streams, discrete research projects, and atomistic medical, public health, and scientific reporting. This proliferation is structured into what some have described as separate silos or bunkers. Because there is little or no communication among silos, there is little consensus among cancer-interested institutions and enterprises about the key questions that need to be answered to achieve the fullest impact possible, let alone how to achieve coordinated action.
Prevention efforts, research about their efficacy, and communication with the public must be as comprehensive, multifaceted, and unfolding over time as the disease itself (52). Successful multilevel, multisector research depends on scientists’ ability to visualize the manifold influences on cancer from society to the cell and to understand the complex interactions that produce worse outcomes for some groups (53). The benefits of transdisciplinary teams that cut across disciplines have been touted for decades—for example, the Institute of Medicine (IOM) recommended in 2006 that National Institutes of Health (NIH) and universities change funding and promotion systems to reward transdisciplinary science (54)—but few such teams have been successfully assembled. Moreover, for researchers and others, operating in this collaborative way is not intuitive. Instead, it requires training across departments, schools, and divisions early in the professional education process.
Absent fundamental organizational change, investigators will regress toward the mean of siloed research, which is familiar and routine. We must consider policy and structural change as a priority within the academy. One U.S. institution, the NIH, is beginning to address the growing urgency to bust silos through initiatives such as the Trans-disciplinary Research in Energetics and Cancer (TREC), funded by the NCI. TREC seeks to link previously separate fields—extending from animal models to the community to the epidemiology of obesity-related cancer burden. They fund groups of investigators who propose mutually informative studies of nutrition, physical activity, energy balance, obesity, and cancer, projects that previously would have operated in separate domains. These initiatives also often draw on other sources of funding that allow for cross-national inquiry and the inclusion of investigators such as urban architects (think pedestrian- and bicycle-friendly design, buildings with inviting stairs centrally located rather than elevators). Success requires that all participants have the opportunity and skills to communicate with one another, ideally bidirectionally.
Producing and sustaining positive change in the nation’s health also requires the engagement and participation of communities, as exemplified by the Illinois Breast Cancer Disparities Act designed to improve mammography standards. Yet, few sustained community-academic partnerships have succeeded, reflecting in part Richmond’s prediction in 1968 that, with NIH’s increased funding, clinical research centers, medical schools, and medical research would focus inward and ignore their community source populations, leading to research that is disconnected from the community and society as a whole (55).
Achieving integration requires mutual respect and two-way communication among academic researchers and community stakeholders working together throughout the research process, with a goal of a sustainable relationship. Communities are much more likely to engage in research if they judge the research to be useful to them and a priority. Cancer centers and academic-community networks can play an important role in coordinating relationships and ensuring that engagement with communities is respectful, meaningful, and effective.
Finally, the inattention to both transdisciplinarity and implementation of scientific knowledge is reflected in faculty governance procedures. Research universities are organized to produce “new knowledge” for discovery and innovation, not for implementation of that knowledge for the benefit of all society (53). In the United States, applied science traditionally has been the province of land-grant universities, which historically have had lower prestige. In research universities, which tend to set standards in biomedical fields, appointment and promotion committees continue to ascribe a lower status to the application of scientific advances to improve health care delivery and community well-being. Development of appointment and promotion guidelines that reward collaborative approaches to research is necessary for this aspect of research and practice to be fostered. In particular, engagement in policy is undervalued; yet policy change is essential for improving population health.
OBSTACLE 8: THE COMPLEXITY OF SUCCESSFUL IMPLEMENTATION
How do we alter policy and practice so that we as a society achieve the reduction in cancer burden that our scientific knowledge base shows is possible? A social strategy built on a scientific knowledge base is a key component of promoting public health, Richmond argues (56). Cancer prevention must be implemented through a coordinated strategy that operates through health care providers, through governmental regulations and policies, and through social and behavioral changes in individuals and communities. It must be sustained over a number of years to improve the health of large numbers of people. As we have discussed, our society and institutions are set up to make such change profoundly difficult. Nevertheless, it has happened before and can happen again.
Tobacco provides an example of successful implementation of multilevel change. Approaches targeting both the provider and the individual have been effective when executed as part of large-scale, state-driven programs such as those in California and Massachusetts. Funded by the California Tobacco Tax and Health Promotion Act of 1988, the California Tobacco Control Program (CTCP) was the first to use a comprehensive, social-norm approach to reducing tobacco use statewide. Despite spending from the tobacco industry in California that was more than five times greater than the total funding of the state tobacco control program, this multifaceted approach proved effective. It consisted of a combination of focused programmatic attention on countering pro-tobacco influences in the community plus regulations reducing secondhand smoke exposure and tobacco availability. The program decreased smoking-attributable cancer mortality in adults 35 years and older to a rate significantly lower than comparable U.S. rates (57).
Regulatory approaches to reduce smoking have been used successfully elsewhere in the United States. These include restriction on who can purchase tobacco products and where individuals can smoke, advertising bans, clear warnings and health information on labels (including pictorial representations of the detrimental effects of smoking), and price increases through taxation (58). These efforts at tobacco control are paying off, and lung cancer rates in the United States are declining among men at a rate of 2% per year (59). The decline in women’s rates will take longer to accrue because they began smoking in large numbers more recently.
The United States is not the only country using regulatory and other measures. The effectiveness of strict restrictions on advertisements and package labels highlighting the harmful effects of tobacco use has been demonstrated in Brazil, where the prevalence of smoking declined from 35% of adults in 1989 to 22% in 2003 after legislative action to reduce tobacco promotion (60). Although tobacco has been critical to Brazil’s economy, the Ministry of Health took multiple approaches to reducing tobacco use. In 2000, a law was enacted that restricted advertisement of tobacco products and prohibited sponsorship of, and merchandising at, cultural and sports events (61). Like the United States, Brazil requires poignant health warnings and images on all tobacco products.
There are many lessons to be gleaned from the successes of tobacco control. First, we should remember that there was a time when smoking was a widespread custom and an easy way to be socially accepted. Laws and ads supported, even promoted, the habit. Efforts to stop smoking began back in the 1960s, 50-plus years ago. The tobacco industry has been a formidable opponent. For the first decades, the spotlight was on smokers; even though tobacco is addictive, the tobacco industry could fight back by focusing on the rights of the smoker to make a choice. In 1980s, anti-smoking forces, supported by science, achieved growing awareness of the severely negative effects of secondhand smoke. The recognition of innocent victims—family members, flight attendants, bartenders, and servers—accelerated change. Damages awarded from successful litigation against the tobacco industry (the Flight Attendant Medical Research Institute, for example) provided funding from sources that did not have to be legislated or renewed, could not be diverted to other purposes, and lasted for many years.
Moreover, smoking is a discrete activity that is possible to differentiate from regular customs and habits and prohibit. The desired action is clear and simple to state: to stop smoking, to eliminate tobacco use. Compare this specificity to the urgent but more complicated societal imperative to eat more healthily and engage in more physical activity throughout life. One can easily see the challenges. But even in this realm, change can, and is, occurring.
CONCLUSION
Who is responsible for applying what we know about cancer prevention to society? Even after we set forth long-term goals for reducing cancer’s burden on society, accountability remains a formidable challenge. Are our public health schools, our medical schools and academic health centers, or our local health departments and administrative structures to be held to account? If yes, by whom? If no, where does this responsibility rest? Health care reform has begun to address these issues, but how will the accountability be implemented and who will decide when it has occurred? With policy changes independent of the science having so much potential influence, who is to be answerable for their successful enactment and implementation?
To achieve prevention of cancer and chronic diseases, we must ask and answer such questions. We must set forth targets that are achievable to sustain the political will and social change that can then accomplish a reduced cancer burden. We need methods for implementation and the resources of academic scientists and public health practitioners, and a means to integrate them.
Moving beyond the obstacles summarized here can speed cancer prevention to achieve maximum benefit for society. More than half of the 572,000 deaths from cancer in the United States in 2011 were caused by one of the key modifiable risk factors described here, and this figure does not even include the large number of people who died from other chronic diseases that can be prevented through the same prevention actions. Evidence-based strategies to effect individual and population behavior changes, public health efforts driven by sound knowledge, legislative support/backing, and social commitment have the potential to rapidly reduce the cancer incidence and mortality by more than 50% in the 21st century. An equally high proportion of cancer globally could be prevented with what we know now.
A renewed focus on and funding of the methods for implementation science and a commitment to an academic and public health infrastructure that supports transdisciplinary research and delivery are necessary to achieve our prevention potential. As the baby boom generation ages, their cancers are becoming clinically discernable. The doubling in cancer cases diagnosed annually and the associated escalating costs of care demand that we act now to reduce this cancer burden for them and the generations that follow. We must vigorously implement what we already know about preventing cancer.
Acknowledgments
We thank H. Dart and P. Cox for technical support.
Funding: NCI grants U54-153460, U54-155496, and P30CA091842 and the Foundation for Barnes-Jewish Hospital. G.A.C. is supported by an American Cancer Society Clinical Research Professorship.
Footnotes
Competing interests: The authors declare that they have no competing interests.
REFERENCES AND NOTES
- 1.Edwards BK, Howe HL, Ries LA, Thun MJ, Rosenberg HM, Yancik R, Wingo PA, Jemal A, Feigal EG. Annual report to the nation on the status of cancer, 1973–1999, featuring implications of age and aging on U.S. cancer burden. Cancer. 2002;94:2766–2792. doi: 10.1002/cncr.10593. [DOI] [PubMed] [Google Scholar]
- 2.Sullivan R, Peppercorn J, Sikora K, Zalcberg J, Meropol NJ, Amir E, Khayat D, Boyle P, Autier P, Tannock IF, Fojo T, Siderov J, Williamson S, Camporesi S, McVie JG, Purushotham AD, Naredi P, Eggermont A, Brennan MF, Steinberg ML, De Ridder M, McCloskey SA, Verellen D, Roberts T, Storme G, Hicks RJ, Ell PJ, Hirsch BR, Carbone DP, Schulman KA, Catchpole P, Taylor D, Geissler J, Brinker NG, Meltzer D, Kerr D, Aapro M. Delivering affordable cancer care in high-income countries. Lancet Oncol. 2011;12:933–980. doi: 10.1016/S1470-2045(11)70141-3. [DOI] [PubMed] [Google Scholar]
- 3.Doll R, Peto R. The Causes of Cancer: Quantitative Estimates of Avoidable Risks of Cancer in the United States Today. New York: Oxford Univ. Press; 1981. [PubMed] [Google Scholar]
- 4.Kenfield SA, Stampfer MJ, Rosner BA, Colditz GA. Smoking and smoking cessation in relation to mortality in women. JAMA. 2008;299:2037–2047. doi: 10.1001/jama.299.17.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM, Vogel V, Robidoux A, Dimitrov N, Atkins J, Daly M, Wieand S, Tan-Chiu E, Ford L, Wolmark N. Tamoxifen for prevention of breast cancer: Report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J. Natl. Cancer Inst. 1998;90:1371–1388. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 6.Rothwell PM, Wilson M, Elwin CE, Norrving B, Algra A, Warlow CP, Meade TW. Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet. 2010;376:1741–1750. doi: 10.1016/S0140-6736(10)61543-7. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention (CDC), State-specific prevalence of cigarette smoking and smokeless tobacco use among adults—United States, 2009. MMWR Morb. Mortal. Wkly. Rep. 2010;59:1400–1406. [PubMed] [Google Scholar]
- 8.National Program of Cancer Registries. 2011 [Google Scholar]
- 9.Jung YS, Na KY, Kim KS, Ahn SH, Lee SJ, Park HK, Cho YU. Nation-wide Korean breast cancer data from 2008 using the breast cancer registration program. J. Breast Cancer. 2011;14:229–236. doi: 10.4048/jbc.2011.14.3.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peto J, Hodgson JT, Matthews FE, Jones JR. Continuing increase in mesothelioma mortality in Britain. Lancet. 1995;345:535–539. doi: 10.1016/s0140-6736(95)90462-x. [DOI] [PubMed] [Google Scholar]
- 11.Burn J, Gerdes AM, Macrae F, Mecklin JP, Moeslein G, Olschwang S, Eccles D, Evans DG, Maher ER, Bertario L, Bisgaard ML, Dunlop MG, Ho JW, Hodgson SV, Lindblom A, Lubinski J, Morrison PJ, Murday V, Ramesar R, Side L, Scott RJ, Thomas HJ, Vasen HF, Barker G, Crawford G, Elliott F, Movahedi M, Pylvanainen K, Wijnen JT, Fodde R, Lynch HT, Mathers JC, Bishop DT. CAPP2 Investigators, Long-term effect of aspirin on cancer risk in carriers of hereditary colorectal cancer: An analysis from the CAPP2 randomised controlled trial. Lancet. 2011;378:2081–2087. doi: 10.1016/S0140-6736(11)61049-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen WY, Rosner B, Colditz GA. Moving forward with breast cancer prevention. Cancer. 2007;109:2387–2391. doi: 10.1002/cncr.22711. [DOI] [PubMed] [Google Scholar]
- 13.U.S. Preventive Services Task Force, Aspirin for the prevention of cardiovascular disease. U.S. Preventive Services Task Force recommendation statement. Ann. Intern. Med. 2009;150:396–404. doi: 10.7326/0003-4819-150-6-200903170-00008. [DOI] [PubMed] [Google Scholar]
- 14.Rothwell PM, Fowkes FG, Belch JF, Ogawa H, Warlow CP, Meade TW. Effect of daily aspirin on long-term risk of death due to cancer: Analysis of individual patient data from randomised trials. Lancet. 2011;377:31–41. doi: 10.1016/S0140-6736(10)62110-1. [DOI] [PubMed] [Google Scholar]
- 15.Yachida S, Jones S, Bozic I, Antal T, Leary R, Fu B, Kamiyama M, Hruban RH, Eshleman JR, Nowak MA, Velculescu VE, Kinzler KW, Vogelstein B, Iacobuzio-Donahue CA. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature. 2010;467:1114–1117. doi: 10.1038/nature09515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Willett W. In: Nutritional Epidemiology. Willett W, editor. New York: Oxford Univ. Press; 1998. [Google Scholar]
- 17.Wei EK, Wolin KY, Colditz GA. Time course of risk factors in cancer etiology and progression. J. Clin. Oncol. 2010;28:4052–4057. doi: 10.1200/JCO.2009.26.9324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maruti SS, Willett WC, Feskanich D, Rosner B, Colditz GA. A prospective study of age-specific physical activity and premenopausal breast cancer. J. Natl. Cancer Inst. 2008;100:728–737. doi: 10.1093/jnci/djn135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berkey CS, Willett WC, Frazier AL, Rosner B, Tamimi RM, Rockett HR, Colditz GA. Prospective study of adolescent alcohol consumption and risk of benign breast disease in young women. Pediatrics. 2010;125:e1081–e1087. doi: 10.1542/peds.2009-2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Su X, Tamimi RM, Collins LC, Baer HJ, Cho E, Sampson L, Willett WC, Schnitt SJ, Connolly JL, Rosner BA, Colditz GA. Intake of fiber and nuts during adolescence and incidence of proliferative benign breast disease. Cancer Causes Control. 2010;21:1033–1046. doi: 10.1007/s10552-010-9532-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int. J. Cancer. 2006;118:3030–3044. doi: 10.1002/ijc.21731. [DOI] [PubMed] [Google Scholar]
- 22.Chang MH, You SL, Chen CJ, Liu CJ, Lee CM, Lin SM, Chu HC, Wu TC, Yang SS, Kuo HS, Chen DS Taiwan Hepatoma Study Group. Decreased incidence of hepatocellular carcinoma in hepatitis B vaccinees: A 20-year follow-up study. J. Natl. Cancer Inst. 2009;101:1348–1355. doi: 10.1093/jnci/djp288. [DOI] [PubMed] [Google Scholar]
- 23.Bogaards JA, Coupé VM, Xiridou M, Meijer CJ, Wallinga J, Berkhof J. Long-term impact of human papillomavirus vaccination on infection rates, cervical abnormalities, and cancer incidence. Epidemiology. 2011;22:505–515. doi: 10.1097/EDE.0b013e31821d107b. [DOI] [PubMed] [Google Scholar]
- 24.Saraiya M, Glanz K, Briss P, Nichols P, White C, Das D. Task Force on Community Preventive Services on reducing Exposure to Ultraviolet Light, Preventing skin cancer: Findings of the Task Force on Community Preventive Services on reducing Exposure to Ultraviolet Light. MMWR Recomm. Rep. 2003;52:1–12. [PubMed] [Google Scholar]
- 25.Heath GW, Brownson RC, Kruger J, Miles KE, Powell KE, Ramsey LT. Task Force on Community Preventive Services, The effectiveness of urban design and land use and transport policies and practices to increase physical activity: A systematic review. J. Phys. Act. Health. 2006;3(Suppl. 1):S55–S76. doi: 10.1123/jpah.3.s1.s55. [DOI] [PubMed] [Google Scholar]
- 26.Montague M, Borland R, Sinclair C. Slip! Slop! Slap! and SunSmart: 1980–2000: Skin cancer control and 20 years of population-based campaigning. Health Educ. Behav. 2001;28:290–305. doi: 10.1177/109019810102800304. [DOI] [PubMed] [Google Scholar]
- 27.Dobbinson SJ, Wakefield MA, Jamsen KM, Herd NL, Spittal MJ, Lipscomb JE, Hill DJ. Weekend sun protection and sunburn in Australia trends (1987–2002) and association with SunSmart television advertising. Am. J. Prev. Med. 2008;34:94–101. doi: 10.1016/j.amepre.2007.09.024. [DOI] [PubMed] [Google Scholar]
- 28.Hill D, Marks R. Health promotion programs for melanoma prevention: Screw or spring? Arch. Dermatol. 2008;144:538–540. doi: 10.1001/archderm.144.4.538. [DOI] [PubMed] [Google Scholar]
- 29.Forster JL, Lazovich D, Hickle A, Sorensen G, Demierre MF. Compliance with restrictions on sale of indoor tanning sessions to youth in Minnesota and Massachusetts. J. Am. Acad. Dermatol. 2006;55:962–967. doi: 10.1016/j.jaad.2006.06.036. [DOI] [PubMed] [Google Scholar]
- 30.Kolata G. New York Times. 2009 [Google Scholar]
- 31.Moses H, III, Dorsey ER, Matheson DH, Thier SO. Financial anatomy of biomedical research. JAMA. 2005;294:1333–1342. doi: 10.1001/jama.294.11.1333. [DOI] [PubMed] [Google Scholar]
- 32.Dancey JE, Monzon J. Ridaforolimus: A promising drug in the treatment of soft-tissue sarcoma and other malignancies. Future Oncol. 2011;7:827–839. doi: 10.2217/fon.11.57. [DOI] [PubMed] [Google Scholar]
- 33.Atkin WS, Edwards R, Kralj-Hans I, Wooldrage K, Hart AR, Northover JM, Parkin DM, Wardle J, Duffy SW, Cuzick J. UK Flexible Sigmoidoscopy Trial Investigators, Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: A multicentre randomised controlled trial. Lancet. 2010;375:1624–1633. doi: 10.1016/S0140-6736(10)60551-X. [DOI] [PubMed] [Google Scholar]
- 34.Rebbeck TR, Kauff ND, Domchek SM. Meta-analysis of risk reduction estimates associated with risk-reducing salpingo-oophorectomy in BRCA1 or BRCA2 mutation carriers. J. Natl. Cancer Inst. 2009;101:80–87. doi: 10.1093/jnci/djn442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khan LK, Sobush K, Keener D, Goodman K, Lowry A, Kakietek J, Zaro S. Centers for Disease Control and Prevention, Recommended community strategies and measurements to prevent obesity in the United States. MMWR Recomm. Rep. 2009;58:1–26. [PubMed] [Google Scholar]
- 36.Rose G. Strategy of prevention: Lessons from cardiovascular disease. Br. Med. J. (Clin. Res. Ed.) 1981;282:1847–1851. doi: 10.1136/bmj.282.6279.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Campa D, Kaaks R, Le Marchand L, Haiman CA, Travis RC, Berg CD, Buring JE, Chanock SJ, Diver WR, Dostal L, Fournier A, Hankinson SE, Henderson BE, Hoover RN, Isaacs C, Johansson M, Kolonel LN, Kraft P, Lee IM, McCarty CA, Overvad K, Panico S, Peeters PH, Riboli E, Sanchez MJ, Schumacher FR, Skeie G, Stram DO, Thun MJ, Trichopoulos D, Zhang S, Ziegler RG, Hunter DJ, Lindstrom S, Canzian F. Interactions between genetic variants and breast cancer risk factors in the breast and prostate cancer cohort consortium. J. Natl. Cancer Inst. 2011;103:1252–1263. doi: 10.1093/jnci/djr265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pharoah PD, Antoniou AC, Easton DF, Ponder BA. Polygenes, risk prediction, and targeted prevention of breast cancer. N. Engl. J. Med. 2008;358:2796–2803. doi: 10.1056/NEJMsa0708739. [DOI] [PubMed] [Google Scholar]
- 39.Danaei G, Vander Hoorn S, Lopez AD, Murray CJ, Ezzati M. Comparative Risk Assessment collaborating group (Cancers), Causes of cancer in the world: Comparative risk assessment of nine behavioural and environmental risk factors. Lancet. 2005;366:1784–1793. doi: 10.1016/S0140-6736(05)67725-2. [DOI] [PubMed] [Google Scholar]
- 40.Parkin DM, Boyd L, Walker LC. The fraction of cancer attributable to lifestyle and environmental factors in the UK in 2010. Br. J. Cancer. 2011;105(Suppl. 2):S77–S81. doi: 10.1038/bjc.2011.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Warnecke RB, Oh A, Breen N, Gehlert S, Paskett E, Tucker KL, Lurie N, Rebbeck T, Goodwin J, Flack J, Srinivasan S, Kerner J, Heurtin-Roberts S, Abeles R, Tyson FL, Patmios G, Hiatt RA. Approaching health disparities from a population perspective: The National Institutes of Health Centers for Population Health and Health Disparities. Am. J. Public Health. 2008;98:1608–1615. doi: 10.2105/AJPH.2006.102525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Honjo K, Tsutsumi A, Kawachi I, Kawakami N. What accounts for the relationship between social class and smoking cessation? Results of a path analysis. Soc. Sci. Med. 2006;62:317–328. doi: 10.1016/j.socscimed.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 43.Hiatt RA, Haslam SZ, Osuch J. Breast Cancer and the Environment Research Centers, The breast cancer and the environment research centers: Transdisciplinary research on the role of the environment in breast cancer etiology. Environ. Health Perspect. 2009;117:1814–1822. doi: 10.1289/ehp.0800120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kogevinas M, Pearce N, Susser M, Boffetta P. Social Inequalities and Cancer. vol. 138. Lyon: International Agency for Research on Cancer; 1997. [Google Scholar]
- 45.Henry KA, Boscoe FP, Johnson CJ, Goldberg DW, Sherman R, Cockburn M. Breast cancer stage at diagnosis: Is travel time important? J. Community Health. 2011;36:933–942. doi: 10.1007/s10900-011-9392-4. [DOI] [PubMed] [Google Scholar]
- 46.Satcher D, Fryer GE, Jr, McCann J, Troutman A, Woolf SH, Rust G. What if we were equal? A comparison of the black-white mortality gap in 1960 and 2000. Health Aff. 2005;24:459–464. doi: 10.1377/hlthaff.24.2.459. [DOI] [PubMed] [Google Scholar]
- 47.Woolf SH, Johnson RE, Fryer GE, Jr, Rust G, Satcher D. The health impact of resolving racial disparities: An analysis of US mortality data. Am. J. Public Health. 2004;94:2078–2081. doi: 10.2105/ajph.94.12.2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Campbell RT, Li X, Dolecek TA, Barrett RE, Weaver KE, Warnecke RB. Economic, racial and ethnic disparities in breast cancer in the US: Towards a more comprehensive model. Health Place. 2009;15:855–864. doi: 10.1016/j.healthplace.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Whitman S, Ansell D, Orsi J, Francois T. The racial disparity in breast cancer mortality. J. Community Health. 2011;36:588–596. doi: 10.1007/s10900-010-9346-2. [DOI] [PubMed] [Google Scholar]
- 50.Watzman N. Sunlight Foundation Reporting Group. vol. 2012. Washington, DC: Sunlight Foundation; 2012. [Google Scholar]
- 51.Angell SY, Silver LD, Goldstein GP, Johnson CM, Deitcher DR, Frieden TR, Bassett MT. Cholesterol control beyond the clinic: New York City’s trans fat restriction. Ann. Intern. Med. 2009;151:129–134. doi: 10.7326/0003-4819-151-2-200907210-00010. [DOI] [PubMed] [Google Scholar]
- 52.Viswanath K. Science and society: The communications revolution and cancer control. Nat. Rev. Cancer. 2005;5:828–835. doi: 10.1038/nrc1718. [DOI] [PubMed] [Google Scholar]
- 53.Gehlert S, Colditz GA. Cancer disparities: Unmet challenges in the elimination of disparities. Cancer Epidemiol. Biomarkers Prev. 2011;20:1809–1814. doi: 10.1158/1055-9965.EPI-11-0628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hernandez L, Blazer D. Genes, Behavior, and the Social Environment: Moving Beyond the Nature/Nurture Debate. Washington, DC: National Academies Press; 2006. [PubMed] [Google Scholar]
- 55.Richmond J. Currents in American Medicine: A Developmental View of Medical Care and Education. Cambridge, MA: Harvard Univ. Press; 1969. [Google Scholar]
- 56.Richmond J, Kotelchuck M. In: Oxford Textbook of Public Health. Holland W, Detel R, Know G, editors. vol. 1. Oxford: Oxford Univ. Press; 1991. [Google Scholar]
- 57.California Tobacco Control Update 2006. Sacramento, CA: CDHS/TCS; 2006. Tobacco Control Section, California Department of Health Services. [Google Scholar]
- 58.Tworek C, Yamaguchi R, Kloska DD, Emery S, Barker DC, Giovino GA, O’Malley PM, Chaloupka FJ. State-level tobacco control policies and youth smoking cessation measures. Health Policy. 2010;97:136–144. doi: 10.1016/j.healthpol.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Edwards BK, Ward E, Kohler BA, Eheman C, Zauber AG, Anderson RN, Jemal A, Schymura MJ, Lansdorp-Vogelaar I, Seeff LC, van Ballegooijen M, Goede SL, Ries LA. Annual report to the nation on the status of cancer, 1975–2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116:544–573. doi: 10.1002/cncr.24760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Monteiro CA, Cavalcante TM, Moura EC, Claro RM, Szwarcwald CL. Population-based evidence of a strong decline in the prevalence of smokers in Brazil (1989–2003) Bull. World Health Organ. 2007;85:527–534. doi: 10.2471/BLT.06.039073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Iglesias R, Jha P, Pinto M, daCosta e Silva V, Godinho J. Tobacco Control in Brazil. Washington, DC: The International Bank for Reconstruction and Development, The World Bank; 2007. [Google Scholar]
- 62.Chang SH, Stoll CR, Colditz GA. Cost-effectiveness of bariatric surgery: Should it be universally available? Maturitas. 2011;69:230–238. doi: 10.1016/j.maturitas.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 63.Lee JE, Willett WC, Fuchs CS, Smith-Warner SA, Wu K, Ma J, Giovannucci E. Folate intake and risk of colorectal cancer and adenoma: Modification by time. Am. J. Clin. Nutr. 2011;93:817–825. doi: 10.3945/ajcn.110.007781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Room R, Babor T, Rehm J. Alcohol and public health. Lancet. 2005;365:519–530. doi: 10.1016/S0140-6736(05)17870-2. [DOI] [PubMed] [Google Scholar]
- 65.Brenner DJ, Hricak H. Radiation exposure from medical imaging: Time to regulate? JAMA. 2010;304:208–209. doi: 10.1001/jama.2010.973. [DOI] [PubMed] [Google Scholar]
- 66.Colditz GA. Decline in breast cancer incidence due to removal of promoter: Combination estrogen plus progestin. Breast Cancer Res. 2007;9:108. doi: 10.1186/bcr1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wolin KY, Carson K, Colditz GA. Obesity and cancer. Oncologist. 2010;15:556–565. doi: 10.1634/theoncologist.2009-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cancer Facts & Figures 2011. Atlanta: American Cancer Society; 2011. American Cancer Society. [Google Scholar]
- 69.Martino S, Cauley JA, Barrett-Connor E, Powles TJ, Mershon J, Disch D, Secrest RJ, Cummings SR. CORE Investigators, Continuing outcomes relevant to Evista: Breast cancer incidence in postmenopausal osteoporotic women in a randomized trial of raloxifene. J. Natl. Cancer Inst. 2004;96:1751–1761. doi: 10.1093/jnci/djh319. [DOI] [PubMed] [Google Scholar]
- 70.Kavanagh AM, Giovannucci EL, Fuchs CS, Colditz GA. Screening endoscopy and risk of colorectal cancer in United States men. Cancer Causes Control. 1998;9:455–462. doi: 10.1023/a:1008884021049. [DOI] [PubMed] [Google Scholar]
- 71.Frazier AL, Colditz GA, Fuchs CS, Kuntz KM. Cost-effectiveness of screening for colorectal cancer in the general population. JAMA. 2000;284:1954–1961. doi: 10.1001/jama.284.15.1954. [DOI] [PubMed] [Google Scholar]
- 72.Chang MH, Chen CJ, Lai MS, Hsu HM, Wu TC, Kong MS, Liang DC, Shau WY, Chen DS. Universal hepatitis B vaccination in Taiwan and the incidence of hepatocellular carcinoma in children. Taiwan Childhood Hepatoma Study Group. N. Engl. J. Med. 1997;336:1855–1859. doi: 10.1056/NEJM199706263362602. [DOI] [PubMed] [Google Scholar]
- 73.Berry DA, Cronin KA, Plevritis SK, Fryback DG, Clarke L, Zelen M, Mandelblatt JS, Yakovlev AY, Habbema JD, Feuer EJ. Cancer Intervention and Surveillance Modeling Network (CISNET) Collaborators, Effect of screening and adjuvant therapy on mortality from breast cancer. N. Engl. J. Med. 2005;353:1784–1792. doi: 10.1056/NEJMoa050518. [DOI] [PubMed] [Google Scholar]
- 74.Mandelblatt JS, Cronin KA, Bailey S, Berry DA, de Koning HJ, Draisma G, Huang H, Lee SJ, Munsell M, Plevritis SK, Ravdin P, Schechter CB, Sigal B, Stoto MA, Stout NK, van Ravesteyn NT, Venier J, Zelen M, Feuer EJ. Breast Cancer Working Group of the Cancer Intervention and Surveillance Modeling Network, Effects of mammography screening under different screening schedules: Model estimates of potential benefits and harms. Ann. Intern. Med. 2009;151:738–747. doi: 10.1059/0003-4819-151-10-200911170-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.National Lung Screening Trial Research Team. Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, Gareen IF, Gatsonis C, Marcus PM, Sicks JD. Reduced lung-cancer mortality with low-dose computed tomographic screening. N. Engl. J. Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang X, Smith-Warner SA, Chan AT, Wu K, Spiegelman D, Fuchs CS, Willett WC, Giovannucci EL. Aspirin use, body mass index, physical activity, plasma C-peptide, and colon cancer risk in US health professionals. Am. J. Epidemiol. 2011;174:459–467. doi: 10.1093/aje/kwr115. [DOI] [PMC free article] [PubMed] [Google Scholar]