Abstract
Of the various genetic factors contributing to the pathogenesis of Parkinson’s disease (PD), only mutations in α-synuclein (α-syn) and LRRK2 genes cause clinical and neuropathological phenotypes closely resembling the sporadic cases. Therefore, studying the pathophysiological functions of these two PD-related genes is particularly informative in understanding the underlying molecular pathogenic mechanism of the disease. PD-related missense and multiplication mutations in α-syn may cause both early- and late-onset PD, whereas various PD-related LRRK2 missense mutations may contribute to the more common late-onset PD. While intensive studies have been carried out to elucidate the pathogenic properties of PD-related mutant α-syn and LRRK2, our knowledge of their normal functions and their potential genetic interplay remains rudimental. In this review, we summarize the progress made regarding the pathophysiological functions of α-syn, LRRK2 and their interaction in PD, based on the available literature and our unpublished observations.
Keywords: 14-3-3, α-synuclein, actin, autophagy, ER, Golgi apparatus, leucine-rich repeat kinase 2, Lewy body, microtubule, mitochondria, Parkinson’s disease, proteasome
Parkinson’s disease
Parkinson’s disease (PD) is the most common degenerative movement disorder, which was initially described by Dr James Parkinson in the 19th century [1]. PD affects more than 1.5% of the population over 65 years old [2,3]. The clinical symptoms of PD include bradykinesia, resting tremor, rigidity and postural instability, with psychiatric and cognitive presentations in some patients. Pathologically, PD is characterized by the progressive death of midbrain dopaminergic (DA) neurons in the substantia nigra pars compacta (SNc) and the formation of abnormal intracellular protein aggregates, named Lewy bodies (LBs) and Lewy neurites (LNs), in the remaining DA neurons [2]. α-synuclein (α-syn) is enriched in LBs and LNs [4]. Interestingly, only familial cases with dominant genetic mutations in α-syn and LRRK2 develop typical PD pathology, including SNc DA neuronal loss and LB/LN formation [5]. Therefore, understanding the pathophysiological functions of α-syn and LRRK2 may provide critical molecular insights into the pathogenesis of PD, which is required to develop any mechanism-based therapeutic strategies to ameliorate this devastating illness.
α-synuclein
More than a decade ago, a substitution of alanine (A) at residue 53 with threonine (T) in α-syn was identified in a familial case of early onset PD, which provided the first evidence of a genetic cause of PD [6]. Following this lead, two other missense mutations in α-syn, A30P and E46K, have subsequently been linked to PD [7,8]. In addition, duplication of an α-syn gene locus region on one allele of chromosome 4, which results in three copies of the α-syn gene, was shown to cause PD with onset at late middle age [9]. Meanwhile, triplication of the α-syn gene locus at one allele or duplication of the α-syn gene loci at both alleles, which results in four copies of the α-syn gene, causes much more severe forms of disease with a much earlier onset and shorter survival time [10]. Together, these genetic studies have strongly established a prominent role of α-syn missense and multiplication mutations in causing PD.
In fact, α-syn is one of only a handful of genes consistently linked to both the etiology and pathogenesis of PD [11]. α-syn is an abundant neuronal protein present in neurons of the SNc and other brain regions, which may make up to 1% of all proteins in the cytosol. Within the neuron, α-syn is almost exclusively located in the presynaptic terminals [11], where it interacts with other presynaptic vesicle proteins, such as synphilin-1, CSPα and SNAREs, and may be involved in neurotransmitter release [12–14]. Accordingly, alterations in neurotransmitter release have been reported in α-syn-deficient and α-syn-overexpressing neurons [15,16].
At the molecular level, α-syn protein perhaps occurs in large part as an α-helically folded homo-tetramer of approximately 58 kDa under native conditions [17]. α-syn exists in either free cytosolic or membrane-bound form [18], approximately 15% is membrane-bound in neurons [19]. Both the N- and C-terminal regions of α-syn are tightly associated with membranes [18], despite the protein lacking a transmembrane domain, lipid anchor, or well-defined membrane-binding domain. α-syn preferentially binds to phospholipid liposomes that contain acidic head groups [20]. Using double labeling, Fortin and colleagues found that α-syn is specifically colocalized with the protein and lipid components of lipid rafts [21]. Lipid rafts are involved in formation of membranous organelles, for example, Golgi apparatus, endoplasmic reticulum (ER), plasma membrane, synaptic vesicles and mitochondria [22]. Overexpression of α-syn can cause Golgi fragmentation [23], mitochondrial swelling [24], lysosome enlargement [25], as well as ER stress [26]. It is possible that α-syn may bind to lipid membranes and alter the bilayer structure, which may alter the membrane curvature and destabilize membrane integrity [27,28]. In line with this notion, lipids also represent a significant portion of LBs, and they have been proposed to derive from degraded membrane organelles [29].
In addition to α-syn, two homologous genes named β- and γ-syn have also been found in mammalian cells [30]. α-syn family genes seem to have overlapping functions, only the α-, β- and γ-syn triple knockout mice display significant alterations in synaptic structure and transmission, and show age-dependent neuronal dysfunction and degeneration [31]. By contrast, α-syn single knockouts or a combination of α-syn with β- or γ-syn double knockout mice lack any obvious phenotypes, suggesting that α-syn itself may not play a crucial role in neuronal function and maintenance [15,32]. On the contrary, overexpression of α-syn produces considerable cytotoxicity and impairs synaptic transmission [16,33]. Together, these observations suggest that α-syn-induced neurodegeneration may not be due to a loss-of-function mechanism, but rather a gain of toxic properties.
LRRK2
Dominantly inherited mutations in the LRRK2 gene are perhaps the most common cause of familial and sporadic PD, which have been identified in approximately 3–5% of familial and 1–3% of sporadic PD cases, although the frequency may vary greatly in different populations [34]. The LRRK2 gene encodes a rather large protein of 2527 amino acids compared with α-syn. LRRK2 contains 12 leucine-rich repeats (LRRs) in the N-terminus, a Roc domain and a MAPKKK domain in the middle, and a WD40 domain in the C-terminus [35]. The putative kinase and GTPase activities of LRRK2 might be important in the pathogenesis of PD. In vitro studies indicate that several PD-specific mutations increase LRRK2 autophosphorylation and phosphorylation of generic protein kinase substrates [36]. For example, the G2019S mutation at the kinase domain of LRRK2 increases its auto-phosphorylation by twofold [37]. Meanwhile, a reciprocal regulation of its GTPase and kinase activities seems to influence the function and cytotoxicity of LRRK2 [35]. While LRRK2 is mainly a cytoplasmic protein, it is also partially associated with membrane organelles such as mitochondria, ER, Golgi apparatus, endosomes and synaptic vesicles [34,35]. This characteristic distribution of LRRK2 may indicate an involvement of LRRK2 in membrane trafficking as suggested by findings in transgenic mouse models [38].
A variety of transgenic mice have been generated to study the function of LRRK2 [36,38]. However, none of these LRRK2 transgenic mice have developed PD-like neuropathology, such as formation of LB-like α-syn-positive inclusions and loss of SNc DA neurons. In addition, none of the LRRK2 knockout mice displayed obvious motor, behavioral or neuropathological phenotypes [39,40]. By contrast, LRRK2 knockout, as well as G2019S and kinase-dead knock-in mice, developed profound pathological lesions in the periphery tissues, such as the kidney and lung of aged animals [37,41], suggesting that LRRK2 is more critically involved in maintaining the normal functions of cells in the kidney and lung, which happen to process robust secretory pathways. A further investigation of LRRK2’s exact role in protein secretion and degradation in kidney and lung cells may provide significant molecular insights into the pathophysiological functions of LRRK2 in neurons and PD. Taken collectively, these observations from LRRK2 knockout and transgenic mice suggest that LRRK2 might play a rather subtle or selective role in modulating the physiological function and survival of neurons. For example, studies from primary cultured neurons derived from LRRK2 transgenic and knockout mice demonstrate an involvement of LRRK2 in axon and den-drite outgrowth during neuronal development, likely through regulating the dynamics of actin assembly or ER/Golgi vesicle trafficking [39,42].
α-synuclein & LRRK2 interplay
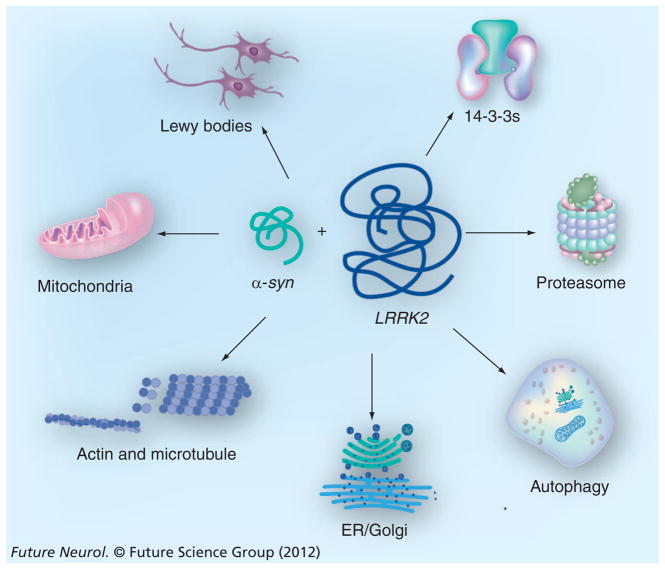
Given the critical involvement of α-syn and LRRK2 in the pathogenesis of PD, many attempts have been made to delineate a potential pathogenic interplay between the two that predisposes SNc DA neurons to be more vulnerable to various cellular stresses seen in PD. Therefore, in this section we will summarize and discuss the potential subcellular organelles and intracellular pathways in which α-syn and LRRK2 may exert their synergistic effects during neurodegeneration (Figure 1).
Figure 1. Intracellular pathways potentially affected by synergistic genetic interplay between α-synuclein and LRRK2.
α-syn: α-synuclein; ER: Endoplasmic reticulum.
Lewy bodies
One of the key pathological hallmarks in PD is the deposition of LBs of which α-syn and its phosphorylated forms are the primary structural components [40,43]. After initial identification of mutant LRRK2 in PD, considerable efforts have been made to examine the presence of the LRRK2 protein in LBs [44,45]. These studies estimated that LRRK2 is present in the periphery of 10–80% of classic LBs. However, these data should be interpreted cautiously, since many of the LRRK2 antibodies used in these early reports have not been tested rigorously regarding their specificity and applicability for immunohistochemistry. LRRK2 was also shown to coimmunoprecipitate with α-syn from soluble protein extracts of postmortem brain tissues with apparent LB pathology [46]. While the association of LRRK2 and α-syn was later confirmed in HEK293 cells transfected with both LRRK2 and α-syn under oxidative stress [47]; a direct biochemical interaction between endogenous α-syn and LRRK2 in neurons remains to be determined.
Since the phospho-S129 of α-syn promotes formation of α-syn filaments and oligomers and is enriched in LBs [43,48], it raises the speculation that LRRK2, especially its G2019S kinase-enhancing mutant, may promote the phosphorylation of α-syn at S129. So far, there is only one published in vitro report showing that LRRK2 and its putative kinase domain-containing fragments have a significant capacity to phosphorylate recombinant α-syn at S129 [49]. However, other studies both in cell cultures and animal models failed to identify any increase of α-syn phosphorylation at S129 when overexpressing either wild-type or PD-related G2019S LRRK2. These findings indicate that LRRK2 may not promote the formation of α-syn aggregates by directly phosphorylating α-syn at S129.
Taken together, the present studies do not support a direct biochemical interaction between α-syn and LRRK2, or direct phosphorylation of α-syn by LRRK2, which contributes to LB formation and neuronal loss.
14-3-3 family proteins
While α-syn may not directly interact with LRRK2, they may coexist in the same protein complex through association with 14-3-3 proteins [50,51]. The 14-3-3 family of proteins consists of seven isoforms in mammalian species. They comprise 1% of total brain proteins and participate in a large variety of cellular functions by interacting with different protein partners [52].
Interestingly, 14-3-3 proteins share structural homology with α-syn [53] and also accumulate in LBs [54]. 14-3-3 proteins and α-syn can also be coimmunoprecipitated from mammalian brains [50]. Moreover, 14-3-3 proteins appear to interact specifically with the phosphorylated form of α-syn [55]. In one line of α-syn transgenic mice, mRNA levels of 14-3-3 isoforms θ and γ were significantly reduced [46]. Under normal physiological conditions, 14-3-3 proteins inhibit apoptosis by binding and inactivating proapoptotic proteins, including the mitochondrial Bcl-2 family member BAD and the transcription factor forkhead [56]. Increased binding to α-syn may potentially sequester 14-3-3 proteins and reduce their anti-apoptotic activity, which may increase neuronal vulnerability to reactive oxygen species generated by endogenous dopamine metabolism [57].
LRRK2 also binds to different isoforms of 14-3-3 proteins upon phosphorylation of 14-3-3 family proteins at residues S910 and S935 [51]. Mutations of these two serine residues to ala-nine prevented 14-3-3 proteins from interacting with LRRK2 [58]. Therefore, LRRK2 protein kinase activity may directly regulate the phosphorylation of 14-3-3 at the S910 and S935 sites, and hence modulate its binding with 14-3-3 isoforms [51]. Indeed, overexpression of the LRRK2 G2019S mutation in cell lines promoted phosphorylation of 14-3-3 at S910/S935 by threefold [58,59]. However, in LRRK2 G2019S BAC-transgenic mice, the level of phospho-S935 14-3-3 was rather decreased compared with the control mice [60]. In addition, overexpression of the other LRRK2 pathogenic mutations, including R1441C, R1441G, R1441H, Y1699C and I2020T, in cell lines also displayed reduced phosphorylation of 14-3-3 at Ser910/Ser935 sites [58,60]. Thereby, LRRK2 may not directly phosphorylate 14-3-3 proteins at S910 or S935. Future studies may also need to further clarify the impact of 14-3-3’s phosphorylation on its interaction with LRRK2. In addition, it is tempting to speculate an indirect interaction between α-syn and LRRK2 through a common adaptor, 14-3-3. Together, the association of 14-3-3 with both α-syn and LRRK2 generates significant interest to further explore the contribution of 14-3-3 family proteins in the pathogenesis of PD.
Actin & microtubule assembly
The cytoskeleton, including actin and micro-tubule networks, is instrumental in establishing and maintaining neuronal morphology and function [61]. The impact of PD-related mutant α-syn and LRRK2 on the dynamics of actin and microtubule assembly has been summarized in a recent review article [39].
Filamentous actin (F-actin) is formed by polymerization of monomeric actin, a globular protein highly conserved across different species [62]. An ATP-dependent rapid remodeling of F-actin is critical in neurite outgrowth and synapse formation as well as plasticity [63]. Actin is found in cytoplasmic α-syn aggregates [64] and α-syn may modulate the polymerization of actin [65]. LRRK2 is also involved in regulating the dynamic arrangement of F-actin assembly. LRRK2 controls the activities of the ezrin, radixin, and moesin (ERM) family proteins via phosphorylation of ERM proteins at residue T558 [66]. The phosphorylated ERM proteins are targeted at the tip of growing neurites, where they tether the underlying F-actin with the cytoplasmic membrane and regulate the dynamics of filopodia protrusion [42]. The LRRK2 G2019S mutation significantly enhances the phosphorylation of ERM proteins and increases the number of phospho-ERM-positive and F-actin-enriched filopodia. This may perturb the homeostasis of phospho-ERM and F-actin in sprouting neurites critical for neuronal morphogenesis. Recently, LRRK2 was also shown to interact with Rac1 and RhoA, two small GTPases that actively participate in the organization of F-actin cytoskeleton during various cellular processes [67]. However, future studies are needed to investigate whether α-syn and LRRK2 act coordinately in regulating the dynamic assembly and disassembly of F-actin in neurons.
Microtubules are built up with α- and β-tubulin heterodimers [68]. α-syn aggregates are suggested to disrupt the integrity of the microtubule network [69]. Meanwhile, LRRK2 has been shown to physically interact with both α- and β-tubulin through its GTPase domain [70,71]. Moreover, the level of free tubulin is significantly increased in the brain extract of LRRK2 knockout mice [71], and dramatically decreased in the brain homogenate of LRRK2 wild-type and G2019S transgenic mice [72], suggesting LRRK2 as a potential stabilizing protein for microtubule assembly. This LRRK2-mediated excessive stabilization of the microtubule network may compromise dynamic assembly and disassembly, and underlie the neurite outgrowing defects observed in neurons overexpressing LRRK2 G2019S mutations [42].
LRRK2 may also regulate the dynamic of the microtubule network through modulating the activities of another microtubule binding protein, tau. Tau regulates the stability and dynamics of microtubule networks [73]. It has been hypothesized that phosphotau does not bind effectively to microtubules and loses the ability to stabilize them, resulting in microtubule breakdown and impairment of axonal transport [74]. Accumulation of phosphotau was found in certain lines of LRRK2 mutant transgenic mice [75–77]. It seems that LRRK2 may play a rather complicated role in organizing the microtubule network. On the one hand, LRRK2 facilitates the polymerization of microtubules by directly binding tubulins. On the other hand, LRRK2 undermines the microtubule assembly through the phosphorylation of tau. More investigations may be required in the future to further elucidate the mechanisms of LRRK2 in regulating the dynamic organization of the microtubule network, especially in the presence of α-syn.
ER/Golgi transport
ER/Golgi-mediated secretory pathways are crucial for neuronal development, function and survival [78]. α-syn may regulate ER–Golgi transport through a small GTPase protein, Rab1 [26]. The structure and function of ER/Golgi are also dynamically regulated by the microtubule network [79]. The disruption of the microtubule lattice by aggregated α-syn may account for the fragmentation of the Golgi apparatus in cells overexpressing α-syn [80]. Interestingly, overexpression of LRRK2 also caused significant fragmentation of the Golgi apparatus [72]. Moreover, coexpression of LRRK2 and α-syn led to more severe and synergistic fragmentation of the Golgi apparatus, which was tightly correlated with the augmentation of α-synuclein accumulation in the soma. By contrast, inhibition of LRRK2 expression prevented the disintegration of the Golgi apparatus in neurons overexpressing α-syn and suppressed the accumulation of α-syn in cell bodies. These findings indicate that overexpression of LRRK2 may promote the improper polymerization of tubulin in neurons, which might cause the fragmentation of the Golgi apparatus and exacerbate α-syn-induced ER–Golgi trafficking defects. This process may form a vicious cycle that further exacerbates the disorganization of microtubule and Golgi networks and abnormal accumulation of α-syn in the soma, which eventually leads to the loss of neurons.
Mitochondria
It has been well known that mitochondrial complex I inhibitors, such as MPTP and rotenone, cause PD-like clinical phenotypes [81], suggesting the importance of maintaining mitochondrial energetic homeostasis in the SNc DA neurons. α-syn-positive aggregates have been shown to induce the damage of mitochondria [24]. While LRRK2 is associated with the outer membrane of mitochondria [82], no apparent structural abnormality was observed in the mitochondria of mouse neurons overexpressing either wild-type or PD-related mutant LRRK2 [72]. However, impaired mitochondrial function and morphology were found in LRRK2 (G2019S) mutant patient tissue [83]. The presence of excess LRRK2 also exacerbated α-syn-induced mitochondrial swellings and superoxide production [72]. It will be interesting to carry out more detailed studies in the future to specify the roles of α-syn and LRRK2 in regulating the biogenesis and functions of mitochondria.
The ubiquitin–proteasome system
The ubiquitin–proteasome system (UPS) is one of the main pathways in cells responsible for the removal and recycling of undesired proteins [84]. While the stability of both α-syn and LRRK2 is regulated by the UPS pathway [85]; α-syn aggregates may cause impairment of UPS activities [86], which may lead to the accumulation of ubiquitinated proteins in neurons. Interestingly, G2019S LRRK2 protein is also sequestered as ubiquitin-positive clusters in neurons of aging animals [72]. The accumulation of G2019S LRRK2 and ubiquitin-positive protein aggregates in aged mice indicate that overexpression of LRRK2, especially the G2019S mutation, may impair UPS activity in neurons. More interestingly, coexpression of PD-related A53T α-syn and G2019S LRRK2 seem to further damage UPS activities and accelerate the deposition of both α-syn and LRRK2 protein aggregates.
Autophagy
Autophagy is the other essential machinery in cells that is responsible for the removal of damaged organelles and large protein aggregates [87]. Various malfunctions of autophagy pathways have been reported in PD, in which both α-syn and LRRK2 are involved [88,89]. The α-syn A53T mutation seems to interfere with the chaperone-mediated autophagy and macroautophagy pathways in cells, which impairs the clearance of α-syn aggregates [90]. LRRK2 is also implicated in regulating autophagy induction and flux rates in cell cultures. In particular, loss of LRRK2 leads to excessive accumulation of marker proteins involved in the induction of autophagy in cells from the kidney and lung [37,41]. However, no overt impairment of the autophagy pathway is observed in LRRK2-deficient neurons. Nonetheless, future studies may focus on identifying the molecular partners of LRRK2 in the autophagy pathway in order to fully understand the contribution of LRRK2 in this fundamental cellular process.
Conclusion & future perspective
Since the identification of PD-related genetic mutations in α-syn and LRRK2, extensive studies have been carried out to investigate the various cellular processes affected by mutant α-syn and LRRK2, which may contribute to the preferential loss of SNc DA neurons in PD. As summarized in this review, PD-related mutant α-syn and LRRK2 target many common intracellular pathways and may exert synergistic effects to exacerbate their respective cytotoxicity. Therefore, a comparative study of α-syn and LRRK2-mediated pathophysiological mechanisms in PD would be instrumental to elucidate a potential common pathogenic pathway critical for the development of PD. Of note, the majority of α-syn and LRRK2-related studies have been performed in cell lines and nonDA neurons. Considering the fact that loss of SNc DA neurons accounts for the main clinical and pathological phenotypes in PD, it may demand a more focused investigation of SNc DA neurons in response to α-syn- and LRRK2-mediated genetic insults. Although midbrain DA neurons only account for a handful of neurons in the brain, they substantially influence almost every aspect of brain activities. These neurons also possess very distinguished morphological and physiological characteristics that are different from many other types of neurons [91]. Therefore, understanding the pathogenic functions of α-syn and LRRK2 and their genetic interplay in midbrain DA neurons may hold the key to finally resolving the mystery of PD and provide a cure for this devastating illness.
Executive summary.
α-synuclein
Genetic studies have strongly established a prominent role of α-synuclein (α-syn) missense and multiplication mutations in causing Parkinson’s disease (PD).
In neurons, α-syn is almost exclusively located in the presynaptic terminals; alterations in neurotransmitter release have been reported in α-syn-deficient and α-syn-overexpressing neurons.
α-syn preferentially binds to phospholipid liposomes that contain acidic head groups.
LRRK2
In vitro studies indicate that several PD-specific mutations increase LRRK2 autophosphorylation and phosphorylation of generic protein kinase substrates.
LRRK2 is mainly a cytoplasmic protein, although also partially associated with membrane organelles.
So far, none of the LRRK2 transgenic mice studied have developed PD-like neuropathology.
α-synuclein & LRRK2 interplay
Lewy body (LB): phosphoserine129 of α-syn is enriched in LBs. Some studies have estimated that LRRK2 is present in the periphery of approximately 10–80% of classic LBs as well. LRRK2 may promote the phosphorylation of α-syn at S129, however, the present studies do not support this.
14-3-3 family proteins: the association of 14-3-3 with both α-syn and LRRK2 generates significant interest to further explore the contribution of 14-3-3 family proteins in the pathogenesis of PD.
Actin and microtubule assembly: both mutant α-syn and LRRK2 are involved in the dynamics of actin and microtubule assembly.
ER–Golgi transport: overexpression of LRRK2 may promote the improper polymerization of tubulin in neurons, which might cause the fragmentation of the Golgi apparatus and exacerbate α-syn-induced ER–Golgi trafficking defects.
Mitochondria: α-syn-positive aggregates have been shown to induce damage of mitochondria; the presence of excess LRRK2 exacerbated α-syn-induced mitochondrial swellings and superoxide production.
The ubiquitin–proteasome system (UPS): both α-syn aggregates and mutant LRRK2 may cause impairment of UPS activities. Coexpression of PD-related A53T α-syn and G2019S LRRK2 seems to further damage UPS activities and accelerate the deposition of both α-syn and LRRK2 protein aggregates.
Autophagy: the α-syn A53T mutation seems to interfere with the chaperone-mediated autophagy and macroautophagy pathways in cells. LRRK2 is also implicated in regulating autophagy induction and flux rates in cell cultures.
Footnotes
For reprint orders, please contact: reprints@futuremedicine.com
Financial & competing interests disclosure
This work is supported by the Intramural Research Program of National Institute on Aging, NIH. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Morris AD. James Parkinson, born April 11, 1755. Lancet. 1955;268(6867):761–763. doi: 10.1016/s0140-6736(55)90558-4. [DOI] [PubMed] [Google Scholar]
- 2.Moore DJ, West AB, Dawson VL, Dawson TM. Molecular pathophysiology of Parkinson’s disease. Annu Rev Neurosci. 2005;28:57–87. doi: 10.1146/annurev.neuro.28.061604.135718. [DOI] [PubMed] [Google Scholar]
- 3.Lees AJ, Hardy J, Revesz T. Parkinson’s disease. Lancet. 2009;373(9680):2055–2066. doi: 10.1016/S0140-6736(09)60492-X. [DOI] [PubMed] [Google Scholar]
- 4.Jellinger KA. Formation and development of Lewy pathology: a critical update. J Neurol. 2009;256(Suppl 3):270–279. doi: 10.1007/s00415-009-5243-y. [DOI] [PubMed] [Google Scholar]
- 5▪▪.Hardy J, Cai H, Cookson MR, Gwinn-Hardy K, Singleton A. Genetics of Parkinson’s disease and parkinsonism. Ann Neurol. 2006;60(4):389–398. doi: 10.1002/ana.21022. Comprehensive review focusing on the genetics of Parkinson’s disease (PD; the ‘PARK’ genes: α-synuclein (α-syn), LRRK2, DJ-1, PINK-1 and parkin) [DOI] [PubMed] [Google Scholar]
- 6▪.Polymeropoulos MH, Lavedan C, Leroy E, et al. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276(5321):2045–2047. doi: 10.1126/science.276.5321.2045. The first evidence of a genetic cause of PD: A53T in α-syn was identified in a familial case of early-onset PD. [DOI] [PubMed] [Google Scholar]
- 7.Krüger R, Kuhn W, Müller T, et al. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson’s disease. Nat Genet. 1998;18(2):106–108. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- 8.Zarranz JJ, Kuhn W, Müller T, et al. The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann Neurol. 2004;55(2):164–173. doi: 10.1002/ana.10795. [DOI] [PubMed] [Google Scholar]
- 9.Chartier-Harlin MC, Kachergus J, Roumier C, et al. Alpha-synuclein locus duplication as a cause of familial Parkinson’s disease. Lancet. 2004;364(9440):1167–1169. doi: 10.1016/S0140-6736(04)17103-1. [DOI] [PubMed] [Google Scholar]
- 10▪ α.Singleton AB, Farrer M, Johnson J, et al. Alpha-synuclein locus triplication causes Parkinson’s disease. Science. 2003;302(5646):841. doi: 10.1126/science.1090278. -syn multiplication mutations cause much more severe forms of the disease with much earlier onset and shorter survival time. [DOI] [PubMed] [Google Scholar]
- 11.Iwai A, Masliah E, Yoshimoto M, et al. The precursor protein of non-A beta component of Alzheimer’s disease amyloid is a presynaptic protein of the central nervous system. Neuron. 1995;14(2):467–475. doi: 10.1016/0896-6273(95)90302-x. [DOI] [PubMed] [Google Scholar]
- 12.Burre J, Sharma M, Tsetsenis T, Buchman V, Etherton MR, Südhof TC. Alpha-synuclein promotes SNARE-complex assembly in vivo and in vitro. Science. 2010;329(5999):1663–1667. doi: 10.1126/science.1195227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chandra S, Gallardo G, Fernández-Chacón R, Schlüter OM, Südhof TC. Alpha-synuclein cooperates with CSPalpha in preventing neurodegeneration. Cell. 2005;123(3):383–396. doi: 10.1016/j.cell.2005.09.028. [DOI] [PubMed] [Google Scholar]
- 14.Murphy DD, Rueter SM, Trojanowski JQ, Lee VM. Synucleins are developmentally expressed, and alpha-synuclein regulates the size of the presynaptic vesicular pool in primary hippocampal neurons. J Neurosci. 2000;20(9):3214–3220. doi: 10.1523/JNEUROSCI.20-09-03214.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abeliovich A, Schmitz Y, Fariñas I, et al. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25(1):239–252. doi: 10.1016/s0896-6273(00)80886-7. [DOI] [PubMed] [Google Scholar]
- 16.Masliah E, Rockenstein E, Veinbergs I, et al. Dopaminergic loss and inclusion body formation in alpha-synuclein mice: implications for neurodegenerative disorders. Science. 2000;287(5456):1265–1269. doi: 10.1126/science.287.5456.1265. [DOI] [PubMed] [Google Scholar]
- 17.Bartels T, Choi JG, Selkoe DJ. Alpha-synuclein occurs physiologically as a helically folded tetramer that resists aggregation. Nature. 2011;477(7362):107–110. doi: 10.1038/nature10324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McLean PJ, Kawamata H, Ribich S, Hyman BT. Membrane association and protein conformation of alpha-synuclein in intact neurons Effect of Parkinson’s disease-linked mutations. J Biol Chem. 2000;275(12):8812–8816. doi: 10.1074/jbc.275.12.8812. [DOI] [PubMed] [Google Scholar]
- 19.Lee HJ, Choi C, Lee SJ. Membrane-bound alpha-synuclein has a high aggregation propensity and the ability to seed the aggregation of the cytosolic form. J Biol Chem. 2002;277(1):671–678. doi: 10.1074/jbc.M107045200. [DOI] [PubMed] [Google Scholar]
- 20.Davidson WS, Jonas A, Clayton DF, George JM. Stabilization of alpha-synuclein secondary structure upon binding to synthetic membranes. J Biol Chem. 1998;273(16):9443–9449. doi: 10.1074/jbc.273.16.9443. [DOI] [PubMed] [Google Scholar]
- 21.Fortin DL, Troyer MD, Nakamura K, Kubo S, Anthony MD, Edwards RH. Lipid rafts mediate the synaptic localization of alpha-synuclein. J Neurosci. 2004;24(30):6715–6723. doi: 10.1523/JNEUROSCI.1594-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000;1(1):31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- 23.Fujita Y, Ohama E, Takatama M, Al-Sarraj S, Okamoto K. Fragmentation of Golgi apparatus of nigral neurons with alpha-synuclein-positive inclusions in patients with Parkinson’s disease. Acta Neuropathol. 2006;112(3):261–265. doi: 10.1007/s00401-006-0114-4. [DOI] [PubMed] [Google Scholar]
- 24.Martin LJ, Pan Y, Price AC, et al. Parkinson’s disease alpha-synuclein transgenic mice develop neuronal mitochondrial degeneration and cell death. J Neurosci. 2006;26(1):41–50. doi: 10.1523/JNEUROSCI.4308-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meredith GE, Totterdell S, Petroske E, Santa Cruz K, Callison RC, Jr, Lau YS. Lysosomal malfunction accompanies alpha-synuclein aggregation in a progressive mouse model of Parkinson’s disease. Brain Res. 2002;956(1):156–165. doi: 10.1016/s0006-8993(02)03514-x. [DOI] [PubMed] [Google Scholar]
- 26.Cooper AA, Gitler AD, Cashikar A, et al. Alpha-synuclein blocks ER–Golgi traffic and Rab1 rescues neuron loss in Parkinson’s models. Science. 2006;313(5785):324–328. doi: 10.1126/science.1129462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Madine J, Doig AJ, Middleton DA. A study of the regional effects of alpha-synuclein on the organization and stability of phospholipid bilayers. Biochemistry. 2006;45(18):5783–5792. doi: 10.1021/bi052151q. [DOI] [PubMed] [Google Scholar]
- 28.Varkey J, Isas JM, Mizuno N, et al. Membrane curvature induction and tubulation are common features of synucleins and apolipoproteins. J Biol Chem. 2010;285(42):32486–32493. doi: 10.1074/jbc.M110.139576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gai WP, Yuan HX, Li XQ, Power JT, Blumbergs PC, Jensen PH. In situ and in vitro study of colocalization and segregation of alpha-synuclein, ubiquitin, and lipids in Lewy bodies. Exp Neurol. 2000;166(2):324–333. doi: 10.1006/exnr.2000.7527. [DOI] [PubMed] [Google Scholar]
- 30.Lavedan C. The synuclein family. Genome Res. 1998;8(9):871–880. doi: 10.1101/gr.8.9.871. [DOI] [PubMed] [Google Scholar]
- 31.Greten-Harrison B, Polydoro M, Morimoto-Tomita M, et al. Alphabetagamma-synuclein triple knockout mice reveal age-dependent neuronal dysfunction. Proc Natl Acad Sci USA. 2010;107(45):19573–19578. doi: 10.1073/pnas.1005005107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chandra S, Fornai F, Kwon HB, et al. Double-knockout mice for alpha- and beta-synucleins: effect on synaptic functions. Proc Natl Acad Sci USA. 2004;101(41):14966–14971. doi: 10.1073/pnas.0406283101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kirik D, Rosenblad C, Burger C, et al. Parkinson-like neurodegeneration induced by targeted overexpression of alpha-synuclein in the nigrostriatal system. J Neurosci. 2002;22(7):2780–2791. doi: 10.1523/JNEUROSCI.22-07-02780.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tan EK, Jankovic J. Genetic testing in Parkinson disease: promises and pitfalls. Arch Neurol. 2006;63(9):1232–1237. doi: 10.1001/archneur.63.9.1232. [DOI] [PubMed] [Google Scholar]
- 35▪▪.Cookson MR. The role of leucine-rich repeat kinase 2 (LRRK2) in Parkinson’s disease. Nat Rev Neurosci. 2010;11(12):791–797. doi: 10.1038/nrn2935. Comprehensive review with a focus on mutations in LRRK2 that affect protein function and affect pathways involved in PD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yue Z, Lachenmayer ML. Genetic LRRK2 models of Parkinson’s disease: dissecting the pathogenic pathway and exploring clinical applications. Mov Disord. 2011;26(8):1386–1397. doi: 10.1002/mds.23737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tong Y, Yamaguchi H, Giaime E, et al. Loss of leucine-rich repeat kinase 2 causes impairment of protein degradation pathways, accumulation of alpha-synuclein, and apoptotic cell death in aged mice. Proc Natl Acad Sci USA. 2010;107(21):9879–9884. doi: 10.1073/pnas.1004676107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johnson SJ, Wade-Martins R. A BACwards glance at neurodegeneration: molecular insights into disease from LRRK2, SNCA and MAPT BAC-transgenic mice. Biochem Soc Trans. 2011;39(4):862–867. doi: 10.1042/BST0390862. [DOI] [PubMed] [Google Scholar]
- 39▪.Parisiadou L, Cai H. LRRK2 function on actin and microtubule dynamics in Parkinson disease. Commun Integr Biol. 2010;3(5):396–400. doi: 10.4161/cib.3.5.12286. Good review that describes how PD-related mutations in LRRK2 protein affect cytosleketal dynamics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Anderson JP, Walker DE, Goldstein JM, et al. Phosphorylation of Ser-129 is the dominant pathological modification of alpha-synuclein in familial and sporadic Lewy body disease. J Biol Chem. 2006;281(40):29739–29752. doi: 10.1074/jbc.M600933200. [DOI] [PubMed] [Google Scholar]
- 41.Herzig MC, Kolly C, Persohn E, et al. LRRK2 protein levels are determined by kinase function and are crucial for kidney and lung homeostasis in mice. Hum Mol Genet. 2011;20(21):4209–4223. doi: 10.1093/hmg/ddr348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parisiadou L, Xie C, Cho HJ, et al. Phosphorylation of ezrin/radixin/moesin proteins by LRRK2 promotes the rearrangement of actin cytoskeleton in neuronal morphogenesis. J Neurosci. 2009;29(44):13971–13980. doi: 10.1523/JNEUROSCI.3799-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fujiwara H, Hasegawa M, Dohmae N, et al. alpha-Synuclein is phosphorylated in synucleinopathy lesions. Nat Cell Biol. 2002;4(2):160–164. doi: 10.1038/ncb748. [DOI] [PubMed] [Google Scholar]
- 44.Alegre-Abarrategui J, Ansorge O, Esiri M, Wade-Martins R. LRRK2 is a component of granular alpha-synuclein pathology in the brainstem of Parkinson’s disease. Neuropathol Appl Neurobiol. 2008;34(3):272–283. doi: 10.1111/j.1365-2990.2007.00888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vitte J, Traver S, Maués De Paula A, et al. Leucine-rich repeat kinase 2 is associated with the endoplasmic reticulum in dopaminergic neurons and accumulates in the core of Lewy bodies in Parkinson disease. J Neuropathol Exp Neurol. 2010;69(9):959–972. doi: 10.1097/NEN.0b013e3181efc01c. [DOI] [PubMed] [Google Scholar]
- 46.Yacoubian TA, Slone SR, Harrington AJ, et al. Differential neuroprotective effects of 14–3–3 proteins in models of Parkinson’s disease. Cell Death Dis. 2010;1:e2. doi: 10.1038/cddis.2009.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qing H, Zhang Y, Deng Y, McGeer EG, McGeer PL. Lrrk2 interaction with alpha-synuclein in diffuse Lewy body disease. Biochem Biophys Res Commun. 2009;390(4):1229–1234. doi: 10.1016/j.bbrc.2009.10.126. [DOI] [PubMed] [Google Scholar]
- 48.Conway KA, Lee SJ, Rochet JC, Ding TT, Williamson RE, Lansbury PT., Jr Acceleration of oligomerization, not fibrillization, is a shared property of both alpha-synuclein mutations linked to early-onset Parkinson’s disease: implications for pathogenesis and therapy. Proc Natl Acad Sci USA. 2000;97(2):571–576. doi: 10.1073/pnas.97.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Qing H, Wong W, McGeer EG, McGeer PL. Lrrk2 phosphorylates alpha synuclein at serine 129: Parkinson disease implications. Biochem Biophys Res Commun. 2009;387(1):149–152. doi: 10.1016/j.bbrc.2009.06.142. [DOI] [PubMed] [Google Scholar]
- 50▪.Xu J, Kao SY, Lee FJ, Song W, Jin LW, Yankner BA. Dopamine-dependent neurotoxicity of alpha-synuclein: a mechanism for selective neurodegeneration in Parkinson disease. Nat Med. 2002;8(6):600–606. doi: 10.1038/nm0602-600. 14-3-3 proteins and α-syn can be coimmunoprecipitated from mammalian brains. [DOI] [PubMed] [Google Scholar]
- 51▪.Dzamko N, Deak M, Hentati F, et al. Inhibition of LRRK2 kinase activity leads to dephosphorylation of Ser(910)/Ser(935), disruption of 14-3-3 binding and altered cytoplasmic localization. Biochem J. 2010;430(3):405–413. doi: 10.1042/BJ20100784. LRRK2 binds to different isoforms of 14-3-3 proteins upon phosphorylation of 14-3-3 family proteins at residues S910 and S935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dougherty MK, Morrison DK. Unlocking the code of 14-3-3. J Cell Sci. 2004;117(Pt 10):1875–1884. doi: 10.1242/jcs.01171. [DOI] [PubMed] [Google Scholar]
- 53.Ostrerova N, Petrucelli L, Farrer M, et al. Alpha-synuclein shares physical and functional homology with 14-3-3 proteins. J Neurosci. 1999;19(14):5782–5791. doi: 10.1523/JNEUROSCI.19-14-05782.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kawamoto Y, Akiguchi I, Nakamura S, Honjyo Y, Shibasaki H, Budka H. 14-3-3 proteins in Lewy bodies in Parkinson disease and diffuse Lewy body disease brains. J Neuropathol Exp Neurol. 2002;61(3):245–253. doi: 10.1093/jnen/61.3.245. [DOI] [PubMed] [Google Scholar]
- 55.McFarland MA, Ellis CE, Markey SP, Nussbaum RL. Proteomics analysis identifies phosphorylation-dependent alpha-synuclein protein interactions. Mol Cell Proteomics. 2008;7(11):2123–2137. doi: 10.1074/mcp.M800116-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yuan J, Yankner BA. Apoptosis in the nervous system. Nature. 2000;407(6805):802–809. doi: 10.1038/35037739. [DOI] [PubMed] [Google Scholar]
- 57.Recchia A, Debetto P, Negro A, Guidolin D, Skaper SD, Giusti P. Alpha-synuclein and Parkinson’s disease. FASEB J. 2004;18(6):617–626. doi: 10.1096/fj.03-0338rev. [DOI] [PubMed] [Google Scholar]
- 58.Nichols RJ, Dzamko N, Morrice NA, et al. 14-3-3 binding to LRRK2 is disrupted by multiple Parkinson’s disease-associated mutations and regulates cytoplasmic localization. Biochem J. 2010;430(3):393–404. doi: 10.1042/BJ20100483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kumar A, Greggio E, Beilina A, et al. The Parkinson’s disease associated LRRK2 exhibits weaker in vitro phosphorylation of 4E-BP compared to autophosphorylation. PLoS ONE. 2010;5(1):e8730. doi: 10.1371/journal.pone.0008730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li X, Wang QJ, Pan N, et al. Phosphorylation-dependent 14-13-13 binding to LRRK2 is impaired by common mutations of familial Parkinson’s disease. PLoS ONE. 2011;6(3):e17153. doi: 10.1371/journal.pone.0017153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mukhopadhyay R, Kumar S, Hoh JH. Molecular mechanisms for organizing the neuronal cytoskeleton. Bioessays. 2004;26(9):1017–1025. doi: 10.1002/bies.20088. [DOI] [PubMed] [Google Scholar]
- 62.Khaitlina SY. Functional specificity of actin isoforms. Int Rev Cytol. 2001;202:35–98. doi: 10.1016/s0074-7696(01)02003-4. [DOI] [PubMed] [Google Scholar]
- 63.Bradke F, Dotti CG. The role of local actin instability in axon formation. Science. 1999;283(5409):1931–1934. doi: 10.1126/science.283.5409.1931. [DOI] [PubMed] [Google Scholar]
- 64.Esposito A, Dohm CP, Kermer P, Bähr M, Wouters FS. Alpha-synuclein and its disease-related mutants interact differentially with the microtubule protein tau and associate with the actin cytoskeleton. Neurobiol Dis. 2007;26(3):521–531. doi: 10.1016/j.nbd.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 65.Sousa VL, Bellani S, Giannandrea M, et al. {alpha}-synuclein and its A30P mutant affect actin cytoskeletal structure and dynamics. Mol Biol Cell. 2009;20(16):3725–3739. doi: 10.1091/mbc.E08-03-0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jaleel M, Nichols RJ, Deak M, et al. LRRK2 phosphorylates moesin at threonine-558: characterization of how Parkinson’s disease mutants affect kinase activity. Biochem J. 2007;405(2):307–317. doi: 10.1042/BJ20070209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chan D, Citro A, Cordy JM, Shen GC, Wolozin B. Rac1 protein rescues neurite retraction caused by G2019S leucine-rich repeat kinase 2 (LRRK2) J Biol Chem. 2011;286(18):16140–16149. doi: 10.1074/jbc.M111.234005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Desai A, Mitchison TJ. Microtubule polymerization dynamics. Annu Rev Cell Dev Biol. 1997;13:83–117. doi: 10.1146/annurev.cellbio.13.1.83. [DOI] [PubMed] [Google Scholar]
- 69.Lee HJ, Khoshaghideh F, Lee S, Lee SJ. Impairment of microtubule-dependent trafficking by overexpression of alpha-synuclein. Eur J Neurosci. 2006;24(11):3153–3162. doi: 10.1111/j.1460-9568.2006.05210.x. [DOI] [PubMed] [Google Scholar]
- 70.Gandhi PN, Wang X, Zhu X, Chen SG, Wilson-Delfosse AL. The Roc domain of leucine-rich repeat kinase 2 is sufficient for interaction with microtubules. J Neurosci Res. 2008;86(8):1711–1720. doi: 10.1002/jnr.21622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gillardon F. Leucine-rich repeat kinase 2 phosphorylates brain tubulin-beta isoforms and modulates microtubule stability – a point of convergence in parkinsonian neurodegeneration? J Neurochem. 2009;110(5):1514–1522. doi: 10.1111/j.1471-4159.2009.06235.x. [DOI] [PubMed] [Google Scholar]
- 72▪▪.Lin X, Parisiadou L, Gu XL, et al. Leucine-rich repeat kinase 2 regulates the progression of neuropathology induced by Parkinson’s-disease-related mutant alpha-synuclein. Neuron. 2009;64(6):807–827. doi: 10.1016/j.neuron.2009.11.006. Reveals an interesting interaction between α-synuclein and LRRK2. LRRK2 modulates age-related neurodegeneration caused by overexpression of α-synuclein in mice. Overexpression of LRRK2 accelerates the progression of α-synuclein-mediated neuropathological changes, whereas deletion of LRRK2 alleviates these alterations. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Drubin DG, Kirschner MW. Tau protein function in living cells. J Cell Biol. 1986;103(6 Pt 2):2739–2746. doi: 10.1083/jcb.103.6.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Johnson GV, Stoothoff WH. Tau phosphorylation in neuronal cell function and dysfunction. J Cell Sci. 2004;117(Pt 24):5721–5729. doi: 10.1242/jcs.01558. [DOI] [PubMed] [Google Scholar]
- 75.Li X, Wang J, Avshalumov MV, et al. Enhanced striatal dopamine transmission and motor performance with LRRK2 overexpression in mice is eliminated by familial Parkinson’s disease mutation G2019S. J Neurosci. 2010;30(5):1788–1797. doi: 10.1523/JNEUROSCI.5604-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li Y, Liu W, Oo TF, et al. Mutant LRRK2(R1441G) BAC transgenic mice recapitulate cardinal features of Parkinson’s disease. Nat Neurosci. 2009;12(7):826–828. doi: 10.1038/nn.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Melrose HL, Dächsel JC, Behrouz B, et al. Impaired dopaminergic neurotransmission and microtubule-associated protein tau alterations in human LRRK2 transgenic mice. Neurobiol Dis. 2010;40(3):503–517. doi: 10.1016/j.nbd.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Horton AC, Ehlers MD. Secretory trafficking in neuronal dendrites. Nat Cell Biol. 2004;6(7):585–591. doi: 10.1038/ncb0704-585. [DOI] [PubMed] [Google Scholar]
- 79.Lippincott-Schwartz J, Donaldson JG, Schweizer A, et al. Microtubule-dependent retrograde transport of proteins into the ER in the presence of brefeldin A suggests an ER recycling pathway. Cell. 1990;60(5):821–836. doi: 10.1016/0092-8674(90)90096-w. [DOI] [PubMed] [Google Scholar]
- 80.Winslow AR, Chen CW, Corrochano S, et al. Alpha-synuclein impairs macroautophagy: implications for Parkinson’s disease. J Cell Biol. 2010;190(6):1023–1037. doi: 10.1083/jcb.201003122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bove J, Prou D, Perier C, Przedborski S. Toxin-induced models of Parkinson’s disease. NeuroRx. 2005;2(3):484–494. doi: 10.1602/neurorx.2.3.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Biskup S, Moore DJ, Celsi F, et al. Localization of LRRK2 to membranous and vesicular structures in mammalian brain. Ann Neurol. 2006;60(5):557–569. doi: 10.1002/ana.21019. [DOI] [PubMed] [Google Scholar]
- 83.Mortiboys H, Johansen KK, Aasly JO, Bandmann O. Mitochondrial impairment in patients with Parkinson disease with the G2019S mutation in LRRK2. Neurology. 2010;75(22):2017–2020. doi: 10.1212/WNL.0b013e3181ff9685. [DOI] [PubMed] [Google Scholar]
- 84.Nandi D, Tahiliani P, Kumar A, Chandu D. The ubiquitin–proteasome system. J Biosci. 2006;31(1):137–155. doi: 10.1007/BF02705243. [DOI] [PubMed] [Google Scholar]
- 85.Wang L, Xie C, Greggio E, et al. The chaperone activity of heat shock protein 90 is critical for maintaining the stability of leucine-rich repeat kinase 2. J Neurosci. 2008;28(13):3384–3391. doi: 10.1523/JNEUROSCI.0185-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tanaka Y, Engelender S, Igarashi S, et al. Inducible expression of mutant α-synuclein decreases proteasome activity and increases sensitivity to mitochondria-dependent apoptosis. Hum Mol Genet. 2001;10:919–926. doi: 10.1093/hmg/10.9.919. [DOI] [PubMed] [Google Scholar]
- 87.Rubinsztein DC, Rubinsztein DC, DiFiglia M, et al. Autophagy and its possible roles in nervous system diseases, damage and repair. Autophagy. 2005;1(1):11–22. doi: 10.4161/auto.1.1.1513. [DOI] [PubMed] [Google Scholar]
- 88.Plowey ED, Chu CT. Synaptic dysfunction in genetic models of Parkinson’s disease: a role for autophagy? Neurobiol Dis. 2011;43(1):60–67. doi: 10.1016/j.nbd.2010.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xilouri M, Stefanis L. Autophagic pathways in Parkinson disease and related disorders. Expert Rev Mol Med. 2011;13:e8. doi: 10.1017/S1462399411001803. [DOI] [PubMed] [Google Scholar]
- 90.Cuervo AM, Stefanis L, Fredenburg R, Lansbury PT, Sulzer D. Impaired degradation of mutant alpha-synuclein by chaperone-mediated autophagy. Science. 2004;305(5688):1292–1295. doi: 10.1126/science.1101738. [DOI] [PubMed] [Google Scholar]
- 91.Bjorklund A, Dunnett SB. Dopamine neuron systems in the brain: an update. Trends Neurosci. 2007;30(5):194–202. doi: 10.1016/j.tins.2007.03.006. [DOI] [PubMed] [Google Scholar]