Abstract
Visual neglect is a multi-component syndrome including prominent attentional disorders. Research on the functional mechanisms of neglect is now moving from the description of dissociations in patients' performance to the identification of the possible component deficits and of their interaction with compensatory strategies. In recent years, the dissection of attentional deficits in neglect has progressed in parallel with increasing comprehension of the anatomy and function of large-scale brain networks implicated in attentional processes. This review focuses on the anatomy and putative functions of attentional circuits in the brain, mainly subserved by fronto-parietal networks, with a peculiar although not yet completely elucidated role for the right hemisphere. Recent results are discussed concerning the influence of a non-spatial attentional function, phasic alertness, on conscious perception in normal participants and on conflict resolution in neglect patients. The rapid rate of expansion of our knowledge of these systems raises hopes for the development of effective strategies to improve the functioning of the attentional networks in brain-damaged patients.
Keywords: attention, neglect, consciousness, parietal lobe, frontal lobe
Taxonomies of attentional processes
Biological organisms live in an environment cluttered with a multitude of objects. To behave in a coherent and goal-driven way, organisms need to select stimuli appropriate to their goals. On the other hand, because of capacity limitations, they must be capable of ignoring other, less important objects. Thus, objects in the world compete for recruiting the organism's attention in order to be the focus of the organism's subsequent behavior. Neural mechanisms of attention resolve this competition by taking into account both the goals of the organisms and the salience of the sensorial stimuli (Desimone and Duncan, 1995). However, attention and its neural correlates cannot be subsumed under a single concept. Attentional phenomena consist of a set of distinct, though interacting, neurocognitive mechanisms. For example, Parasuraman (1998) identified at least three independent but interacting components of attention: (1) selection, that is, mechanisms determining more extensive processing of some input rather than another; (2) vigilance, the capacity of sustaining attention over time; (3) control, the ability of planning and coordinating different activities. The concept of spatial selective attention refers operationally to the advantage in speed and accuracy of processing for objects lying in attended regions of space as compared to objects located in non-attended regions (Posner, 1980). In ecological settings, agents usually orient toward important stimuli by turning their gaze, head and trunk toward them (Sokolov, 1963). This is done in order to align the stimulus with the part of the sensory surface with highest resolution (e.g., the retinal fovea). This allows further perceptual processing of the detected stimulus, for example its classification as a useful or as a dangerous object. Even very simple artificial organisms display orienting behavior when their processing resources are insufficient to process the whole visual scene in parallel (Di Ferdinando et al., 2007).
Spatial selective attention must allow an organism to successfully cope with a continuously changing environment, while maintaining its goals. This flexibility calls for mechanisms that (A) allow for the processing of novel, unexpected events, that could be either advantageous or dangerous, in order to respond appropriately with either approaching or avoidance behavior; (B) allow for the maintenance of finalized behavior in spite of distracting events (Allport, 1989). For example, attention can be directed to an object in space either in a relatively reflexive way (e.g., when a honking car attracts the attention of a pedestrian) or in a more controlled mode (e.g., when the pedestrian monitors the traffic light waiting for the “go” signal to appear). It is thereby plausible that different attentional processes serve these two partially conflicting goals. A traditional distinction in experimental psychology refers to more exogenous processes for orienting attention to novel events (Yantis, 1995), as opposed to more endogenous orienting processes, which would be responsible for directing the organism's attention toward relevant targets despite the presence of distractors in the environment (Laberge et al., 2000). A further important notion concerns the fact that attention can not only be directed to a region of space, but also (and perhaps more importantly) to visual objects in space (Egly et al., 1994; Valdes-Sosa et al., 1998). Exogenous attention directed on an object part automatically spreads to the entire object (Macquistan, 1997).
Posner and Petersen (1990) have further refined the taxonomy of attention by proposing to distinguish the orienting processes of spatial attention from alerting and executive control. Executive control requires both monitoring and conflict solving, such as in flanker paradigms, where participants have to respond to targets while inhibiting the processing of adjacent flankers (Eriksen and Eriksen, 1974). Alerting mechanisms prepare the system for fast reactions by means of a change in the internal state, sometimes at the expense of motor control (Posner and Petersen, 1990; Callejas et al., 2005). Two types of alerting have been described: tonic alerting refers to a sustained activation over a period of several minutes, whereas phasic alerting refers to a non-specific activation occurring when a warning signal is presented a few hundred milliseconds prior to a target (Sturm and Willmes, 2001; Callejas et al., 2005).
Architecture of attentional circuits in the brain
Today, we know a fair amount of detailed information on the anatomy, functions, dynamics, and pathology of the brain networks that subserve the orienting of gaze and attention in the human brain1. Important components of these networks include the dorsolateral prefrontal cortex (PFC) and the posterior parietal cortex (PPC). Physiological studies indicate that these two structures show interdependence of neural activity. In the monkey, analogous PPC and PFC areas show coordinated activity when the animal selects a visual stimulus as a saccade target (Buschman and Miller, 2007). Importantly, PFC and PPC show distinctive dynamics and seem to use two different “languages” when attention is selected by the stimulus (bottom-up or exogenous orienting) or when it is directed by more top-down (or endogenous) goals. In particular, bottom-up signals appear first in the parietal cortex and are characterized by an increase of fronto-parietal coherence in the gamma band (25–100 Hz), whereas top-down signals emerge first in the frontal cortex and tend to synchronize in the beta band (12–30 Hz) (Buschman and Miller, 2007).
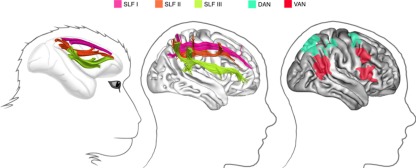
Functional MRI studies in healthy human participants (reviewed by Corbetta and Shulman, 2002) indicate the existence of multiple fronto-parietal networks for spatial attention (Figure 1, right panel).
Figure 1.
Fronto-parietal networks linked by the three branches of the superior longitudinal fasciculus. Left: in the monkey brain (from Schmahmann and Pandya, 2006): middle: in the human right hemisphere (from Thiebaut de Schotten et al., 2011); right: attentional networks in the right hemisphere, according to Corbetta and Shulman (2002).
A dorsal attentional network (DAN), composed by the intraparietal sulcus/superior parietal lobule and the frontal eye field/dorsolateral PFC, shows increased blood oxygenation level dependent (BOLD) responses during the cue—target period. As a consequence, the DAN is supposed to be important for spatial orienting. Functional MRI also demonstrated a more a ventral attentional network (VAN), which includes the temporoparietal junction and the ventral frontal cortex (inferior and middle frontal gyri), and shows increased BOLD responses when participants have to respond to invalidly cued targets. Thus, the VAN is considered important for detecting unexpected but behaviorally relevant events. Importantly, the DAN is bilateral and symmetric, whereas the VAN is strongly lateralized to the right hemisphere.
Not surprisingly given the postulated architecture of these networks, PFC and PPC are directly and extensively interconnected. In particular, three distinct fronto-parietal long-range pathways can be identified in the monkey on the basis of cortical terminations and course (Petrides and Pandya, 1984; Schmahmann and Pandya, 2006) (see Figure 1, left panel). Recently, advanced tractography techniques and post-mortem dissections demonstrated that a similar architecture seems to exist in the human brain (Thiebaut de Schotten et al., 2011) (see the middle panel in Figure 1). In humans, the most dorsal branch (SLF I) originates from BA 5 and 7 and projects to BA 8, 9, and 32. In contrast, the middle pathway (SLF II) originates in BA 39 and 40 within the inferior parietal lobule (IPL) and ends in prefrontal BA 8 and 9. Lastly, the most ventral pathway (SLF III) originates in BA 40 and terminates in BA 44, 45, and 47. These results permitted to fit anatomical pathways to the fMRI evidence on attentional networks mentioned above. Thiebaut de Schotten et al. (2011) were able to show that the SLF III connects brain regions within the VAN, whereas the DAN is connected by the human homologue of SLF I. The SLF II connects the parietal component of the VAN to the prefrontal component of the DAN, thus allowing direct communication between ventral and DANs. Importantly, in good agreement with asymmetries of BOLD response during fMRI, with larger right hemisphere response for the VAN and more symmetrical activity for the DAN (Corbetta and Shulman, 2002), the SLF III (connecting the VAN) is anatomically larger in the right hemisphere than in the left hemisphere, whereas the SLF I (connecting the DAN) is more symmetrically organized. The lateralization of the SLF II is instead strongly correlated to behavioral signs of right hemisphere specialization for visuospatial attention such as pseudo-neglect in line bisection (i.e., small leftwards deviations of the subjective midline produced by normal individuals) (Bowers and Heilman, 1980; Jewell and Mccourt, 2000; Toba et al., 2011), and asymmetries in the speed of detection between the right and the left hemifield (Thiebaut de Schotten et al., 2011).
Impaired attention after brain damage: visual neglect
The neglect syndrome
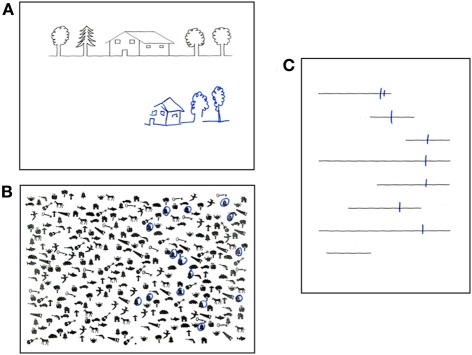
Temporary inactivation of the SLF II in the human right hemisphere impairs the symmetrical distribution of visual attention (Thiebaut de Schotten et al., 2005). Damage to SLF networks in the right hemisphere is frequently associated to a disabling condition known as left visual neglect (Bartolomeo, 2006, 2007; Doricchi et al., 2008; Thiebaut de Schotten et al., 2008). About half of the patients with a lesion in the right hemisphere suffer from neglect for the left side of space (Azouvi et al., 2006). The cause is most often vascular strokes, but signs of neglect may also be observed as a consequence of brain tumors (Hughlings Jackson, 1876/1932; Bartolomeo, 2011) and of neurodegenerative conditions, such as Alzheimer disease (D'Erme et al., 1991 (abstract); Bartolomeo et al., 1998) or posterior cortical atrophy (Andrade et al., 2010; Migliaccio et al., 2011). Neglect patients are unaware of events occurring in a portion (usually the left half) of their environment, sometimes up to the dramatic extent of “forgetting” to eat from the left part of their dish or of bumping into obstacles situated on their left. Patients with left neglect also display a tendency to look to right-sided details as soon as a visual scene deploys, as if their attention were “magnetically” attracted by these details (Gainotti et al., 1991). They are usually unaware of their deficits (anosognosia), and often obstinately deny being hemiplegic. Patients with left brain damage may also show signs of right-sided neglect, albeit more rarely and usually in a less severe form (Bartolomeo et al., 2001a; Beis et al., 2004). Neglect is a substantial source of handicap and disability for patients, and entails a poor functional outcome. Diagnosis is important, because effective rehabilitation strategies are available, and there are promising possibilities for pharmacological treatments (Bartolomeo, 2007). Furthermore, in many cases the “negative” nature of neglect deficits (impaired active exploration of a part of space) renders the diagnosis difficult or impossible if signs of neglect are not searched for. This is unfortunate, because simple paper-and-pencil tests can easily make the diagnosis at patient's bedside (Figure 2).
Figure 2.
Performance of a patient with left spatial neglect on paper-and-pencil tests. (A) copy of a linear drawing with omission of left-sided elements; (B) target cancellation task, with omission of left-sided targets (bells); (C) bisection of horizontal lines, with rightward deviation of the bisection mark and complete omission of one left-sided line.
Attention and neglect
In addition to its clinical importance, neglect also raises important issues concerning the brain mechanisms of consciousness, perception and attention. In particular, the study of patients with visual neglect has given a substantial contribution to the analysis of attentional processes and of their neural substrates (Bartolomeo and Chokron, 2002; Corbetta and Shulman, 2011). Neglect is characterized, among other symptoms, by severe problems in orienting attention toward left-sided objects (Bartolomeo and Chokron, 2002; Rastelli et al., 2008). Typically, however, neglect patients' deficits of spatial attention are not generalized, but concern first and foremost exogenous orienting (see Bartolomeo and Chokron, 2002, for review), with a relative sparing of endogenous orienting (Bartolomeo et al., 2001b). For example, Rastelli et al. (2008) demonstrated that the onset, but not the offset, of right-sided visual objects was able to induce a pathological attentional bias in neglect patients (see also D'Erme et al., 1992). Thus, it is right-sided objects (and not spatial regions) that tend to capture patients' attention, consistent with the peculiar relationships between object-based and exogenous forms of attention (Macquistan, 1997) (see Section Taxonomies of attentional processes above)2.
Importantly, recent accumulating evidence from behavioral, neurophysiologic, neuropsychological and neuroimaging experiments in normal participants (reviewed by Chica and Bartolomeo, 2012) indicate that while endogenous attention has weak influence on subsequent conscious perception of near-threshold stimuli, exogenous attention appears instead to be a necessary, although not sufficient, step in the development of reportable visual experiences. Thus, there is an impressive convergence of findings between the striking spatial unawareness shown by neglect patients, their severe impairment of exogenous orienting of attention, and the importance of exogenous attention for conscious visual perception in normal individuals (Bartolomeo, 2008b).
How do these notions map on the hypotheses concerning the organization of the attention networks in the brain? A plausible model of intra-and inter-hemispheric interactions in neglect (He et al., 2007) stipulates that damage to right hemisphere VAN causes a functional imbalance between the left and right DANs, with a hyperactivity of the left dorsal fronto-parietal network, which would provoke an attentional bias toward right-sided objects and neglect of left-sided items. Consistent with this hypothesis, suppressive TMS on left fronto-parietal networks correlated with an improvement of patients' performance on cancellation tests (Koch et al., 2008). However, an alternative proposal has been made recently by Singh-Curry and Husain (2009) on the role of the right IPL, which is not fully captured by the Corbetta and Shulman (2002) model. In particular, the authors argued that the VAN is not only dedicated to salience detection in a stimulus-driven way but is also responsible for maintaining attention on goals or task demands, which is a top-down process. In support of this proposal, functional MRI has suggested a role for the inferior frontal junction (parts of BA 9, 44, 6) in mediating interactions between bottom-up and top-down attention (Asplund et al., 2010). Furthermore, TPJ, the caudal node of the VAN, demonstrates increased BOLD response for behaviorally relevant distractors, but not for non-relevant but highly salient ones (Indovina and Macaluso, 2007). Thus, deficits in these non-spatial aspects of attention may lead to an exacerbation of the spatial bias in neglect patients (Husain and Nachev, 2007).
Another important characteristic of neglect-related deficits is that spatial attention and gaze are prone to be captured by right-sided objects (Gainotti et al., 1991), often in a repeated fashion. For example, in cancellation tasks patients may keep cancelling the same right-sided lines over and over again. Perhaps normal individuals do not show this perseverative behavior because of processes inhibiting repeated orientations toward the same event. When two consecutive visual events occur at the same spatial location, there can be an early facilitation to respond to the second event. However, when the interval between the two events is longer than 300 ms, responses to the second event are typically slower that those to the first. This phenomenon, dubbed inhibition of return (IOR, Posner et al., 1985; Klein, 2000; Lupiáñez et al., 2006), is thus important for thoroughly exploring the visual environment, by avoiding repeated processing of the same location (Klein, 1988). IOR occurs both with manual responses (such as a keypress) and with saccades to peripheral visual stimuli. Not surprisingly, IOR can be abnormal in visual neglect (Bartolomeo et al., 1999). When pressing a key in response to peripheral visual targets which were occasionally repeated on the same side of space, patients with left neglect presented abnormal facilitation, instead of IOR, for repeated right-sided items, i.e., for items appearing in their supposedly normal hemispace (Bartolomeo et al., 1999). Other patients with right hemisphere damage but without neglect had, instead, normal IOR for both sides of space (Bartolomeo et al., 1999). These results were later confirmed in neglect patients with cue-target paradigms (Bartolomeo et al., 2001b; Lupiáñez et al., 2004; Sieroff et al., 2007). Patients with parietal damage also demonstrated decreased IOR (but not facilitation) on the ipsilesional side, even in the absence of neglect signs (Vivas et al., 2003, 2006). These results are important in suggesting that cortical networks including the right parietal lobe, which are typically dysfunctional in neglect patients (Mort et al., 2003; Thiebaut de Schotten et al., 2005; Bartolomeo et al., 2007; He et al., 2007), are implicated in the occurrence of IOR. However, in these studies eye movements were not controlled; if patients looked at ipsilesional first targets or cues (a frequent occurrence in right brain-damaged patients, Gainotti et al., 1991), they received the second stimulus on the fovea; then fast responses to foveal stimuli could have offset IOR. To address these questions, Bourgeois et al. (2012) explored IOR with central fixation and manual responses (covert attention, Experiment 1), as well as IOR generated by saccadic responses (overt attention, Experiment 2). Bourgeois et al. used a target-target paradigm similar to the one used in the seminal study on IOR in neglect (Bartolomeo et al., 1999), while eye movements were monitored at all times. Neglect patients' performance was compared to that of right brain-damaged patients without neglect. Confirming the previous results obtained by Bartolomeo et al. (1999), neglect patients demonstrated facilitation, instead of inhibition, for repeated right-sided targets with manual responses. However, they had normal IOR for the same right-sided targets with saccadic responses. All neglect patients had damage to the supramarginal gyrus in the right parietal lobe, or to its connections with the ipsilateral PFC. Bourgeois et al. (2012) concluded that IOR with manual responses relies on fronto-parietal attentional networks in the right hemisphere, whose functioning is typically impaired in neglect patients. Saccadic IOR may instead depend on circuits less likely to be damaged in neglect, such as the retinotectal visual pathway.
Perceptual asymmetries in neglect
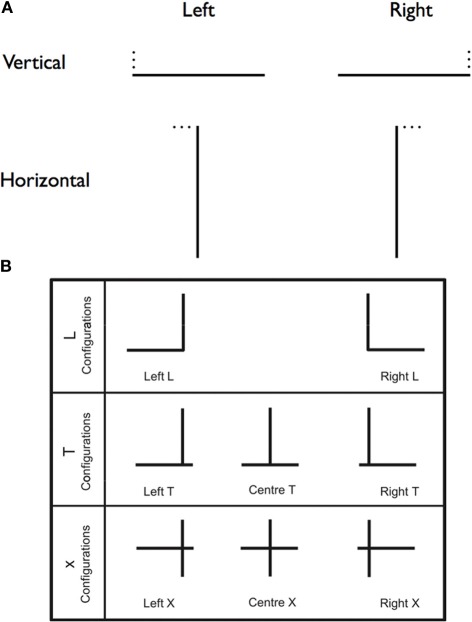
As these results indicate, the multiform character of visual neglect calls for finely articulated models of attentional deficits in this condition. One important question concerning spatial attention in neglect is: are rightward attentional capture and leftward orienting deficits two (consecutive) sides of the same coin, or should they be considered as distinct components of neglect behavior? To answer this question, Charras et al. (2010) asked neglect patients to draw the horizontal segment of left- or right-directed Ls, on the basis of a given vertical segment (Figure 3A).
Figure 3.
Neglect and length estimation. (A) A schematic depiction of the stimuli used by Charras et al. (2010). A single black line, either horizontal or vertical (40 mm long, 1 mm thick), was printed in the center of the sheet. Participants performed a line extension task in which they were to draw either a horizontal or a vertical line to complete an L figure. The missing line was located either to the left or right of the presented line. The position of the missing line was indicated by three small black dots. (B) Schematic depiction of the stimuli used by Charras et al. (2012). The L configurations enabled to test leftwards and rightrightwards biases separately. In the T and X configurations, there was a left/right competition between the horizontal line segments left and right of the bisection line. The results showed that, in the T configuration, the vertical line was overestimated, while in the X configuration, the horizontal line was overestimated.
Neglect patients drew longer left-directed segments than right-directed segments. However, comparison with controls' performance revealed that neglect patients did over-extend horizontal lines toward the left, but did not under-extend rightwards lines. This result invites the conclusion that the left–right imbalance observed in length estimation resulted more from left impairment in stimulus processing than from right attentional capture. However, in a different series of patients, Urbanski and Bartolomeo (2008) found that right attentional capture exerted by the right extremity of horizontal lines did have an important role in patients' performance in bisection-related tasks. Their patients were selected on the basis of the presence of a pathological rightward deviation on line bisection. However, when they had to set the left endopoint of an imaginary line on the basis of a central point, their performance depended on the presence/absence of a (presumably attention-capturing) right endpoint. The two virtual segments were asymmetric, mimicking ordinary line bisection, when the right endpoint was visible, but much more symmetrical when it was not. To account for the apparent discrepancy between the outcome of these two studies, Charras et al. (2010) noted that in their L-shaped figures there was no right-sided horizontal line whose extremity could capture patients' attention (see Gainotti et al., 1991), which presumably led to the absence of right overestimation. In this sense, Charras et al. (2010) results are perfectly consistent with the effects of right attentional capture effect in imaginary line bisection described by Urbanski and Bartolomeo (2008).
In a second study, Charras et al. (2012) were able to confirm and refine their previous conclusions. Patients were asked to estimate the length of left- and right-sided segments with L-, T-, or cross-shaped (X) configurations (see Figure 3B). When there was no competition between left and right horizontal segments, such as in the L configurations, the left-right imbalance resulted from left underestimation, in the absence of right overestimation, thus confirming the previous results (Charras et al., 2010). Similar results occurred with the T configurations, when emphasis was put on the vertical dimension of the stimulus (as shown by participants' strong tendency to overestimate the vertical portion of the stimulus), thus presumably preventing left-right integration of the horizontal segments. However, when left- and right-segments competed to be integrated in a single percept, as in the X configurations, then, right attentional capture did contribute to patients' performance. Interestingly, the presence of left homonymous hemianopia worsened left underestimations, but did not modulate right overestimations. Based on these results, Charras et al. (2012) proposed the existence of distinct neural bases for right overestimation, resulting from the activity of an isolated left hemisphere (see the section on interhemispheric disconnection in Bartolomeo et al., 2007), and left underestimation, dependent on impaired functioning of right hemisphere attentional networks (Bartolomeo, 2006). In different patients, these two component deficits might have different weights, perhaps depending on individual differences in anatomical asymmetries of fronto-parietal networks linked by SLF II and III (Thiebaut de Schotten et al., 2011).
Neglect and non-spatial attention: the role of alertness
Thus far, we have examined the role of different sorts of imbalance of spatial attention mechanisms in neglect. However, other attentional capacities have been shown to be impaired in neglect patients (Husain and Rorden, 2003). For example, it has long been shown that non-spatial aspects of attentional mechanisms, such as alerting, can be defective in neglect, and contribute in substantial ways to patients' patterns of performance (Robertson, 2001). Thus, a further question of interest is: given the complex patterns of interaction between selective attention, alerting, executive functions and perceptual consciousness in normal individuals (Callejas et al., 2005; Kusnir et al., 2011), what happens when brain damage intervenes?
First of all, it is worth noting that there are relatively underexplored links between alerting and perceptual consciousness in normal individuals. For example, the manipulation of phasic alertness in healthy participants has been shown to affect perceptual discriminations and conscious perception of targets presented near the threshold of conscious perception (Kusnir et al., 2011). In this study, near-threshold visual targets were presented, accompanied or not by a short acoustic tone. Acoustic tones (which increase phasic alerting) ameliorated both speed (as manifested in decreased response times to discriminate targets) and discrimination performance (as manifested in increased accuracy) when the target was presented in a temporally non-predictive manner (Kusnir et al., 2011). This constitutes a piece of evidence in favor of the idea that phasic alerting can directly affect perceptual processing, rather than just motor readiness. Phasic auditory alerting also improved the subjective perception of near-threshold visual stimuli, perhaps through the activation of right hemisphere fronto-parietal networks whose dysfunction may determine visual unawareness in neglect patients (Bartolomeo, 2006). This is consistent with observations suggesting that visual neglect patients with extensive right hemisphere damage show, in addition to spatial deficits, non-spatial deficits in sustaining alertness (Robertson et al., 1998). There is evidence from neuroimaging that tonic alertness, like spatial attention, relies on fronto-parietal networks in the right hemisphere (Sturm and Willmes, 2001). In contrast, the attentional system underlying phasic alertness depends on ascending thalamic-mesencephalic, noradrenergic projections from the locus coeruleus (Mesulam, 1981; Posner et al., 1987), as well as additional left-hemisphere cortical networks (Sturm and Willmes, 2001). All these structures are typically intact in visual neglect patients. Thus, it has been proposed that in visual neglect ascending subcortical projections may phasically activate what is spared of the fronto-parietal cortical networks subserving spatial attention and alerting in the damaged right hemisphere, thus shifting spatial attention leftwards and compensating for neglect deficits (Robertson et al., 1998).
The importance of the interplay between attentional networks implicated in alerting, orienting and executive control has been explored in a group of patients with right hemisphere damage (Chica et al., 2011). Patients were evaluated by using a modified computerized battery test (Attention Network Test, ANT), originally designed to determine the functional independence and efficiency of the three attentional networks (Fan et al., 2002). The introduction of an alerting tone before the occurrence of the visual cue permits to assess the efficiency and independence of each network, but also their interactions. If the attentional networks interact, the phasic alerting produced by the tone could ameliorate neglect patients' orienting deficits, who might be faster and/or more accurate for validly cued left-targets. Better orienting might in turn be able to improve conflict resolution at the attended location. The results of the Chica et al. (2011) study demonstrated that modulating alertness is an important way of improving basic mechanisms typically impaired in neglect. In particular, neglect patients' orienting abilities improved after the phasic alerting tone, which enhanced conflict resolution in the neglected hemispace. However, three patients out of 16 were not able to benefit from auditory alerting tones. These patients had damage implicating the right insula and the underlying white matter. The right insula has been associated with sustained attention (Thakral and Slotnick, 2009) and has important connections to the anterior cingulated cortex (Augustine, 1996), a structure crucial for cognitive control and conflict resolution (Botvinick et al., 1999; Fan et al., 2003). Thus, the Chica et al. (2011) results suggest that conflict resolution can be improved in neglect patients by modulating alerting and orienting, provided that structures critical for conflict resolution such as the insula are spared by the lesion.
Imaginal neglect
To further complicate the semiotics of spatial neglect, about a third of neglect patients may also neglect the left part of their mental images (Bartolomeo et al., 1994). When describing places from memory, these patients omit to mention the left side of the mental space (Figure 4), thus demonstrating “imaginal” neglect (Bisiach and Luzzatti, 1978).
Figure 4.
Imaginal neglect. In their seminal paper, Bisiach and Luzzatti (1978) reported two left neglect patients who, when asked to imagine and describe from memory familiar surroundings (the Piazza del Duomo in Milan), omitted to mention left-sided details regardless of the imaginary vantage point that they assumed.
However, not all patients with visual neglect show imaginal neglect, perhaps because imagined details have less attention-capturing power than real ones (Bartolomeo et al., 1994). Imaginal neglect can also occur in the absence of signs of perceptual neglect, either at onset or, perhaps more commonly, as a result of selective compensation for the perceptual aspects of the syndrome. Patients often learn with time (and possibly the help of people around them) to explore more thoroughly their visual environment. However, compensation may be more difficult to obtain in the more abstract imaginal domain, which is rarely the object of rehabilitation or of more informal reminders to “look to your left” (Bartolomeo and Chokron, 2001). Thus, similar to other domains of visual mental imagery (Bartolomeo, 2002, 2008a), several studies have reported the existence of double dissociations between imaginal and perceptual neglect (Anderson, 1993; Guariglia et al., 1993; Beschin et al., 1997; Coslett, 1997; Ortigue et al., 2001).
However, the study of imaginal neglect raises peculiar methodological problems. Often, very different task are used to evaluate spatial perception and spatial imagery. In particular, in several studies, paper-and-pencil tests were used for perception and description from memory for imagery (Rode and Perenin, 1994; Rode et al., 2007). Moreover, description from memory might rely more on verbal semantic memory than on visual imagery, and thus produce symmetrical descriptions even in the presence of imaginal neglect (Rode et al., 2004). To encourage the use of a visual mental strategy, a response time “geographical” test was devised (Bartolomeo et al., 2005), with strictly comparable perceptual and imaginal conditions (Bourlon et al., 2008, 2011a). In different tasks, participants either saw towns/regions on a map of France or heard their names, and pressed one of two keys according to the stimulus location (left or right of Paris). Interestingly, when normal participants performed such a task, their eye movements mimicked those produced with real displays, thus lending support to the hypothesis that similar attentional mechanisms may be engaged in perception and in mental imagery (Bourlon et al., 2011b). In patients, however, the results obtained with these tasks confirmed the rarity of imaginal neglect with respect to perceptual neglect.
In a recent case report of imaginal neglect (Rode et al., 2010), structural and diffusion MRI demonstrated damage to several white matter tracts in the right hemisphere and to the splenium of corpus callosum. The same study reported on a second right-brain-damaged patient, who showed signs of perceptual but not imaginal neglect, and had damage to the same intrahemispheric tracts; the callosal connections, however, were spared. Imaginal neglect might thus result from the association of fronto-parietal dysfunction, which impairs orienting toward left-sided items (see Bartolomeo et al., 2007) and additional posterior callosal disconnection, which might prevent the symmetrical processing of spatial information from long-term memory.
In clinical settings, drawing from memory is often used to assess imaginal abilities and then directly compared to drawing copying. However, visual feedback provided by drawing may influence final performance by inducing an attentional capture of the right-sided details the patient has just drawn (Chokron et al., 2004). To address this issue, recent studies employed drawing without visual feedback, e.g., while blindfolded (Chokron et al., 2004) or by using a pen which leaves no visible traces on the sheet (Cristinzio et al., 2009). While in general patients show more neglect with visual feedback than without visual feedback (Chokron et al., 2004), thus confirming the attention-capturing effect of right-sided visual items (Bartolomeo et al., 1994), one recent case report (Cristinzio et al., 2009) demonstrated the opposite effect, perhaps as a consequence of additional working memory impairment (Wojciulik et al., 2004). In conclusion, a possibility to account for the rarity of imaginal neglect is that this form of neglect might depend on additional deficits of top-down processes, such as endogenous attention or active rehearsal of spatial knowledge, that are typically less impaired than exogenous attention in patients with perceptual neglect (Bourlon et al., 2011a).
The anatomy of visual neglect
Signs of visual neglect have been traditionally related to damage to the IPL (Vallar and Perani, 1986; Mort et al., 2003). More recent evidence suggested that neglect signs do not result from focal cortical lesions, but correlate with dysfunction of large-scale networks, whose nodes include the PPC, the lateral prefrontal cortex (LPFC), the TPJ and the occipital lobe (Bartolomeo et al., 2007; Doricchi et al., 2008). As mentioned before, these cortical nodes show increased BOLD response during spatial orienting of attention (Nobre, 2001; Corbetta and Shulman, 2002; Bartolomeo et al., 2008). Consistent with the hypothesis of a causal link between neglect signs and impairment of large-scale fronto-parietal networks in the right hemisphere (Bartolomeo, 2006), accumulating evidence has demonstrated an associated injury to white matter pathways connecting these networks in monkey studies (Gaffan and Hornak, 1997) and in human neglect patients with vascular damage (Urbanski et al., 2008, 2011; Chechlacz et al., 2010; Verdon et al., 2010) or neurosurgical lesions (Thiebaut de Schotten et al., 2005; Shinoura et al., 2009; Roux et al., 2011). It must be noted that in all these studies on human brain-damaged patients the lesions affected both the gray and the white matter. However, a recent single case report demonstrated that severe, if transitory, neglect signs can result from small lesions restricted to the white matter and affecting components of the SLF (Ciaraffa et al., 2012).
Discussion
Putting things together: towards a neural model of attentional interactions in neglect
Several neural models have been proposed to explain neglect, but no single model can plausibly account for all the complex and sometimes contradictory features of this syndrome. A perusal of the vast literature on neglect invites the conclusion that the refinement of behavioral analysis has not yet been matched by completely satisfactory neural models of neglect-related deficits and compensatory processes. We outline here some ideas which could offer starting points for the enterprise of mapping behavioral deficits to brain networks.
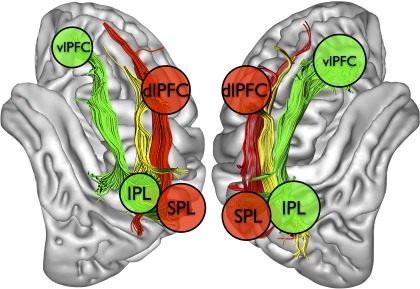
Despite the obvious links between left neglect and dysfunction of large-scale fronto-parietal networks in the right hemisphere (Bartolomeo, 2006, 2007; Bartolomeo et al., 2007; Doricchi et al., 2008), the most severe and persistent signs of left neglect typically occur after retro-Rolandic lesions. This apparent paradox may be explained by the architecture of fronto-parietal connections in the human brain (Thiebaut de Schotten et al., 2011) (Figure 5; see also Figure 3).
Figure 5.
Schematic depiction of fronto-parietal attentional networks for visuospatial processing in the two hemispheres, based on Corbetta and Shulman (2002) and Thiebaut de Schotten et al. (2011). IPL and SPL, inferior and superior parietal lobules. dlPFC and vlPFC, dorsolateral and ventrolateral prefrontal cortex.
As mentioned in Section Architecture of attentional circuits in the brain, the SLF II, whose caudal cortical origin is in part shared with that of the SLF III in the IPL, connects the parietal component of the VAN to the prefrontal component of the DAN (Thiebaut de Schotten et al., 2011). Thus, it is plausible that damage to the IPL (Mort et al., 2003), when accompanied by injury to the underlying white matter (Doricchi and Tomaiuolo, 2003; Verdon et al., 2010), can produce severe and persisting signs of neglect because it can jointly disrupt the functioning of both the VAN (through SLF III disconnection) and its communication with the DAN (through SLF II damage). On the other hand, less extensive lesions, perhaps sparing a significant part of SLF II, might allow for intra-hemispheric compensation mechanisms relying on the possibility of communication between VAN and DAN offered by SLF II. In this case, an initial imbalance between the dorsal fronto-parietal networks, with the left hemisphere DAN being relatively more active than its right hemisphere counterpart, might subside after the acute phase, with consequent recovery from neglect signs (Corbetta et al., 2005).
Another possible mechanism of neglect recovery might depend on inter-hemispheric interactions. Individual variability in the asymmetry of SLF II and III, which only recently is starting to be explored (Thiebaut de Schotten et al., 2011), could account for different patterns of recovery/compensation. It is possible that patients who happen to have a relatively large SLF III in the left hemisphere may use resources pertaining to a left-hemisphere homologue of the right-sided VAN to partially compensate for neglect signs. Along similar lines, one might speculate that the larger the left-hemisphere SLF II, the better the communication between the DAN and the left hemisphere homologue of the VAN. A relatively efficient left homologue of the VAN might control the ipsilateral VAN and ensure a relatively functional exploration of the whole space after right brain damage, thus leading to (apparent) recovery from neglect.
If these considerations are true, however, neglect compensation by using alternative (left-hemisphere-based) attentional routes is likely to be partial and subject to task demands. Indeed, it has repeatedly been shown that even patients who do not demonstrate anymore neglect on paper-and-pencil tests often show lateralized impairments on more demanding, time-constrained tasks (Posner et al., 1984; Bartolomeo, 1997, 2000; Bonato et al., 2010). This evidence is consistent with the common clinical observation of chronic patients who perform perfectly on paper-and-pencil tasks but, as soon as they exit the testing room, start again bumping into left-sided obstacles.
Conclusions
Attentional processes, mainly subserved by frontoparietal brain networks, with a peculiar although not yet completely elucidated role for the right hemisphere, are at the basis of our capacity to actively explore the external world. Their impairment as a result of brain damage can hamper the conscious perception of objects in space, and is a source of significant disability for patients. Our knowledge of these systems is still too limited to enable us to offer specific interventions for the whole range of attentional impairments, but it is expanding at fast pace, raising hopes for the development of effective strategies to improve the functioning of the attentional networks in brain-damaged patients.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Supported by postdoctoral grants from the Neuropôle de Recherche Francilen (NeRF), Marie Curie Intra-European Program (FP7), and Ramón y Cajal fellowship from the Spanish Ministry of Education and Science to Ana B. Chica.
Footnotes
1The relationship between attention and gaze shifts is a debated one. According to the so-called “premotor theory” (Rizzolatti et al., 1987), an attention shift always entails the programming of an eye movement, which can then be executed (overt attention) or not (covert attention). Consistent with this view, nodes of the fronto-parietal attentional networks such as the FEF and the IPS do contribute to saccade programming (Corbetta, 1998).
2Instances have been described of “object-based” neglect, whereby patients fail to process information coming from the intrinsic left side of an object, whether or not it corresponds to the left of patient's midline. However, the left-right border is variable in neglect, and some of these cases have been reinterpreted as examples of relative egocentric neglect (Driver and Pouget, 2000).
References
- Allport D. A. (1989). “Visual attention,” in Foundations of Cognitive Science, ed Posner M. I. (Cambridge, MA: MIT Press; ), 631–687 [Google Scholar]
- Anderson B. (1993). Spared awareness for the left side of internal visual images in patients with left-sided extrapersonal neglect. Neurology 43, 213–216 [DOI] [PubMed] [Google Scholar]
- Andrade K., Samri D., Sarazin M., Cruz De Souza L., Cohen L., Thiebaut de Schotten M., Dubois B., Bartolomeo P. (2010). Visual neglect in posterior cortical atrophy. BMC Neurol. 10, 68 10.1186/1471-2377-10-68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asplund C. L., Todd J. J., Snyder A. P., Marois R. (2010). A central role for the lateral prefrontal cortex in goal-directed and stimulus-driven attention. Nat. Neurosci. 13, 507–512 10.1038/nn.2509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine J. R. (1996). Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res. Rev. 22, 229–244 10.1016/S0165-0173(96)00011-2 [DOI] [PubMed] [Google Scholar]
- Azouvi P., Bartolomeo P., Beis J-M., Perennou D., Pradat-Diehl P., Rousseaux M. (2006). A battery of tests for the quantitative assessment of unilateral neglect. Restor. Neurol. Neurosci. 24, 273–285 [PubMed] [Google Scholar]
- Bartolomeo P. (1997). The novelty effect in recovered hemineglect. Cortex 33, 323–332 [DOI] [PubMed] [Google Scholar]
- Bartolomeo P. (2000). Inhibitory processes and compensation for spatial bias after right hemisphere damage. Neuropsychol. Rehabil. 10, 511–526 [Google Scholar]
- Bartolomeo P. (2002). The relationship between visual perception and visual mental imagery: a reappraisal of the neuropsychological evidence. Cortex 38, 357–378 [DOI] [PubMed] [Google Scholar]
- Bartolomeo P. (2006). A parieto-frontal network for spatial awareness in the right hemisphere of the human brain. Arch. Neurol. 63, 1238–1241 10.1001/archneur.63.9.1238 [DOI] [PubMed] [Google Scholar]
- Bartolomeo P. (2007). Visual neglect. Curr. Opin. Neurol. 20, 381–386 10.1097/WCO.0b013e32816aa3a3 [DOI] [PubMed] [Google Scholar]
- Bartolomeo P. (2008a). The neural correlates of visual mental imagery: an ongoing debate. Cortex 44, 107–108 10.1016/j.cortex.2006.07.001 [DOI] [PubMed] [Google Scholar]
- Bartolomeo P. (2008b). Varieties of attention and of consciousness: evidence from neuropsychology. Psyche 14 Available Online at http://www.theassc.org/vol_14_2008 [Google Scholar]
- Bartolomeo P. (2011). The quest for the ‘critical lesion site’ in cognitive deficits: problems and perspectives. Cortex 47, 1010–1012 10.1016/j.cortex.2010.11.007 [DOI] [PubMed] [Google Scholar]
- Bartolomeo P., Bachoud-Levi A. C., Azouvi P., Chokron S. (2005). Time to imagine space: a chronometric exploration of representational neglect. Neuropsychologia 43, 1249–1257 10.1016/j.neuropsychologia.2004.12.013 [DOI] [PubMed] [Google Scholar]
- Bartolomeo P., Chokron S. (2001). “Levels of impairment in unilateral neglect,” in Handbook of Neuropsychology 2nd edn, eds Boller F., Grafman J. (Amsterdam: Elsevier Science Publishers; ), 67–98 [Google Scholar]
- Bartolomeo P., Chokron S. (2002). Orienting of attention in left unilateral neglect. Neurosci. Biobehav. Rev. 26, 217–234 10.1016/S0149-7634(01)00065-3 [DOI] [PubMed] [Google Scholar]
- Bartolomeo P., Chokron S., Gainotti G. (2001a). Laterally directed arm movements and right unilateral neglect after left hemisphere damage. Neuropsychologia 39, 1013–1021 10.1016/S0028-3932(01)00042-2 [DOI] [PubMed] [Google Scholar]
- Bartolomeo P., Sieroff E., Decaix C., Chokron S. (2001b). Modulating the attentional bias in unilateral neglect: the effects of the strategic set. Exp. Brain Res. 137, 432–444 [DOI] [PubMed] [Google Scholar]
- Bartolomeo P., Chokron S., Sieroff E. (1999). Facilitation instead of inhibition for repeated right-sided events in left neglect. Neuroreport 10, 3353–3357 [DOI] [PubMed] [Google Scholar]
- Bartolomeo P., Dalla Barba G., Boissé M. T., Bachoud-Lévi A. C., Degos J. D., Boller F. (1998). Right-side neglect in Alzheimer's disease. Neurology 51, 1207–1209 [DOI] [PubMed] [Google Scholar]
- Bartolomeo P., D'Erme P., Gainotti G. (1994). The relationship between visuospatial and representational neglect. Neurology 44, 1710–1714 [DOI] [PubMed] [Google Scholar]
- Bartolomeo P., Thiebaut de Schotten M., Doricchi F. (2007). Left unilateral neglect as a disconnection syndrome. Cereb. Cortex 45, 3127–3148 10.1093/cercor/bhl181 [DOI] [PubMed] [Google Scholar]
- Bartolomeo P., Zieren N., Vohn R., Dubois B., Sturm W. (2008). Neural correlates of primary and reflective consciousness of spatial orienting. Neuropsychologia 46, 348–361 10.1016/j.neuropsychologia.2007.07.005 [DOI] [PubMed] [Google Scholar]
- Beis J. M., Keller C., Morin N., Bartolomeo P., Bernati T., Chokron S., Leclercq M., Louis-Dreyfus A., Marchal F., Martin Y., Perennou D., Pradat-Diehl P., Prairial C., Rode G., Rousseaux M., Samuel C., Sieroff E., Wiart L., Azouvi P. (2004). Right spatial neglect after left hemisphere stroke: qualitative and quantitative study. Neurology 63, 1600–1605 [DOI] [PubMed] [Google Scholar]
- Beschin N., Cocchini G., Della Sala S., Logie R. (1997). What the eyes perceive, the brain ignores: a case of pure unilateral representational neglect. Cortex 33, 3–26 [DOI] [PubMed] [Google Scholar]
- Bisiach E., Luzzatti C. (1978). Unilateral neglect of representational space. Cortex 14, 129–133 [DOI] [PubMed] [Google Scholar]
- Bonato M., Priftis K., Marenzi R., Umiltà C., Zorzi M. (2010). Increased attentional demands impair contralesional space awareness following stroke. Neuropsychologia 48, 3934–3940 10.1016/j.neuropsychologia.2010.08.022 [DOI] [PubMed] [Google Scholar]
- Botvinick M., Nystrom L. E., Fissell K., Carter C. S., Cohen J. D. (1999). Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature 402, 179–181 10.1038/46035 [DOI] [PubMed] [Google Scholar]
- Bourgeois A., Chica A. B., Migliaccio R., Thiebaut de Schotten M., Bartolomeo P. (2012). Cortical control of inhibition of return: evidence from patients with inferior parietal damage and visual neglect. Neuropsychologia 50, 800–809 10.1016/j.neuropsychologia.2012.01.014 [DOI] [PubMed] [Google Scholar]
- Bourlon C., Duret C., Pradat-Diehl P., Azouvi P., Loeper-Jény C., Merat-Blanchard M., Levy C., Chokron S., Bartolomeo P. (2011a). Vocal response times to real and imagined stimuli in spatial neglect: a group study and single-case report. Cortex 47, 536–546 10.1016/j.cortex.2010.03.004 [DOI] [PubMed] [Google Scholar]
- Bourlon C., Oliviero B., Wattiez N., Pouget P., Bartolomeo P. (2011b). Visual mental imagery: what the head's eye tells the mind's eye. Brain Res. 1367, 287–297 10.1016/j.brainres.2010.10.039 [DOI] [PubMed] [Google Scholar]
- Bourlon C., Pradat-Diehl P., Duret C., Azouvi P., Bartolomeo P. (2008). Seeing and imagining the “same” objects in unilateral neglect. Neuropsychologia 46, 2602–2606 10.1016/j.neuropsychologia.2008.04.002 [DOI] [PubMed] [Google Scholar]
- Bowers D., Heilman K. M. (1980). Pseudoneglect: effects of hemispace on a tactile line bisection task. Neuropsychologia 18, 491–498 [DOI] [PubMed] [Google Scholar]
- Buschman T. J., Miller E. K. (2007). Top-down versus bottom-up control of attention in the prefrontal and posterior parietal cortices. Science 315, 1860–1862 10.1016/j.ijpsycho.2009.05.011 [DOI] [PubMed] [Google Scholar]
- Callejas A., Lupiáñez J., Funes M. J., Tudela P. (2005). Modulations among the alerting, orienting and executive control networks. Exp. Brain Res. 167, 27–37 10.1007/s00221-005-2365-z [DOI] [PubMed] [Google Scholar]
- Charras P., Lupiáñez J., Bartolomeo P. (2010). Assessing the weights of visual neglect: a new approach to dissociate defective symptoms from productive phenomena in length estimation. Neuropsychologia 48, 3371–3375 10.1016/j.neuropsychologia.2010.07.006 [DOI] [PubMed] [Google Scholar]
- Charras P., Lupiáñez J., Migliaccio R., Toba M. N., Pradat-Diehl P., Duret C., Bartolomeo P. (2012). Dissecting the component deficits of perceptual imbalance in visual neglect: evidence from horizontal-vertical length comparisons. Cortex 48, 540–552 10.1016/j.cortex.2011.01.008 [DOI] [PubMed] [Google Scholar]
- Chechlacz M., Rotshtein P., Bickerton W. L., Hansen P. C., Deb S., Humphreys G. W. (2010). Separating neural correlates of allocentric and egocentric neglect: distinct cortical sites and common white matter disconnections. Cogn. Neuropsychol. 27, 277–303 10.1080/02643294.2010.519699 [DOI] [PubMed] [Google Scholar]
- Chica A. B., Bartolomeo P. (2012). Attentional routes to conscious perception. Front. Psychol. 3:1 10.3389/fpsyg.2012.00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chica A. B., Thiebaut de Schotten M., Toba M. N., Malhotra P., Lupiáñez J., Bartolomeo P. (2011). Attention networks and their interactions after right-hemisphere damage. Cortex [Epub ahead of print]. 10.1016/j.cortex.2011.01.009 [DOI] [PubMed] [Google Scholar]
- Chokron S., Colliot P., Bartolomeo P. (2004). The role of vision in spatial representation. Cortex 40, 281–290 [DOI] [PubMed] [Google Scholar]
- Ciaraffa F., Castelli G., Parati E. A., Bartolomeo P., Bizzi A. (2012). Visual neglect as a disconnection syndrome? A confirmatory case report. Neurocase (in press). [DOI] [PubMed] [Google Scholar]
- Corbetta M. (1998). Frontoparietal cortical networks for directing attention and the eye to visual locations: identical, independent, or overlapping neural systems? Proc. Natl. Acad. Sci. U.S.A. 95, 831–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M., Kincade M. J., Lewis C., Snyder A. Z., Sapir A. (2005). Neural basis and recovery of spatial attention deficits in spatial neglect. Nat. Neurosci. 8, 1603–1610 10.1038/nn1574 [DOI] [PubMed] [Google Scholar]
- Corbetta M., Shulman G. L. (2002). Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 3, 201–215 10.1093/cercor/bhm051 [DOI] [PubMed] [Google Scholar]
- Corbetta M., Shulman G. L. (2011). Spatial neglect and attention networks. Annu. Rev. Neurosci. 34, 569–599 10.1146/annurev-neuro-061010-113731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coslett H. B. (1997). Neglect in vision and visual imagery: a double dissociation. Brain 120, 1163–1171 10.1093/brain/120.7.1163 [DOI] [PubMed] [Google Scholar]
- Cristinzio C., Bourlon C., Pradat-Diehl P., Trojano L., Grossi D., Chokron S., Bartolomeo P. (2009). Representational neglect in “invisible” drawing from memory. Cortex 45, 313–317 10.1016/j.cortex.2008.03.013 [DOI] [PubMed] [Google Scholar]
- D'Erme P., Bartolomeo P., Masullo C. (1991). Alzheimer's disease presenting with visuo-spatial disorders. Ital. J. Neurol. Sci. 12, 117 [abstract]. [Google Scholar]
- D'Erme P., Robertson I., Bartolomeo P., Daniele A., Gainotti G. (1992). Early rightwards orienting of attention on simple reaction time performance in patients with left-sided neglect. Neuropsychologia 30, 989–1000 10.1016/0028-3932(92)90050-V [DOI] [PubMed] [Google Scholar]
- Desimone R., Duncan J. (1995). Neural mechanisms of selective visual attention. Annu. Rev. Neurosci. 18, 193–222 10.1146/annurev.ne.18.030195.001205 [DOI] [PubMed] [Google Scholar]
- Di Ferdinando A., Parisi D., Bartolomeo P. (2007). Modeling orienting behavior and its disorders with “ecological” neural networks. J. Cogn. Neurosci. 19, 1033–1049 10.1162/jocn.2007.19.6.1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doricchi F., Thiebaut de Schotten M., Tomaiuolo F., Bartolomeo P. (2008). White matter (dis) connections and gray matter (dys) functions in visual neglect: gaining insights into the brain networks of spatial awareness. Cortex 44, 983–995 10.1016/j.cortex.2008.03.006 [DOI] [PubMed] [Google Scholar]
- Doricchi F., Tomaiuolo F. (2003). The anatomy of neglect without hemianopia: a key role for parietal-frontal disconnection? Neuroreport 14, 2239–2243 10.1097/01.wnr.0000091132.75061.64 [DOI] [PubMed] [Google Scholar]
- Driver J., Pouget A. (2000). Object-centered visual neglect, or relative egocentric neglect? J. Cogn. Neurosci. 12, 542–545 [DOI] [PubMed] [Google Scholar]
- Egly R., Driver J., Rafal R. D. (1994). Shifting visual attention between objects and locations: evidence from normal and parietal lesion patients. J. Exp. Psychol. Gen. 123, 161–177 [DOI] [PubMed] [Google Scholar]
- Eriksen B. A., Eriksen C. W. (1974). Effects of noise letters upon the identification of a target letter in a non-search task. Percept. Psychophys. 16, 143–149 [Google Scholar]
- Fan J., Flombaum J. I., Mccandliss B. D., Thomas K. M., Posner M. I. (2003). Cognitive and brain consequences of conflict. Neuroimage 18, 42–57 10.1006/nimg.2002.1319 [DOI] [PubMed] [Google Scholar]
- Fan J., Mccandliss B. D., Sommer T., Raz A., Posner M. I. (2002). Testing the efficiency and independence of attentional networks. J. Cogn. Neurosci. 14, 340–347 10.1162/089892902317361886 [DOI] [PubMed] [Google Scholar]
- Gaffan D., Hornak J. (1997). Visual neglect in the monkey. Representation and disconnection. Brain 120, 1647–1657 10.1093/brain/120.9.1647 [DOI] [PubMed] [Google Scholar]
- Gainotti G., D'erme P., Bartolomeo P. (1991). Early orientation of attention toward the half space ipsilateral to the lesion in patients with unilateral brain damage. J. Neurol. Neurosurg. Psychiatry 54, 1082–1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guariglia C., Padovani A., Pantano P., Pizzamiglio L. (1993). Unilateral neglect restricted to visual imagery. Nature 364, 235–237 10.1038/364235a0 [DOI] [PubMed] [Google Scholar]
- He B. J., Snyder A. Z., Vincent J. L., Epstein A., Shulman G. L., Corbetta M. (2007). Breakdown of functional connectivity in frontoparietal networks underlies behavioral deficits in spatial neglect. Neuron 53, 905–918 10.1016/j.neuron.2007.02.013 [DOI] [PubMed] [Google Scholar]
- Hughlings Jackson J. (1876/1932). “Case of large cerebral tumour without optic neuritis and with left hemiplegia and imperception,” in Selected Writings of John Hughlings Jackson, ed Taylor J. (London: Hodden and Stoughton; ), 146–152 [Google Scholar]
- Husain M., Nachev P. (2007). Space and the parietal cortex. Trends Cogn. Sci. 11, 30–66 10.1016/j.tics.2006.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain M., Rorden C. (2003). Non-spatially lateralized mechanisms in hemispatial neglect. Nat. Rev. Neurosci. 4, 26–36 10.1038/nrn1005 [DOI] [PubMed] [Google Scholar]
- Indovina I., Macaluso E. (2007). Dissociation of stimulus relevance and saliency factors during shifts of visuospatial attention. Cereb. Cortex 17, 1701–1711 10.1093/cercor/bhl081 [DOI] [PubMed] [Google Scholar]
- Jewell G., Mccourt M. E. (2000). Pseudoneglect: a review and meta-analysis of performance factors in line bisection tasks. Neuropsychologia 38, 93–110 10.1016/S0028-3932(99)00045-7 [DOI] [PubMed] [Google Scholar]
- Klein R. M. (1988). Inhibitory tagging system facilitates visual search. Nature 334, 430–431 10.1038/334430a0 [DOI] [PubMed] [Google Scholar]
- Klein R. M. (2000). Inhibition of return. Trends Cogn. Sci. 4, 138–147 10.1016/S1364-6613(00)01452-2 [DOI] [PubMed] [Google Scholar]
- Koch G., Oliveri M., Cheeran B., Ruge D., Lo Gerfo E., Salerno S., Torriero S., Marconi B., Mori F., Driver J., Rothwell J. C., Caltagirone C. (2008). Hyperexcitability of parietal-motor functional connections in the intact left-hemisphere of patients with neglect. Brain 131, 3147–3155 10.1093/brain/awn273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusnir F., Chica A. B., Mitsumasu M. A., Bartolomeo P. (2011). Phasic auditory alerting improves visual conscious perception. Conscious. Cogn. 20, 1201–1210 10.1016/j.concog.2011.01.012 [DOI] [PubMed] [Google Scholar]
- Laberge D., Auclair L., Siéroff E. (2000). Preparatory attention: experiment and theory. Conscious. Cogn. 9, 396–434 10.1006/ccog.1999.0429 [DOI] [PubMed] [Google Scholar]
- Lupiáñez J., Decaix C., Sieroff E., Chokron S., Milliken B., Bartolomeo P. (2004). Independent effects of endogenous and exogenous spatial cueing: inhibition of return at endogenously attended target locations. Exp. Brain Res. 159, 447–457 10.1007/s00221-004-1963-5 [DOI] [PubMed] [Google Scholar]
- Lupiáñez J., Klein R. M., Bartolomeo P. (2006). Inhibition of return: twenty years after. Cogn. Neuropsychol. 23, 1003–1014 10.1080/02643290600588095 [DOI] [PubMed] [Google Scholar]
- Macquistan A. D. (1997). Object-based allocation of visual attention in response to exogenous, but not endogenous, spatial precues. Psychon. Bull. Rev. 4, 512–515 [Google Scholar]
- Mesulam M. M. (1981). A cortical network for directed attention and unilateral neglect. Ann. Neurol. 10, 309–325 10.1002/ana.410100402 [DOI] [PubMed] [Google Scholar]
- Migliaccio R., Agosta F., Toba M. N., Samri D., Corlier F., De Souza L. C., Chupin M., Sharman M., Gorno-Tempini M. L., Dubois B., Filippi M., Bartolomeo P. (2011). Brain networks in posterior cortical atrophy: a single case tractography study and literature review. Cortex [Epub ahead of print]. 10.1016/j.cortex.2011.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mort D. J., Malhotra P., Mannan S. K., Rorden C., Pambakian A., Kennard C., Husain M. (2003). The anatomy of visual neglect. Brain 126, 1986–1997 10.1093/brain/awg200 [DOI] [PubMed] [Google Scholar]
- Nobre A. C. (2001). The attentive homunculus: now you see it, now you don't. Neurosci. Biobehav. Rev. 25, 477–496 10.1016/S0149-7634(01)00028-8 [DOI] [PubMed] [Google Scholar]
- Ortigue S., Viaud-Delmon I., Annoni J. M., Landis T., Michel C., Blanke O., Vuilleumier P., Mayer E. (2001). Pure representational neglect after right thalamic lesion. Ann. Neurol. 50, 401–404 [DOI] [PubMed] [Google Scholar]
- Parasuraman R. (1998). “The attentive brain: issues and prospects,” in The Attentive Brain, ed Parasuraman R. (Cambridge, MA: The MIT Press; ), 3–15 [Google Scholar]
- Petrides M., Pandya D. N. (1984). Projections to the frontal cortex from the posterior parietal region in the rhesus monkey. J. Comp. Neurol. 228, 105–116 10.1002/cne.902280110 [DOI] [PubMed] [Google Scholar]
- Posner M. I. (1980). Orienting of attention. Q. J. Exp. Psychol. 32, 3–25 [DOI] [PubMed] [Google Scholar]
- Posner M. I., Inhoff A. W., Friedrich F. J., Cohen A. (1987). Isolating attentional mechanisms: a cognitive-anatomical analysis. Psychobiology 15, 107–112 [Google Scholar]
- Posner M. I., Petersen S. E. (1990). The attention system of the human brain. Annu. Rev. Neurosci. 13, 25–42 10.1146/annurev.ne.13.030190.000325 [DOI] [PubMed] [Google Scholar]
- Posner M. I., Rafal R. D., Choate L. S., Vaughan J. (1985). Inhibition of return: neural basis and function. Cogn. Neuropsychol. 2, 211–228 [Google Scholar]
- Posner M. I., Walker J. A., Friedrich F. J., Rafal R. D. (1984). Effects of parietal injury on covert orienting of attention. J. Neurosci. 4, 1863–1874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastelli F., Funes M. J., Lupiáñez J., Duret C., Bartolomeo P. (2008). Left neglect: is the disengage deficit space- or object-based? Exp. Brain Res. 187, 439–446 10.1007/s00221-008-1316-x [DOI] [PubMed] [Google Scholar]
- Rizzolatti G., Riggio L., Dascola I., Umilta C. (1987). Reorienting attention across the horizontal and vertical meridians: evidence in favor of a premotor theory of attention. Neuropsychologia 25, 31–40 [DOI] [PubMed] [Google Scholar]
- Robertson I. H. (2001). Do we need the “lateral” in unilateral neglect? Spatially nonselective attention deficits in unilateral neglect and their implications for rehabilitation. Neuroimage 14, S85–S90 10.1006/nimg.2001.0838 [DOI] [PubMed] [Google Scholar]
- Robertson I. H., Mattingley J. B., Rorden C., Driver J. (1998). Phasic alerting of neglect patients overcomes their spatial deficit in visual awareness. Nature 395, 169–172 10.1162/jocn.2006.18.8.1368 [DOI] [PubMed] [Google Scholar]
- Rode G., Cotton F., Revol P., Jacquin-Courtois S., Rossetti Y., Bartolomeo P. (2010). Representation and disconnection in imaginal neglect. Neuropsychologia 48, 2903–2911 10.1016/j.neuropsychologia.2010.05.032 [DOI] [PubMed] [Google Scholar]
- Rode G., Perenin M. T. (1994). Temporary remission of representational hemineglect through vestibular stimulation. Neuroreport 5, 869–872 [DOI] [PubMed] [Google Scholar]
- Rode G., Revol P., Rossetti Y., Boisson D., Bartolomeo P. (2007). Looking while imagining: the influence of visual input on representational neglect. Neurology 68, 432–437 10.1212/01.wnl.0000252936.54063.b0 [DOI] [PubMed] [Google Scholar]
- Rode G., Rossetti Y., Perenin M. T., Boisson D. (2004). Geographic information has to be spatialized to be neglected: a representational neglect case. Cortex 40, 391–397 [DOI] [PubMed] [Google Scholar]
- Roux F. E., Dufor O., Lauwers-Cances V., Boukhatem L., Brauge D., Draper L., Lotterie J. A., Demonet J. F. (2011). Electrostimulation mapping of spatial neglect. Neurosurgery 69, 1218–1231 10.1227/NEU.0b013e31822aefd2 [DOI] [PubMed] [Google Scholar]
- Schmahmann J. D., Pandya D. N. (2006). Fiber Pathways of the Brain. New York, NY: Oxford University Press [Google Scholar]
- Shinoura N., Suzuki Y., Yamada R., Tabei Y., Saito K., Yagi K. (2009). Damage to the right superior longitudinal fasciculus in the inferior parietal lobe plays a role in spatial neglect. Neuropsychologia 47, 2600–2603 10.1016/j.neuropsychologia.2009.05.010 [DOI] [PubMed] [Google Scholar]
- Sieroff E., Decaix C., Chokron S., Bartolomeo P. (2007). Impaired orienting of attention in left unilateral neglect: a componential analysis. Neuropsychology 21, 94–113 10.1037/0894-4105.21.1.94 [DOI] [PubMed] [Google Scholar]
- Singh-Curry V., Husain M. (2009). The functional role of the inferior parietal lobe in the dorsal and ventral stream dichotomy. Neuropsychologia 47, 1434–1448 10.1016/j.neuropsychologia.2008.11.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolov E. N. (1963). Higher nervous functions: the orienting reflex. Annu. Rev. Physiol. 25, 545–580 10.1146/annurev.ph.25.030163.002553 [DOI] [PubMed] [Google Scholar]
- Sturm W., Willmes K. (2001). On the functional neuroanatomy of intrinsic and phasic alertness. Neuroimage 14, S76–S84 10.1006/nimg.2001.0839 [DOI] [PubMed] [Google Scholar]
- Thakral P. P., Slotnick S. D. (2009). The role of parietal cortex during sustained visual spatial attention. Brain Res. 1302, 157–166 10.1016/j.brainres.2009.09.031 [DOI] [PubMed] [Google Scholar]
- Thiebaut de Schotten M., Dell'acqua F., Forkel S. J., Simmons A., Vergani F., Murphy D. G. M., Catani M. (2011). A lateralized brain network for visuospatial attention. Nat. Neurosci. 14, 1245–1246 10.1038/nn.2905 [DOI] [PubMed] [Google Scholar]
- Thiebaut de Schotten M., Kinkingnéhun S. R., Delmaire C., Lehéricy S., Duffau H., Thivard L., Volle E., Lévy R., Dubois B., Bartolomeo P. (2008). Visualization of disconnection syndromes in humans. Cortex 44, 1097–1103 10.1016/j.cortex.2008.02.003 [DOI] [PubMed] [Google Scholar]
- Thiebaut de Schotten M., Urbanski M., Duffau H., Volle E., Levy R., Dubois B., Bartolomeo P. (2005). Direct evidence for a parietal-frontal pathway subserving spatial awareness in humans. Science 309, 2226–2228 10.1126/science.1116251 [DOI] [PubMed] [Google Scholar]
- Toba M. N., Cavanagh P., Bartolomeo P. (2011). Attention biases the perceived midpoint of horizontal lines. Neuropsychologia 49, 238–346 10.1016/j.neuropsychologia.2010.11.022 [DOI] [PubMed] [Google Scholar]
- Urbanski M., Bartolomeo P. (2008). Line bisection in left neglect: the importance of starting right. Cortex 44, 782–793 10.1016/j.cortex.2007.04.001 [DOI] [PubMed] [Google Scholar]
- Urbanski M., Thiebaut de Schotten M., Rodrigo S., Catani M., Oppenheim C., Touzé E., Chokron S., Méder J.-F., Lévy R., Dubois B., Bartolomeo P. (2008). Brain networks of spatial awareness: evidence from diffusion tensor imaging tractography. J. Neurol. Neurosurg. Psychiatry 79, 598–601 10.1136/jnnp.2007.126276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanski M., Thiebaut de Schotten M., Rodrigo S., Oppenheim C., Touzé E., Méder J. F., Moreau K., Loeper-Jeny C., Dubois B., Bartolomeo P. (2011). DTI-MR tractography of white matter damage in stroke patients with neglect. Exp. Brain Res. 208, 491–505 10.1007/s00221-010-2496-8 [DOI] [PubMed] [Google Scholar]
- Valdes-Sosa M., Bobes M. A., Rodriguez V., Pinilla T. (1998). Switching attention without shifting the spotlight object-based attentional modulation of brain potentials. J. Cogn. Neurosci. 10, 137–151 [DOI] [PubMed] [Google Scholar]
- Vallar G., Perani D. (1986). The anatomy of unilateral neglect after right-hemisphere stroke lesions. A clinical/CT-scan correlation study in man. Neuropsychologia 24, 609–622 10.1016/0028-3932(86)90001-1 [DOI] [PubMed] [Google Scholar]
- Verdon V., Schwartz S., Lovblad K. O., Hauert C. A., Vuilleumier P. (2010). Neuroanatomy of hemispatial neglect and its functional components: a study using voxel-based lesion-symptom mapping. Brain 133, 880–894 10.1093/brain/awp305 [DOI] [PubMed] [Google Scholar]
- Vivas A. B., Humphreys G. W., Fuentes L. J. (2003). Inhibitory processing following damage to the parietal lobe. Neuropsychologia 41, 1531–1540 10.1016/S0028-3932(03)00063-0 [DOI] [PubMed] [Google Scholar]
- Vivas A. B., Humphreys G. W., Fuentes L. J. (2006). Abnormal inhibition of return: a review and new data on patients with parietal lobe damage. Cogn. Neuropsychol. 23, 1049–1064 10.1080/02643290600588400 [DOI] [PubMed] [Google Scholar]
- Wojciulik E., Rorden C., Clarke K., Husain M., Driver J. (2004). Group study of an “undercover” test for visuospatial neglect: invisible cancellation can reveal more neglect than standard cancellation. J. Neurol. Neurosurg. Psychiatry 75, 1356–1358 10.1136/jnnp.2003.021931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yantis S. (1995). “Attentional capture in vision,” in Converging Operations in the Study of Visual Selective Attention, edsKramer A. F., Coles G. H., Logan G. D. (Washington, DC: American Psychological Association; ), 45–76 [Google Scholar]