Abstract
BACKGROUND
Abnormal plasma fibrin architecture is a major determinant of both premature coronary artery disease (CAD) and hypofibrinolysis. The presence of the FXIIIVal34Leu genetic variant increases and accelerates fibrin stabilization. Its association with premature CAD remains controversial.
AIM
To evaluate fibrin clot structure/function in patients with premature CAD compared to healthy controls and whether the presence of the FXIII Val34Leu variant is an independent correlate of both impaired fibrinolysis and premature CAD.
METHODS
Fibrin phenotype and FXIII Val34Leu genetic variant were determined in a cohort of 242 young patients (<45 years) who survived an MI and compared to 242 healthy controls matched for age and gender. Fibrin clot stiffness (elastic modulus) and response to rt-PA mediated fibrinolysis (clot lysis time and fibrinolysis rate) were measured using the Hemodyne analyser and by confocal microscopy. The effect of FXIII Val34Leu on long term survival was also evaluated.
RESULTS
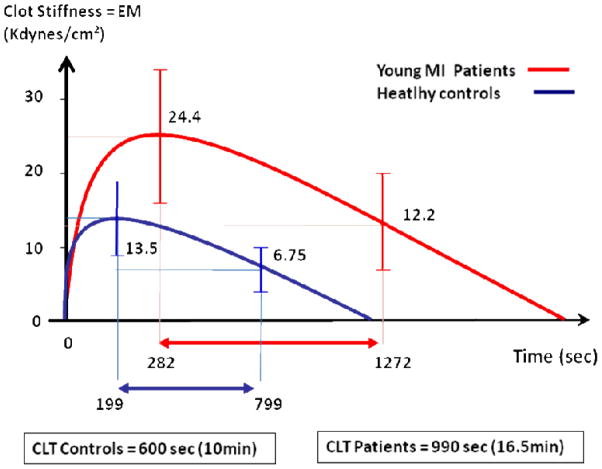
CAD patients produced stiffer fibrin clots as compared to healthy controls (24.7±16 vs. 13.6±6 kdynes/cm2; p<0.0001) and displayed a reduced response to fibrinolysis with longer clot lysis time (16.5±12 vs. 10±7 min; p<0.0001) and lower fibrinolysis rate (8.3±7. vs. 14.7±19 sec−1×10−4; p<0.0001). Factor XIII Val34Leu presence led to a stepwise decrease in fibrinolysis rate with a gene dose effect in both patients (9.4±8 vs. 6.9±7 vs. 5.5±4 sec−1×10−4, for wildtype, heterozygous and homozygous, respectively, p value for all =0.02) and healthy controls, suggesting an effect independent from CAD. A similar impact of the Factor XIII Val34Leu substitution was observed on clot lysis time. Increased clot stiffness and hypofibrinolysis were both independent correlates of premature CAD. Factor XIII Val34Leu presence was neither protective of premature CAD (adjOR 0.83 [0.49–1.4] nor did it impact long term clinical outcome during a median follow-up of 6.3 years (±2.4).
CONCLUSIONS
Stiff fibrin that is more resistant to fibrinolysis is a major determinant of premature CAD. Presence of the factor XIII Leu34 genetic variant provides a pharmacogenetic resistance to fibrinolysis ex-vivo but neither relates to premature CAD nor to recurrent acute coronary events.
Keywords: Atherothrombosis, Coronary Disease, Factor XIII Val34Leu, Fibrin structure, Fibrinogen, Hypofibrinolysis, Pharmacogenetic, Thrombosis
INTRODUCTION
Fibrinogen is a well established cardiovascular risk factor and a major predictor of clot architecture 1, 2. Recent investigations have demonstrated that abnormal fibrin physical properties characterized by the formation of stiff plasma fibrin with subsequent hypofibrinolysis are independent correlates of premature CAD 3. However, determinants of this particular phenotype remain little explored. 4, 5,6. Activated factor XIII covalently cross links fibrin to increase clot stability, leading to a stiffer three-dimensional structure that is more resistance to fibrinolysis7. The FXIII Leu34 genetic variant, present in 40% of the caucasian population, is one ofthe most relevant functional polymorphisms described in the haemostatic system, as it increases and accelerates fibrin stabilization. A single investigation has reported that the presence of this variant was associated with failure of fibrinolytic therapy in ST-elevation myocardial infarction (STEMI) presenters8. However, whether the presence of the FXIII Leu34 variant by itself is a determinant of both CAD and long-term clinical outcome remains subject to controversies7–12. Indeed, regulation of fibrin structure and function depends on a complex gene-environment interactions resulting in possible conflicting results13 and we believe that rather than just the effect of a single polymorphism, the impact of genetics on therapeutics seems to be more important in a clinical setting14.
Prior familial history is a strong risk factor of premature CAD, suggesting a potential genetic susceptibility, so in the present investigation we have conducted a large case-control study comparing patients with established premature CAD with healthy controls matched for age and gender, in order to (1) confirm in a large cohort that abnormal fibrin phenotype was a determinant of premature CAD (2) test the hypothesis that presence of FXIII Leu34 is an independent predictor of both premature CAD and recurrent thrombotic events (3) test the hypothesis that the presence of FXIII Leu34 provides pharmacogenetic resistance to fibrinolysis ex-vivo.
METHODS
Study design and eligibility
Between April 1, 1996, and April 1, 2006, 242 consecutive patients presenting a myocardial infarction (MI) and aged < 45 years at the time of the first coronary events entered the ongoing AFIJI registry (Appraisal of risk Factors in young Ischemic patients Justifying aggressive Intervention), a prospective web-based registry which was dedicated to the management of premature coronary artery disease, including risk factors and genetic profiling. This programme was designed in 1996 to identify classic and unusual risk factors, as well as genetic factors affecting complex phenotypes of coronary thrombosis, and to implement an educational programme and optimize medical treatment to prevent new coronary events. To be eligible, patients (aged >18 and <45 years) needed to have an established coronary disease defined by an episode of myocardial infarction (ST-elevation or non-ST-elevation myocardial infarction) and genotyping for the Factor XIII Val34Leu polymorphism. In order to create a case-control study, we matched these 242 patients to 242 healthy controls for age and gender. Healthy controls were recruited from a primary prevention program run at the same hospital and could enter the study if they were without known diabetes or hypertension and without personal history of cardiovascular disease. All patients and controls gave informed consent according to the study protocol and all the results obtained were for research purposes only. This study was promoted by the Assistance Publique - Hopitaux de Paris (AP-HP) and was reviewed by the Institutional Review Board Ethics Committee of the Pitié-Salpêtrière Hospital in Paris (CPP), France.
Blood Sampling and Data Collection
Blood sampling for healthy volunteers was performed when they entered the primary prevention program after being checked for exclusion criteria and was performed for patients after stabilization more than 3 months after the last acute coronary event. All blood samples were collected by venipuncture of the forearm and collected in a 4.5 ml tube with of 0.129 M of citrate (Vacutainer™, Becton-Dickinson, Oxford, England). Citrated samples were centrifuged at 3000g for 20 minutes, and aliquots of 0.6 mL of plasma supernatant were stored at − 80°C until assay. A fasting blood sample was taken for plasma determination of classic and rare risk factors of coronary artery disease and for DNA analysis. Medical history and risk factors were assessed. Blood pressure was measured to the nearest 2 mm Hg and calculated as a mean of 3 consecutive readings. Hypertension was identified as an average blood pressure >140/90 mm Hg. Individuals were qualified as diabetic if on prior antidiabetic drugs or if fasting glycemia >1.26 g/L twice. Smoking status was recorded as active or not. Familial history of coronary artery disease was defined as any coronary event that occurred in first-degree relatives of the patient before 60 years of age in men and 65 years in women. Dyslip-idemia was defined in patients and control with: total CT > 6.2 mmol/L and TG >2.25 mmol/L or if they were under a lipid lowering treatment. Follow-up was done every 6 months in a dedicated outpatient’s clinic with a detailed report of drug treatment.
Genotyping and biological risk factors
Genotyping of the Factor XIII Val34Leu (G103T) polymorphisms was performed using polymerase chain reaction (PCR)-restriction fragment length polymorphism assays. Probe and primers for Factor XIII Val34Leu genotyping were the following: Forward Primer GTA AAG TCA AAAATG TCA GAA AC and Reverse Primer GTT GAC GCC CAG GGG CAC CG with a premix 2.0 mM MgCl2 premix at 94°C for 5 min, then 35 cycles (94° 20 sec + fixation primers 58°C 20 sec + 72°C 20 sec) and 10 min at 72°C. Enzymatic digestion occurred overnight at 37°C with Hin P I and samples were separated by SDS Page. In addition to the classic cardiovascular risk factors, we systematically measured plasma concentrations of biomarkers, which have previously been associated with prognosis of coronary artery disease. Plasma fibrinogen was measured by the Clauss method.
Fibrin clot viscoelastic-properties and response to rt-PA mediated fibrinolysis
Clots were formed in a thermostable 37°C plastic plate by recalcifing 500 μL of platelet poor plasma by adding CaCl2 (6 mM final concentration) and 10 μL of purified recombinant human tissue factor (Innovin, Dade Behring). To facilitate lysis, we simultaneously added 10 μL of tissue plasminogen activator (rt-PA) (Boehringer Ingelheim, Germany) at a final concentration of 0.5 nM. Viscoelastic properties of plasma clots were measured with the Hemodyne RM-2 Analyser (Hemodyne, Richmond, VA) using the theory of the torsion pendulum as previously described 3. Recordings of free oscillations allowed the calculation of the maximum elastic modulus (EM) (dynes per centimeter squared), which reflects the stiffness of the clot, and fibrinolysis was assessed by continuous monitoring of the EM and expressed as the rate of lysis (in per seconds). The accuracy of this assay has been shown to be similar to the determination of the lysis front velocity (or fiber lysis rate). Clot lysis time (CLT) was defined as the time needed for the maximum EM to decrease by 50%. The intra-individual variability of this technique was calculated to 9.8% on pooled plasma from 15 healthy controls.
Confocal Microscopy
A confocal microscopy sub analysis was performed in a sample of n=22 patients representative of each genotype as previously described 15. Fibrin clots were prepared using PPP mixed with 0.075 mM final concentration of Alexa 488-labeled human fibrinogen (Molecular Probes, molar ratio of dye:protein = 8:1). This preparation was soaked into dedicated micro-chambers after addition of 0.4 μL of purified recombinant human tissue factor (Innovin, Dade Behring) and CaCl2 to a final concentration of 20 mM to allow clotting. After 20 minutes incubation in a moist atmosphere at 37°C, labelled specimens were examined with a Leica confocal laser scanning microscope (Carl Zeiss, Inc) linked to an inverted microscope equipped with a 63X, 1.2 NA water lens using a 488 nm Argon or a 633 nm HeNe laser. A stack of 10 μm was obtained by scanning every 0.5μm, starting at 50 μm above the cover-slip. A volume of 5 μl of rt-PA (0.5 nM final concentration) was loaded at the edge of the labelled fibrin clot, whose edge was visualized by the confocal microscopy. Scanning was performed every 15 seconds. Gain and offset were adjusted for each image so that 1% of total pixels were saturated and 1% of pixels were zero. Lysis front velocity and the rate of fiber digestion were determined from reconstructed confocal microscope images which were combined in videos. Lysis speed was calculated by dividing the distance of the front lysis progression by the time to obtain a full lysis of the stack.
Outcomes
The primary endpoint was a composite of cardiovascular death, non-fatal myocardial infarction, and urgent revascularisation. Cardiovascular death was regarded as any death with a demonstrable cardiovascular cause or any death not clearly attributable to a non-cardiovascular cause. Myocardial infarction was defined as a recent ischaemic symptom with electrocardiographic abnormalities in the ST segment (depression or elevation of at least 0·1 mV) and a positive troponin concentration as defined locally. Urgent revascularisation was defined as a coronary (any vessel) revascularisation (any type percutaneous coronary intervention or coronary artery bypass graft) decided(?) within 24 h of a recurrent episode of myocardial ischaemia. Additionally, we assessed stent thrombosis on the basis of definitions from the Academic Research Consortium, with the pre-specified key endpoint of definite (i.e, total occlusion originating in or within 5 mm of the stent) or visible thrombus within the stent or within 5 mm of the stent in the presence of an acute ischaemic clinical syndrome within 48 h.
Statistical Analysis
Continuous variables were expressed as mean ± standard deviation and categorical variables were expressed as percentages. The comparability between groups was assessed using Mann–Whitney test for continuous variables and Fisher exact test for qualitative variables, respectively. The level was set at 0.05. Correlations between fibrinogen and clot stiffness was tested by using Spearman correlation coefficients. Potential associations between fibrinolysis rate or fibrin stiffness and covariates were first tested in univariate analysis by using the Spearman correlation for continuous variables and the Wilcoxon rank-sum test for categorical variables. All variables with a probability value of <0.15 were then included in a multivariate analysis of covariance. Relationships between premature CAD and covariates were first studied using a univariate conditional logistic regression for matched pair’s data. Uni-variate variables with a probability value of <0.15 were then included in a forward multivariate logistic regression for matched pairs data. All p values are two-sided. Estimates for hazard ratios and corresponding 95% CI were obtained for every significant outcome factor. The three genotype categories were collapsed into two categories, resulting in a dominant model of inheritance: Val34/Val34 (non-carriers) versus Val34/Leu34 and Leu34/Leu34 (carriers) for FXII Val34Leu allele. The time to first cardiovascular event was then compared between genotypes (carriers vs. non-carriers of FXII 34Leu allele) with log-rank tests. Follow-up of patients was censored(?) at the date of the first recurrent event corresponding to the primary endpoint. In event-free patients, follow-up ceased(??) at the date of the last follow-up visit. Analyses were performed independently by the biostatistics department of our university hospital, with SAS Software v.8 (SAS Institute, Cary, NC, 1999) and with GraphPad Prism version 5.00 for Windows, GraphPad Software, San Diego California).
Role of the funding source
This study had no industrial funding and study design, data collection, data analysis, interpretation of data, or writing of the report were all under the responsibility of the authors who had full access to all the data in the study.
RESULTS
Population baseline characteristics
A total of 242 patients who were survivors of MI entered the secondary prevention programme and 242 controls matched for age and gender entered the study. Measurements of clot stiffness and response to fibrinolysis was performed in all patients but genotyping was only available for 228 patients (94.2%) and 219 controls (90.5%). The majority of patients were young males with a high prevalence of smoking habits and familial history of CAD. Premature CAD patients had a higher body mass index than healthy controls (Table 1). Other conventional risk factors for coronary artery disease were infrequent and absent from the healthy control group. The initial presentation was predominantly STEMI, underlying the importance of acute coronary thrombosis in this population. CAD disease was single vessel in about two-thirds of patients. Percutaneous coronary intervention with stent placement was done in most patients (73.0%), with bare-metal stents used more frequently than drug-eluting stents (54.0% vs. 32%) and with no stents in 14%. As opposed to healthy controls, almost all patients of the premature CAD patients were on long-term treatment with aspirin (97.3%), clopidogrel (71%) and statin (95%). The majority were also on β-blocker (91.1%) and angiotensin converting enzyme inhibitor (74.5%). Fibrinogen concentration, a major determinant of clot stiffness and response to fibrinolysis was not different between the control group and patients, as opposed to other classical biomarkers (Table 2).
Table 1.
Baseline Clinical Characteristics (NA=not applicable)
| Controls (n=242) | Patients (n=242) | p | |
|---|---|---|---|
| Baseline Characteristics | |||
| Age (y) | 39.1 ± 5.3 | 39.1 ± 5.3 | NA |
| Male gender | 215 (88.8%) | 215 (88.8%) | NA |
| Risk Factors | |||
| Body Mass Index (kg/m2) | 24.3 ± 3.4 | 25.5 ± 3.6 | 0.002 |
| Familial CAD | 31 (12.8%) | 105 (43.4%) | <0.0001 |
| Active smoker | 74 (30.4%) | 128 (52.8%) | <0.0001 |
| Prior or active smoker | 133 (55.1%) | 199 (82.4%) | <0.0001 |
| Hypertension | 0 | 35 (14.6%) | NA |
| Known diabetes | 0 | 24 (9.9%) | NA |
| Dyslipidemia or lipid lowering treatment | 73 (30.2%) | 173 (71.7%) | <0.0001 |
| Clinical Presentation | |||
| ST-segment elevation MI | 0 | 189 (78%) | NA |
| Non ST-segment elevation MI | 0 | 53 (22%) | NA |
| Coronary revascularization | 0 | 182 (75.1%) | NA |
Table 2.
Haemostasis biomarkers and fibrin properties
| Controls (n=242) | Patients (n=242) | p | |
|---|---|---|---|
| Haemostasis biomarkers | |||
| Fibrinogen (mg/mL) | 3.1 ± 0.5 | 3.2 ± 0.9 | NS |
| D-Dimers (ng/ml) | 221.4 ± 194 | 364.7 ± 592 | <0.001 |
| Factor VII (%) | 119.13 ± 26.0 | 106.4 ± 28.3 | <0.001 |
| Factor II (%) | 104.7 ± 11.6 | 98.3 ± 16.8 | <0.001 |
| Triglyceride (mmol/L) | 1.1 ± 0.7 | 1.74 ±1.1 | <0.05 |
| Total Cholesterol (mmol/L) | 5.7 ± 1 | 5.5± 1.6 | <0.05 |
| Fibrin physical properties and fibrinolysis | |||
| Maximum EM (G′) (kdynes/cm2) | 13.5 ± 5.8 | 24.5 ± 16.4 | <0.0001 |
| Clot Lysis Time (min) | 10.3 ± 7.2 | 16.4 ± 12.6 | <0.0001 |
| Fibrinolysis rate (sec−1) × 10−4 | 14.5 ± 1.6 | 8.4 ± 0. 8 | <0.0001 |
Genotype distribution of Factor XIII Val34Leu variant and outcomes
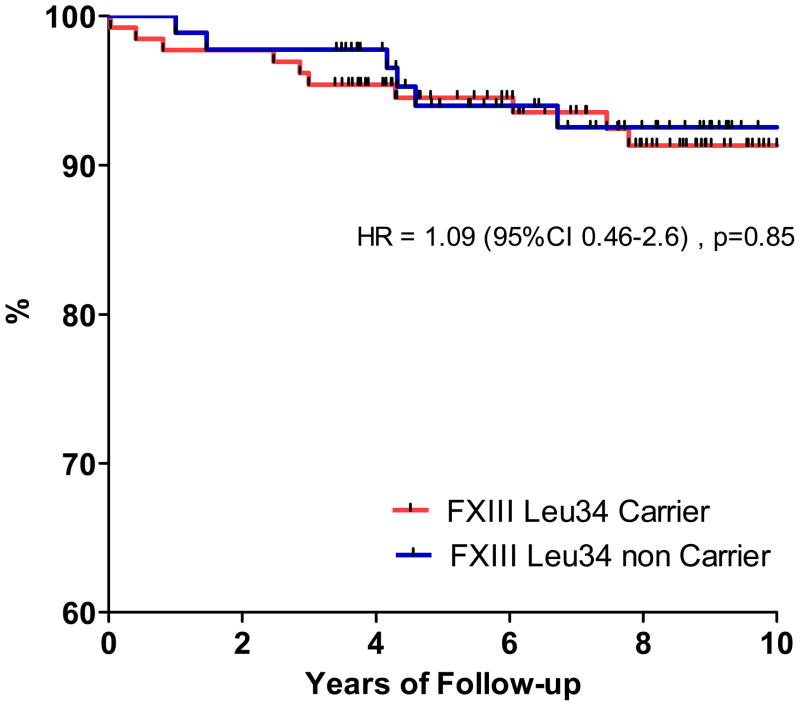
Distributions of Factor XIII Val34Leu genotypes were similar between patients and controls (Table 3). Baseline demographics, clinical presentation and therapy did not differ significantly between wild-type homozygous patients and those carrying the Leu 34 allelic variant (data not shown). There was no deviation from the expected proportions of genotypes in the population predicted by the Hardy-Weinberg equilibrium for polymorphisms. The mean duration of patients’ follow-up was 6.3 years (±2.4), with a maximal length of 10 years. A single patient was lost and 21 major adverse cardiac events were recorded, 13 in FXIII Leu carriers and 8 in non carriers. Figure 1 shows the Kaplan-Meier curve for survival free from the composite primary endpoint with no differences between carriers and non-carriers.
Table 3.
Genotype distribution
| Genotype | Controls n= 242 | Patients n= 242 | p |
|---|---|---|---|
| Factor XIII (G103T) | |||
| Wild Type GG (Val/Val) | 52.9 % (128) | 58.3 % (141) | 0.23 |
| Heterozygous GT (Val/Leu) | 40.9 % (99) | 35.9 % (87) | 0.26 |
| Homozygous TT (Leu/Leu) | 6.2 % (15) | 5.8 % (14) | 0.84 |
| Leu34 Carrier | 47.1 % (114) | 41.7 % (101) | 0.23 |
Figure 1.
Long term outcomes according to the presence of the FXIII Leu34 genetic variant. Kaplan Meier curves represent survival free from CV events (Death/MI/Revasc)
Fibrin Physical Properties and Hypofibrinolysis
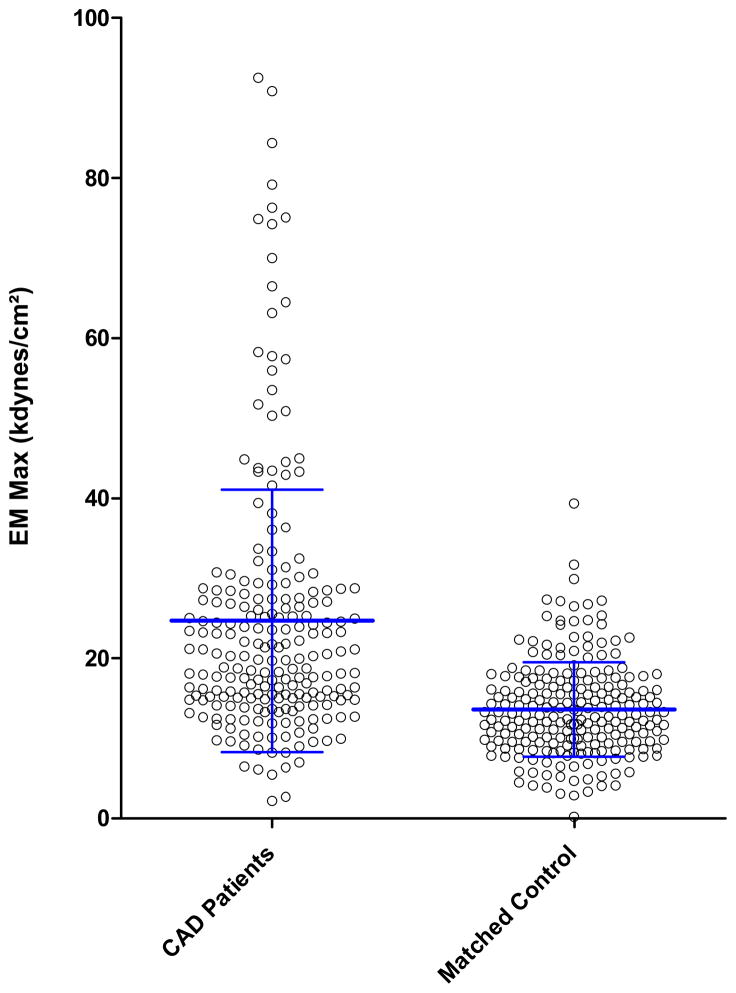
Patients with premature CAD produced two-fold stiffer plasma fibrin clot than controls as represented by the maximum EM, a measure of fibrin clot storage modulus (G′) (Figure 2). Continuous monitoring of EM allowed real-time assessment of rt-PA mediated fibrinolysis after full fibrin stabilization by activated Factor XIII. There was a 59% increase in clot lysis time among patients vs. controls suggesting that altered fibrin properties from young CAD patients led to reduced fibrinolysis. Indeed, fibrinolysis rate, which is normalized according to fibrin stiffness, was reduced by 42% in patients vs. controls (Table 2). This was found irrespective of plasma fibrinogen concentration, which did not differ between patients and controls and which significantly correlated with clot stiffness (r=0.43; 95% CI 0.35–0.51, p<0.001) but not with fibrinolysis rate (r=0.07; 95% CI 0.35–0.51p=0.09) in both groups.
Figure 2.
Measure of fibrin viscoelastic properties by maximum elastic modulus, a measure of fibrin clot stiffness in 242 premature CAD patients and in 242 matched healthy controls. Central line represents the mean and bars the standard deviation.
Impact of Factor XIII Leu34 allele on fibrinolysis
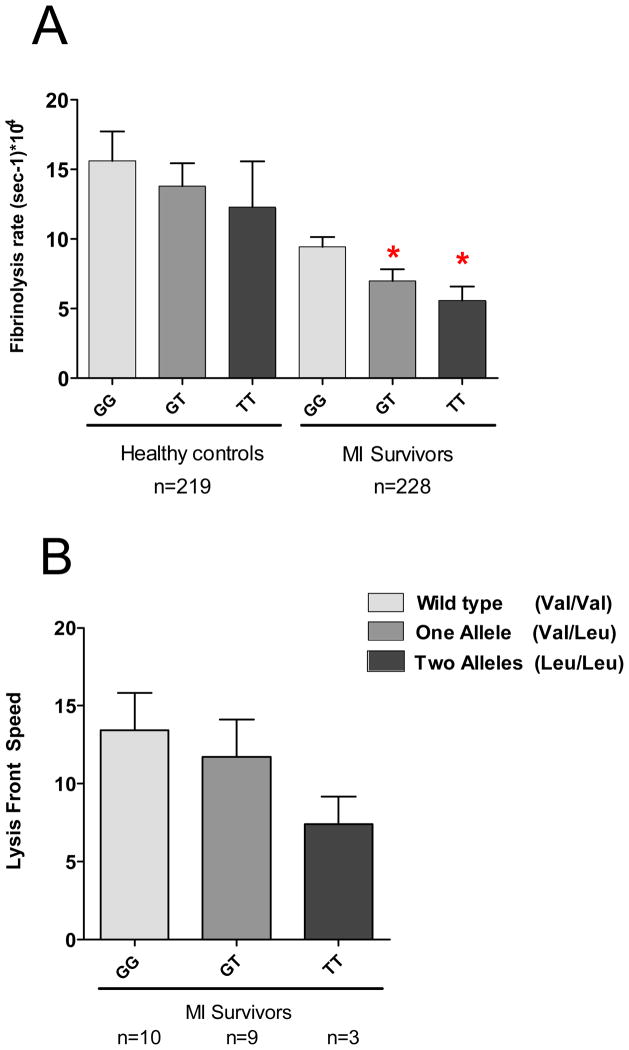
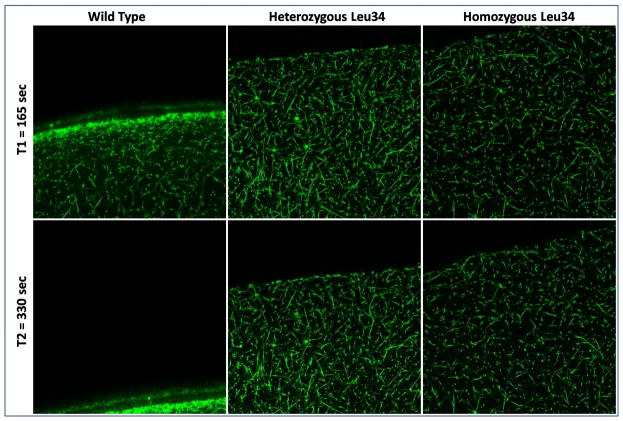
The FXIII Leu34 allele reduced response to rt-PA mediated fibrinolysis in patients with an even more pronounced effect in homozygous carriers (Figure 4). This genotype-phenotype interaction was observed in both patients and controls, indicating an effect independent of premature CAD. The detrimental effect of the FXIII Leu34 allele was also confirmed by measuring the lysis front velocity visualized by confocal microscopy (figure 4B and figure 5) in a subgroup of 22 patients. The progression of the lysis front was dramatically reduced in hetero and homozygous carriers of FXIII Leu 34 variant with a clear gene dose effect.
Figure 4.
Impact of the presence of FXIII Leu34 variant on rt-PA mediated fibrinolysis assessed by continuous monitoring of either EM (A) or lysis front velocity in young post-MI patients and controls (B). * indicates p<0.05 when compared to the more common variant.
Figure 5.
Impact of the presence of the FXIII Leu34 variant on extrinsinc fibrinolysis assessed by lysis front motion visualized by confocal microscopy with Alexa 488-labeled human fibrinogen. Each frame is taken every 15 sec and represents a stack of 50μm×50μm×10μm. Time refers to rt-PA loading.
Figure 5 bis. Videos of lysis according to the genotype for FXIII Val34Leu variant. Corresponding to the images taken in figure 5. Total movie lengths are 4.25 minutes for the wild type and 30 minutes each for the heterozygous and the homozygous.
Independent predictors of premature CAD, fibrin clot stiffness and hypofibrinolysis
As expected, classical cardiovascular risk factors, including heredity, smoking, dyslipidemia and overweight, were significantly related to premature CAD and to hypofibrinolysis after univariate analysis (table 4). After multivariate analysis. fibrin clot stiffness (EM) and response to rt-PA mediated fibrinolysis (CLT and Fibrinolysis rate) were both found to characterize premature CAD.
Table 4.
Independent predictors of Premature CAD
| Premature CAD | |||
|---|---|---|---|
| p | Adj. Odds Ratio1 [95% CI] | ||
| Univariate | Multivariate1 | ||
| Age | NA | NA | - |
| Gender | NA | NA | - |
| Diabetes | NA | NA | - |
| Hypertension | NA | NA | - |
| Heredity | <0.0001 | 0.0045 | 4.0 [1.5–10.2] |
| Smoking | <0.0001 | 0.0069 | 3.7 [1.4–9.7] |
| Dyslipidemia | <0.0001 | <0.0001 | 9.8 [3.5–28] |
| BMI | 0.002 | NS | - |
| Fibrinogen | NS | NS | - |
| EM (kdynes/cm2) | <0.0001 | <0.0001 | 1.2 [1.1–1.3]* per units |
| Fibrinolysis Rate | <0.0001 | <0.0001 | ?? [-] per units |
| CLT (sec) | <0.0001 | 0.0012 | 1.001 [1.001–1.002]* per units |
| Variable | Adj. Odds Ratio1 [95% CI] | p | |
|---|---|---|---|
| FXIII Val34Leu (G103T) | GG (Val-Val) | - | - |
| GT (Val-Leu) | 0.834 [0.470 – 1.467] | NS | |
| TT (Leu-Leu) | 0.531 [0.152 – 1.790] | NS | |
| FXIII | Allele T (Leu) | 0.828 [0.490 1.399] | NS |
adjusted for fibrinogen concentration, and cardiovascular risk factors such as heredity, smoking status, overweight, dyslipidemia and diabetes.
Covariance analysis showed that diabetes and fibrinogen concentration were the only predictors of clot stiffness with a 78% increase in EM (p=0.001) in diabetics patients and 30% increment in EM (p<0.001) for an increase of 1g/L in plasma fibrinogen concentration. The presence of the Factor XIII Leu34 allele did not impact clot stiffness but was the unique independent correlate of hypofibrinolysis after multivariable analysis and was associated with a 32% decrease (p=0.016) in fibrinolysis rate with a gene-dose effect. Fibrinolysis was decreased by 26% (p=0.03) and 42% (p= 0.02) in heterozygous and homozygous carriers vs. wildtype, respectively. Fibrinogen level was neither related to premature CAD nor to fibrinolysis rate.
Discussion
Fibrin is the major protein constituent of the blood clot16. Its structure/function represents a key phenotype arising from activation of the fluid phase of coagulation and it has been shown to be altered in venous thromboembolism 17 and evidence, although limited and from small populations, suggests a role in atherothrombosis. Factors influencing clot architecture are numerous and the mechanisms by which they lead to increased cardiovascular risk are subject to controversies18. We report here the largest case-control study establishing that altered fibrin phenotype and the resulting defective fibrinolysis are independent correlates of premature CAD in young post-MI patients. The novelty of the present investigation is that the presence of factor XIII Leu34 genetic variant, the most powerful determinant of altered fibrinolysis, was indeed correlated to a resistance to fibrinolysis ex-vivo with a gene-dose effect, but was associated neither with premature MI nor with recurrent acute coronary events.
A relationship exists between fibrin clot structure and fibrinolysis. such that clots made of thin fibers, with increased density and a more tightly knit cross-linked structure, are more resistant to fibrinolysis15. These clots are stiffer, have thinner fibers, and begin polymerization more quickly than matched controls7. Previous studies suggest that both environmental and genetic factors influence fibrin clot structure.
In the present investigation, we establish that risk factors, including active smoking, dyslipidemia and heritability of MI, are independent predictors of premature CAD but do not impact fibrin clot visco-elastic properties. In accordance with previous investigations, we found that diabetes has a strong correlation with altered fibrin properties and is the only classical risk factor significantly associated with increased fibrin stiffness19. However, its low prevalence in our particular study population accounts why it is not an independent correlate of premature CAD. Conversely, plasma fibrinogen level was strongly associated with altered fibrin properties but not with premature CAD. These findings suggest a unique and complex role of increased fibrin stiffness as a potential link between impaired fibrinolysis and increased risk of CAD20.
Of major importance, the dramatic differences observed in clot stiffness and response to fibrinolysis between patients and controls persisted after adjustment for fibrinogen and cardiovascular risk factors. This further suggested that the mechanisms by which lifestyle changes translate into increased cardiovascular risk remains to be established. Indeed, our study was conducted three months after the acute MI and risk factors, including lack of exercise, overweight and smoking, were controlled in the vast majority of our study population. This prompted us to investigate the role of a genetic variant on top of cardiovascular risk factors, although major influences that affect fibrin structure/function appear to be environmental with little effect of heritability21.
The impact of the common variant in the Factor XIIIA gene (Val34Leu) or FXIII Leu34 variant was investigated for several reasons. Factor XIII, when activated by thrombin, covalently crosslinks fibrin multimer strands, thereby enhancing the mechanical strength of the clot and increasing the resistance to fibrinolysis22. This polymorphism is the main functional polymorphism influencing FXIII activation23 and is associated with fibrinolysis failure in STEMI patients8. We report no significant difference in the distribution of the FXIII Val34Leu polymorphism between patients and controls, suggesting no interaction with the premature CAD. Our results rather indicate a protective effect (OR 0.83; 95%CI 0.49–1.4) and are consistent with previous investigations24–27 and two recent meta-analysis of retrospective studies of 8,743 and 12,399 patients reporting odds ratios of 0.79 (95% CI 0.66–0.93) and 0.81 (95% CI 0.70 – 0.92) respectively28,29. Conversely, there was no effect of this genetic variant on the occurrence of new acute coronary events in young post MI patients, despite a median follow-up of more than 6 years. These results add to the debate on the effect of this genetic variant on the risk of cardiovascular events, which is poorly understood.
Paradoxically, we demonstrate here that the possession of this genetic variant, results in a resistance to fibrinolysis ex-vivo in patients, but also in healthy volunteers. This pharmacogenetic resistance was found with two different techniques, one evaluating intrinsic fibrinolysis and the other extrinsic fibrinolysis. These results are complementary to the results of the EuroCLOT study, where the Leu34 allele of the FXIII Val34Leu polymorphism was associated with slightly prolonged lysis times using high throughput turbidimetric assays30. This latter result conflicts with the reported protective effect of Leu34, while supporting the pharmacogenetic effect reported in the study of Marin et al. showing less effective thrombolytic therapy after acute MI in subjects possessing Leu348. Our study brings a path-ophysiological explanation and appears to be a relevant approach to resolve this issue, given the documented resistance to fibrinolysis ex-vivo associated with this genetic variant.
How this polymorphism relates to the risk of vascular disease remains to be fully elucidated. We believe that it is likely that the FXIII Leu34 variant interacts strongly with patient response to fibrinolysis in terms of pharmacodynamics but that its impact on the development or the progression of CAD is limited or absent. However, the pharmacogenetic effect of Leu34 becomes an important factor when patients suffering from a STEMI are treated by lytic therapy, exposing them to a reduced response. Our experimental study allowed the demonstration of this pharmacogenetic interaction for both intrinsic and extrinsic fibrinolysis, which likely occurs during lytic therapy with a gene-dose effect.
Several limitations should be acknowledged. First, morphological analysis of the 3D architecture of the fibrin network could not be evaluated in the entire study population due to obvious technical reasons. However, we presume that our previous findings showing fibrin with thinner and shorter fibrin fibers would apply to the present population with similar baseline characteristics3. Then, although platelets have a critical impact on fibrin assembly, fibrin mechanical properties and fibrinolysis, their effect was not evaluated and could not be routinely assessed given the limited timing required for such studies.
In conclusion, we demonstrated that altered fibrin clot structure/function characterized by a stiff fibrin clot more resistant to fibrinolysis is a major determinant of premature CAD, a particular phenotype which is under the influence of a gene-environment interactions. Our study also provides new insight into consequences of the presence of the FXIII Leu34 genetic variant, which is strongly associated with resistance to fibrinolysis in a gene-dose dependent manner, especially relevant to lytic therapy, but neither relates to premature CAD nor to recurrent acute coronary events. This pharmacogenetic resistance to fibrinolysis should be more intensively investigated and integrated into future pharmacological trials, including fibrinolytic treatment.
Figure 3.
Graphic representation of differences in response to rt-PA mediated fibrinolysis expressed as mean Clot Lysis Time (CLT) between young MI patients and healthy controls.
Acknowledgments
Funding:
This study was supported by the French Federation for Medical Research (FRM) and partially by NIH HL30954 and HL090774.
We thank Ghalia Anzana, Delphine Brugier, Sophie Galier, Vanessa Gallois and Nicolas Vignolles for their expert technical assistance. We are grateful to BTR-AFM for storage of samples.
ABBREVIATIONS
- ACS
Acute Coronary Syndrome
- CAD
Coronary Artery Disease
- CLT
Clot Lysis Time
- EM
Maximum Elastic Modulus
- LMWH
Low Molecular Weight Heparin
- MACE
Major Cardiovascular Events
- MI
Myocardial Infarction
- PAI-1
Plasminogen Activating Inhibitor
- PCI
Percutaneous Coronary Intervention
- rt-PA
recombinant tissue Plasminogen Activator
- STEMI
ST Segment Elevation Myocardial Infarction
- TnI
Troponin I
- UFH
Unfractionned Heparin
References
- 1.Ernst E, Resch KL. Fibrinogen as a cardiovascular risk factor: a meta-analysis and review of the literature. Ann Intern Med. 1993 Jun 15;118(12):956–963. doi: 10.7326/0003-4819-118-12-199306150-00008. [DOI] [PubMed] [Google Scholar]
- 2.Ryan EA, Mockros LF, Weisel JW, et al. Structural origins of fibrin clot rheology. Biophys J. 1999 Nov;77(5):2813–2826. doi: 10.1016/S0006-3495(99)77113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collet JP, Allali Y, Lesty C, et al. Altered fibrin architecture is associated with hypofibrinolysis and premature coronary atherothrombosis. Arterioscler Thromb Vasc Biol. 2006 Nov;26(11):2567–2573. doi: 10.1161/01.ATV.0000241589.52950.4c. [DOI] [PubMed] [Google Scholar]
- 4.Collet JP, Soria J, Mirshahi M, et al. Dusart syndrome: a new concept of the relationship between fibrin clot architecture and fibrin clot degradability: hypofibrinolysis related to an abnormal clot structure. Blood. 1993 Oct 15;82(8):2462–2469. [PubMed] [Google Scholar]
- 5.Collet JP, Montalescot G, Soria J, et al. Pharmacological remodeling of the thrombus architecture. Blood Coagul Fibrinolysis. 1999 Feb;10( Suppl 1):S49–53. [PubMed] [Google Scholar]
- 6.Fatah K, Silveira A, Tornvall P, et al. Proneness to formation of tight and rigid fibrin gel structures in men with myocardial infarction at a young age. Thromb Haemost. 1996 Oct;76(4):535–540. [PubMed] [Google Scholar]
- 7.Mills JD, Ariens RA, Mansfield MW, et al. Altered fibrin clot structure in the healthy relatives of patients with premature coronary artery disease. Circulation. 2002 Oct 8;106(15):1938–1942. doi: 10.1161/01.cir.0000033221.73082.06. [DOI] [PubMed] [Google Scholar]
- 8.Marin F, Gonzalez-Conejero R, Lee KW, et al. A pharmacogenetic effect of factor XIII valine 34 leucine polymorphism on fibrinolytic therapy for acute myocardial infarction. J Am Coll Cardiol. 2005 Jan 4;45(1):25–29. doi: 10.1016/j.jacc.2004.09.051. [DOI] [PubMed] [Google Scholar]
- 9.Hancer VS, Diz-Kucukkaya R, Bilge AK, et al. The association between factor XIII Val34Leu polymorphism and early myocardial infarction. Circ J. 2006 Mar;70(3):239–242. doi: 10.1253/circj.70.239. [DOI] [PubMed] [Google Scholar]
- 10.Marin F, Corral J, Roldan V, et al. Factor XIII Val34Leu polymorphism modulates the prothrombotic and inflammatory state associated with atrial fibrillation. J Mol Cell Cardiol. 2004 Sep;37(3):699–704. doi: 10.1016/j.yjmcc.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 11.Franco RF, Pazin-Filho A, Tavella MH, et al. Factor XIII val34leu and the risk of myocardial infarction. Haematologica. 2000 Jan;85(1):67–71. [PubMed] [Google Scholar]
- 12.Marin F, Roldan V, Sogor F. Factor XIII Val34Leu polymorphism and premature myocardial infarction. Rev Esp Cardiol. 2002 Oct;55(10):1106–1107. doi: 10.1016/s0300-8932(02)76767-5. [DOI] [PubMed] [Google Scholar]
- 13.Lim BC, Ariens RA, Carter AM, et al. Genetic regulation of fibrin structure and function: complex gene-environment interactions may modulate vascular risk. Lancet. 2003 Apr 26;361(9367):1424–1431. doi: 10.1016/S0140-6736(03)13135-2. [DOI] [PubMed] [Google Scholar]
- 14.Collet JP, Hulot JS, Pena A, et al. Cytochrome P450 2C19 polymorphism in young patients treated with clopidogrel after myocardial infarction: a cohort study. Lancet. 2009 Jan 24;373(9660):309–317. doi: 10.1016/S0140-6736(08)61845-0. [DOI] [PubMed] [Google Scholar]
- 15.Collet JP, Park D, Lesty C, et al. Influence of fibrin network conformation and fibrin fiber diameter on fibrinolysis speed: dynamic and structural approaches by confocal microscopy. Arterioscler Thromb Vasc Biol. 2000 May;20(5):1354–1361. doi: 10.1161/01.atv.20.5.1354. [DOI] [PubMed] [Google Scholar]
- 16.Weisel JW. The mechanical properties of fibrin for basic scientists and clinicians. Biophys Chem. 2004;112:267–276. doi: 10.1016/j.bpc.2004.07.029. [DOI] [PubMed] [Google Scholar]
- 17.Undas A, Zawilska K, Ciesla-Dul M, et al. Altered fibrin clot structure/function in patients with idiopathic venous thromboembolism and in their relatives. Blood. 2009 Nov 5;114(19):4272–4278. doi: 10.1182/blood-2009-05-222380. [DOI] [PubMed] [Google Scholar]
- 18.Scott EM, Ariens RAS, Grant PJ. Genetic and Environmental Determinants of Fibrin Structure and Function Relevance to Clinical Disease. Atheroscler Thromb Vasc Biol. 2004;24:1558–1566. doi: 10.1161/01.ATV.0000136649.83297.bf. [DOI] [PubMed] [Google Scholar]
- 19.Jorneskog G, Egberg N, Fagrell B, et al. Altered properties of the fibrin gel structure in patients with IDDM. Diabetologia. 1996;39:1519–1523. doi: 10.1007/s001250050607. [DOI] [PubMed] [Google Scholar]
- 20.Mannila MN, Eriksson P, Ericsson CG, et al. Epistatic and pleiotropic effects of polymorphisms in the fibrinogen and coagulation factor XIII genes on plasma fibrinogen concentration, fibrin gel structure and risk of myocardial infarction. Thromb Haemost. 2006 Mar;95(3):420–427. doi: 10.1160/TH05-11-0777. [DOI] [PubMed] [Google Scholar]
- 21.Dunn EJ, Ariens RA, De Lange M, et al. Genetics of fibrin clot structure: a twin study. Blood. 2004;103:1735–1740. doi: 10.1182/blood-2003-07-2247. [DOI] [PubMed] [Google Scholar]
- 22.Ariens RA, Lai TS, Weisel JW, et al. Role of factor XIII in fibrin clot formation and effects of genetic polymorphisms. Blood. 2002;100:743–754. doi: 10.1182/blood.v100.3.743. [DOI] [PubMed] [Google Scholar]
- 23.de Lange M, Andrew T, Snieder H, et al. Joint linkage and association of six single-nucleotide polymorphisms in the factor XIII-A subunit gene point to V34L as the main functional locus. Arterioscler Thromb Vasc Biol. 2006;26(8):1914–1919. doi: 10.1161/01.ATV.0000231538.60223.92. [DOI] [PubMed] [Google Scholar]
- 24.Endler G, Funk M, Haering D, et al. Is the factor XIII 34Val/Leu polymorphism a protective factor for cerebrovascular disease? Br J Haematol. 2003 Jan;120(2):310–314. doi: 10.1046/j.1365-2141.2003.04047.x. [DOI] [PubMed] [Google Scholar]
- 25.Roldan V, Corral J, Marin F, et al. Role of factor XIII Val34Leu polymorphism in patients <45 years of age with acute myocardial infarction. Am J Cardiol. 2003 May 15;91(10):1242–1245. doi: 10.1016/s0002-9149(03)00274-1. [DOI] [PubMed] [Google Scholar]
- 26.Rallidis LS, Politou M, Komporozos C, et al. Factor XIII Val34Leu polymorphism and the risk of myocardial infarction under the age of 36 years. Thromb Haemost. 2008 Jun;99(6):1085–1089. doi: 10.1160/TH07-12-0755. [DOI] [PubMed] [Google Scholar]
- 27.Bronic A, Ferencak G, Zadro R, et al. Impact of FXIII-A Val34Leu polymorphism on coronary artery disease in Croatian patients. Mol Biol Rep. 2009 Jan;36(1):1–5. doi: 10.1007/s11033-007-9144-9. [DOI] [PubMed] [Google Scholar]
- 28.Shafey M, Anderson JL, Scarvelis D, et al. Factor XIII Val34Leu variant and the risk of myocardial infarction: a meta-analysis. Thromb Haemost. 2007 Apr;97(4):635–641. [PubMed] [Google Scholar]
- 29.Voko Z, Bereczky Z, Katona E, et al. Factor XIII Val34Leu variant protects against coronary artery disease. A meta-analysis. Thromb Haemost. 2007 Mar;97(3):458–463. [PubMed] [Google Scholar]
- 30.Carter AM, Cymbalista CM, Spector TD, et al. Heritability of clot formation, morphology, and lysis: the EuroCLOT study. Arterioscler Thromb Vasc Biol. 2007 Dec;27(12):2783–2789. doi: 10.1161/ATVBAHA.107.153221. [DOI] [PubMed] [Google Scholar]