Abstract
HIV associated neurological disorders (HAND) are a mounting problem despite the advent of highly active antiretroviral therapy. To address mechanisms of HAND we used an SIV pigtailed macaque model to study innate immune responses in brain that suppress viral replication during acute infection. We previously reported that during acute infection in brain, noncanonical type I interferon signaling occurs, where interferon beta mRNA is induced while interferon alpha is simultaneously suppressed. Two downstream interferon stimulated genes, MxA and TRAIL, also show differential expression patterns. Here we show that differential signaling is due to interactions between macrophages and astrocytes. Astrocytes produce high levels of CCL2 upon SIV infection, which binds to CCR2 receptors on macrophages, leading to a selective suppression of interferon alpha and the ISG TRAIL, while simultaneously inducing interferon beta and MxA. The interactions between chemokine and cytokine pathways are a novel finding that may specifically occur in the CNS.
Introduction
Type I interferons (IFNs) are the first line of defense against viral infections, including HIV and SIV. Canonical type I IFN signaling begins with the production of interferon beta (IFNβ) upon stimulation of pattern recognition receptors (PRRs). IFNβ is then secreted and binds the interferon receptor in an autocrine and paracrine manner where it activates the JAK/STAT pathway, leading to the expression of interferon alpha (IFNα) and a plethora of antiviral interferon stimulated genes (ISGs). IFNα is then secreted and binds to the IFN receptor where it perpetuates the IFN signaling cascade in a positive feedback loop (1).
Our group has made the novel discovery that type I IFN signaling in the central nervous system (CNS) in response to SIV infection occurs in a more complex, noncanonical manner (2). Using an accelerated, consistent pigtailed macaque SIV model of HIV associated neurological disease, we have shown that while IFNβ is induced during acute SIV infection in brain, IFNα is simultaneously down regulated and associated with a suppression of signaling modulators downstream of the IFN receptor, including Tyk2, STAT1 and IRF7. This results in differential regulation of ISGs as well, with MxA being induced along with IFNβ, while TRAIL is suppressed along with IFNα. These results indicate that suppression of JAK/STAT signaling leading to IFNα and TRAIL does not result in complete shutdown of antiviral signaling, since IFNβ and MxA are still induced. Rather, accessory signaling pathways downstream of the IFN receptor exist which enables IFNβ to up regulate a subset of antiviral genes, such as MxA. Other groups have also found that despite the suppression of the JAK/STAT pathway, IFNβ still up regulates a subset of antiviral ISGs through alternative pathways, such as MAPK and PI3K (3). These results reveal that highly regulated mechanisms controlling IFN signaling occur during acute SIV infection in the brain, where the JAK/STAT pathway leading to IFNα and TRAIL is suppressed, while other IFNβ stimulated pathways downstream of the IFN receptor are induced.
Why this differential regulation occurs in the CNS and not in peripheral tissues, such as the lung, is most likely a consequence of the effects IFNs and their ISGs have in the brain. Whereas IFNβ has been associated with neuroprotection and the stimulation of anti-inflammatory mediators, IFNα and Tyk2 have been associated with neurotoxicity, inflammation, cell death and neuroinflammatory diseases such as HIV dementia and multiple sclerosis (4–10). TRAIL is a gene associated with cell death as well. Therefore, this differential pattern of IFN signaling in brain may be an evolutionary mechanism to induce an antiviral response mediated by IFNβ without inducing the inflammatory and destructive effects mediated by IFNα, TRAIL and the JAK/STAT pathway.
In order to investigate the mechanism behind this differential IFN signaling, we examined the two cell types involved in HIV and SIV infection in brain, macrophages and astrocytes. HIV and SIV infection of the CNS occurs during early stages of disease, as indicated by production of viral mRNA and protein both in brain tissue and CSF (11–13). This occurs via a “Trojan Horse” mechanism, where infection of certain subpopulations of peripheral monocytes results in immune activation and upregulation of adhesion molecules such as VCAM-1, facilitating transmigration across the blood brain barrier (BBB) (14–16). These monocytes accumulate in the perivascular regions and undergo differentiation into macrophages, which is where early HIV and SIV replication takes place (17–20). While induction of cytokines such as IFNβ in macrophages have been shown to be protective against SIV infection in the brain (21, 22), uncontrolled viral replication can lead to the production of pro-inflammatory cytokines, excitatory amino acids, and molecules mediating oxidative stress, all of which ultimately lead to neuronal death (23, 24). Astrocytes are the most numerous cells in the brain and have been reported to express relatively high levels of TLR3 and the downstream signaling adaptor molecules, indicating that astrocytes, as well as macrophages, play a major role in the immunological response to infection (25–27). HIV/SIV infection in astrocytes has been reported in brain tissue of humans and macaques, as well as in vitro (13, 28). Upon infection, astrocytes are the major producers of monocyte chemotactic protein (MCP-1), or CCL2, which is a chemokine that recruits monocytes expressing CCR2 into the CNS (14). Certain populations of macrophages/microglia in brain have been shown to express CCR2 during SIV/HIV infection (29). Importantly, CCL2 has also been reported to protect neurons from apoptosis as well as inhibit glutamate production in response to HIV infection, making it an important neuroinflammatory modulator (30).
Perhaps the most significant immunological role astrocytes play in the CNS is their immunomodulatory effects on macrophages and microglia. Microglia, the resident macrophages of the brain, have been shown to exhibit increased inflammatory activity in vitro than observed in pathologic conditions in the brain (31, 32), largely because microglia are usually cultured alone, which does not accurately portray the CNS microenvironment. However, by coculturing astrocytes with macrophages or by exchanging supernatants, it was found that astrocytes release soluble factors that attenuate the inflammatory response in macrophages. These effects include reducing the expression of inducible nitric oxide synthetase and IL-12 (33, 34), increasing expression of antioxidant enzymes (35), and inhibiting IFNγ-induced microglial activation (36). Also, HIV-infected monocytes that interact with astrocytes have been reported to secrete decreased levels of TNF-α and eicoisanoids upon LPS stimulation (37).
Since astrocytes have been shown to attenuate the inflammatory response in macrophages, we examined if these cells could selectively down modulate IFNα, since this cytokine is a potent neuroinflammatory mediator. We found that the differential IFN response observed in vivo is attributed to astrocytic production of CCL2. When SIV infected macrophages are exposed to CCL2, there is specific suppression of IFNα and TRAIL, while maintaining an upregulation of IFNβ and MxA. This not only illustrates the novel role CCL2 plays in selectively suppressing IFNα expression in SIV infected macrophages, but also suggests that the immunomodulatory effects that astrocytes exert on macrophages are a key factor in maintaining an anti-inflammatory environment during acute viral infection in the brain.
Materials and Methods
Cell culture and infections
Macrophages
Primary pigtailed macaque monocyte derived macrophages (MDMs) were prepared from whole blood as previously described (38). On day 7, cells were washed 3 times with sterile phosphate buffered saline (PBS) to remove non adherent cells. On day 8, cells were infected with SIV/17E-Fr, which was grown using macaque producer cells, using an MOI of 0.05 for 6 hours and then washed 5 times with PBS. Cells were then cultured with normal macrophage media, which consists of Dulbecco’s modified Eagle’s medium (DMEM) (Invitrogen, Grand Island, N.Y.), 10% fetal bovine serum (FBS), 10mM HEPES (Invitrogen), 2 mM L-glutamine (Invitrogen), and 0.5mg/mL gentamicin (Invitrogen), unless otherwise indicated. SIV infected and timepoint specific uninfected controls were harvested at 24, 48 and 72 hours post infection. RNA was isolated using the RNeasy kit (Qiagen, Valencia California) and then treated with turbo DNAse (Ambion) according to manufacturer’s instructions. Protein lysates were made using RIPA buffer (0.25% Na deoxycholate, 0.1% SDS, 25 mM Tris pH 7.4, 150 mM NaCl, 1 mM EDTA, and 1% Nonidet P-40 in water) and spiked with 1mM DTT, 0.1 mM PMSF, protease inhibitor cocktail (Calbiochem, Darmstadt, Germany) and phosphotase inhibitor cocktails (Calbiochem) just before use.
Astrocytes
Primary rhesus macaque astrocytes (Cambrex, Walkersville MD) were cultured as previously described (39). Cells were infected with SIV/17E-Fr at an MOI of 0.05 for 6 hours and then washed 3 times with sterile PBS. Cells were then cultured in D10 (DMEM, 10% FBS, 10 mM HEPES, 2 mM L-glutamine, 0.5 mg/mL gentamicin, and 2 mM Na pyruvate). Half of the media was replaced with fresh D10 every 5 days for 30 days. Infected and uninfected cells were harvested on days 5–7, 10, 14, 21 and 25. RNA was isolated using the RNeasy kit and treated with DNAse as described above. Protein lysates were made as indicated above. For polyI:C and IFNβ treated astrocytes: cells were treated with either 10 ug/mL of polyIC, or 100 units/mL of IFNB. Cells were harvested at 24, 48 and 72 hours post treatment and RNA was isolated as described above.
Supernatant exchange reactions
Primary MDMs were infected as indicated above. After washing off virus, cells were cultured with either normal macrophage media, supernatants from uninfected astrocytes, or supernatants from infected astrocytes. Astrocytes supernatants, including time-point specific uninfected controls, were collected at 14–21 days p.i. when cells were harvested. Ultracentrifugation was done at 40,000 RPM for 2 hours at 4°C on both sets of supernatants using the Sorvall Discovery 100SE (rotor TH641) (Thermo-Fischer Scientific) in order to pellet virus and cellular debris. Supernatants were then passed through a 0.22 micron filter unit and a p27 analysis by ELISA (Zeptometrix) was done to verify no infectious virus was present. Infected and uninfected cells were harvested 48 hours p.i. and RNA was isolated as described above.
Quantitative reverse transcriptase PCR
All primer and probe sequences as well as qRT-PCR protocols are described elsewhere (2). All genes were normalized to 18s rRNA. SIV infected samples were expressed as a fold induction over each sample’s respective uninfected control. In the case where uninfected samples were given a treatment, all treated samples were expressed as a fold induction over untreated controls.
Western blots and antibodies
All western blot protocols and antibodies used are described elsewhere (2). Quantitations were performed using ImageQuantTL 7.0 software on scanned images.
CCL2 ELISA
CCL2 was measured in the supernatants of SIV infected and UI macrophages (48 hr p.i.), as well as SIV infected and UI astrocytes (14 days p.i.) using the Quantikine Human CCL2 ELISA (R&D Systems, Minneapolis, Minnesota) according to the manufacturers protocol.
CCL2 Neutralization
Infected astrocyte supernatants were incubated with 50ug/mL of anti-human CCL2 neutralizing antibody (R&D Systems) or nonspecific goat IgG for 1 hour at room temperature. The supernatants were then added to uninfected and SIV infected MDMs after virus had been washed off. Cells were harvested 48 hours p.i. and RNA was isolated using the RNeasy kit (Qiagen) as described above.
Chemokine and growth factor treatment
Uninfected and SIV infected MDMs were treated or untreated with either 100ng/mL of recombinant human CCL2 (ProSpec East Brunswick, New Jersey), 0.2 ng/mL of recombinant human TGFβ (R&D Systems), or 10ng/mL of recombinant human CCL3 (ProSpec). Cells were harvested 48 hours p.i. and RNA was isolated using the RNeasy kit (Qiagen) as described above.
CCR2 inhibitor: Propagermanium
Bis(2-carboxyethylgermanium(IV) sesquioxide) was purchased from Sigma Aldrich (St. Louis, Missouri) and made into a 10mg/mL stock solution in water. Other commonly used names: propagermanium and 2-carboxyethylgermasesquioxane. A final concentration of 100 uM was used to treat infected and uninfected MDMs. For samples that were treated with both propagermanium and CCL2, cells were incubated with propagermanium 45 minutes before adding CCL2.
Statistical analysis
For mRNA comparisons, either one-way analysis of variance (ANOVA) with Bonferroni post test for multiple comparisons or two-way ANOVA with Bonferroni post test for multiple comparisons was performed depending on the experimental conditions. For protein comparisons which only consisted of 2 groups (uninfected versus infected), an unpaired t test was performed. Exact statistical analysis performed for each graph is outlined in the figure legends. Results were considered significant for p<0.05.
Results
Type I IFN signaling is up regulated in SIV infected MDMs
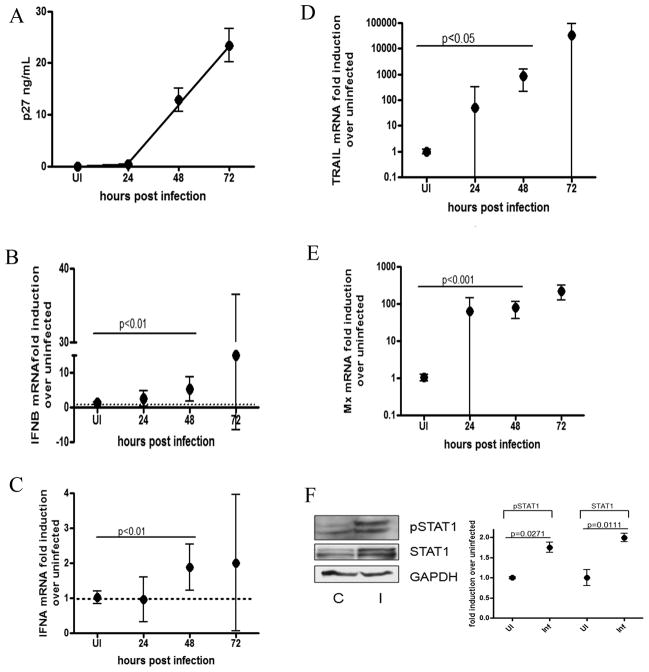
Since macrophages are the major target cells responsible for SIV replication in brain, we examined SIV infection of pigtailed macaque macrophages in vitro. Macaque monocyte derived macrophages (MDMs) were infected with SIV (MOI 0.05) and harvested at 24, 48 and 72 hours post infection (p.i.). Levels of Gag p27 were measured in cell supernatants to quantify viral production (Figure 1A) and quantitative reverse transcriptase PCR (qRT-PCR) was done for IFNα and IFNβ. We found that at 48 hours p.i. both IFNs β and α mRNA are significantly up regulated, 5 fold (p<0.01) and 2 fold (p<0.01), respectively (Figures 1B and 1C), coinciding with the start of viral detection in the supernatant. mRNA levels of two ISGs, TRAIL and MxA, were also quantitated. We show that at 48 hours p.i, both genes are significantly upregulated (p<0.05, p<0.001 respectively). (Figures 1D and 1E), contrary to our in vivo observations where MxA mRNA is upregulated in the same pattern as IFNB, while TRAIL, like IFNα, is not induced (2). Because the 48 hour timepoint exhibited the least amount of variability in gene expression between replicates, this timepoint was chosen for subsequent experiments. By 72 hours p.i., cell death started occurring in infected cells, which may have accounted for the high degree of variability. At 48 hours p.i., the transcription factor STAT1, which is phosphorylated upon IFN receptor activation, is not only translationally upregulated (p=0.0111), but also has an increase in tyrosine phosphorylation (Y701) (p=0.0271) (Figure 1F). Both pSTAT1 and STAT1 are induced by about 75% upon SIV infection, indicating that there is a translational and post translational up regulation of interferon signaling upon SIV infection. These results indicate that both IFNs α and β are upregulated in SIV infected MDMs in vitro, and downstream IFN signaling is activated.
Figure 1. Type I IFN signaling is up regulated in SIV infected macrophages.
Primary pigtailed macaque MDMs were infected with SIV and RNA was isolated at 24, 48 and 72 hours p.i. (A) Levels of p27 were measured in the supernatants by ELISA. (B–E) qRT-PCR analysis was done for (B) IFNβ, (C) IFNα, (D) TRAIL, (E) and MxA. All qRT-PCR data is expressed as a fold induction over uninfected controls. All genes are normalized to 18s rRNA. Data is expressed as the mean of 3 independent experiments (each done with duplicate technical replicates) from 2 donor macaques with 95% confidence interval. One-way ANOVA with Bonferroni post test for multiple comparisons was performed for mRNA comparisons. Significant (p<0.05) pairwise comparisons are shown. (F) Protein lysates were made from UI controls (C) and SIV infected (I) MDM at 48 hours p.i. and probed for GAPDH, STAT1 and pSTAT1 (Y701) via western blot. Quantitations were performed on 3 replicate western blots normalized to GAPDH. Blot shown is representative of all experiments. An unpaired t test was done to compare uninfected and SIV infected samples for each protein.
IFN signaling is downregulated in SIV infected astrocytes
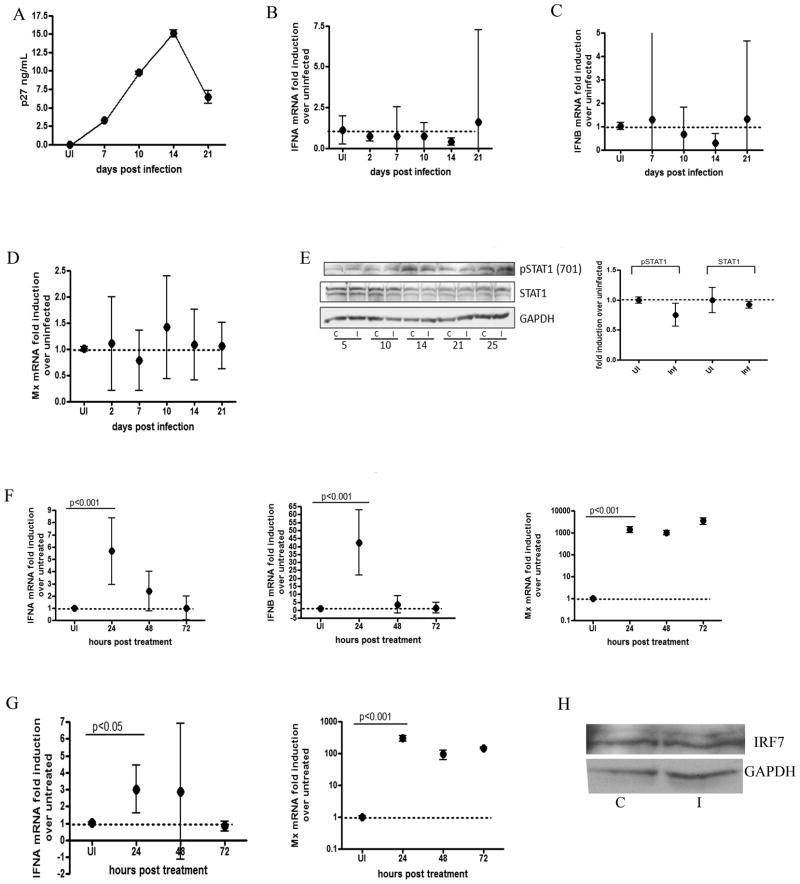
Primary rhesus astrocytes were infected with SIV (MOI 0.05) and harvested at 2, 7, 10, 14 and 21 days p.i. for mRNA analysis. As we and other groups have shown (39, 40), while virus infection in macrophages takes place during the course of days, infection in astrocytes takes longer because restrictive factors specific to this cell type severely slows down viral replication in vitro. (Figures 1A and 2A). Unlike macrophages, which displayed an activated IFN response, no significant induction of IFNα, IFNβ or MxA mRNA was observed for any of the timepoints (Figures 2A–2D), nor was there an increase in STAT1 protein or phosphorylation status in infected cells (Figure 2E). TRAIL mRNA was undetectable. To determine why this inhibition of IFN was occurring, we measured IRF7 mRNA and protein, since this is an important transcription factor in IFN alpha and beta production that is both transcriptionally and translationally induced upon viral infection (41). IRF7 mRNA was undetectable in infected and uninfected cells, and there was no detectable change in protein at 14 days p.i. (Figure 2H). To ensure that the astrocytes were immunologically functional, uninfected cells were treated with polyI:C to test pathogen sensing activity, and with IFNβ to test stimulation through the IFN receptor. Both treatments significantly upregulated all gene expression by 24 hours post treatment (Figures 2F and 2G), indicating that the astrocytes were able to induce a full innate immune response. These results indicate that the IFN response is specifically downregulated in SIV infected astrocytes.
Figure 2. Type I IFN signaling is down regulated in SIV infected astrocytes.
Primary rhesus astrocytes were infected with SIV. RNA was isolated at 2, 7, 10, 14 and 21 days p.i. (A) p27 levels were measured in the supernatants by ELISA. (B–D) qRT-PCR analysis was done for (B) IFNα, (C) IFNβ and (D) MxA. TRAIL mRNA was undetectable. All qRT-PCR data is expressed as a fold induction over uninfected controls. All genes are normalized to 18s rRNA. Data is expressed as the mean of 3 independent experiments (each done with duplicate technical replicates) with 95% confidence interval. (E) Protein lysates were made at 5, 10, 14, 21 and 25 days p.i. from uninfected controls (C) and SIV infected (I) primary rhesus astrocytes and probed for GAPDH, STAT1, and pSTAT1 (Y701) via western blot. Quantitations were performed on 4 replicate western blots at 14 days p.i normalized to GAPDH. Blot shown is representative of all experiments. (F) Astrocytes were stimulated with polyI:C (10ug/mL) and RNA was isolated at 24, 28 and 72 hours p.i. qRT-PCR was done on IFNα, IFNβ and Mx. Values are normalized to 18s rRNA and are expressed as a fold induction over untreated controls. (G) Primary rhesus astrocytes were treated with IFNB (100 U/mL) and RNA was isolated 24, 48 and 72 hours p.i. qRT-PCR was done for IFNA and Mx. Values are normalized to 18s rRNA and are expressed as a fold induction over untreated controls. Data for astrocyte treatments is expressed as the means of duplicate independent experiments (each done with 3 technical replicates) with 95% confidence interval. (H) Western blot for IRF7 and GAPDH were performed on control (c) and infected (I) astrocytes 14 days p.i. Blot is representative of 3 replicates. For all mRNA comparisons, one-way ANOVA with Bonferroni post test for multiple comparisons was performed. Significant (p<0.05) pairwise comparisons are shown.
Astrocytes release soluble factors that specifically suppress IFNα induction in SIV infected macrophages
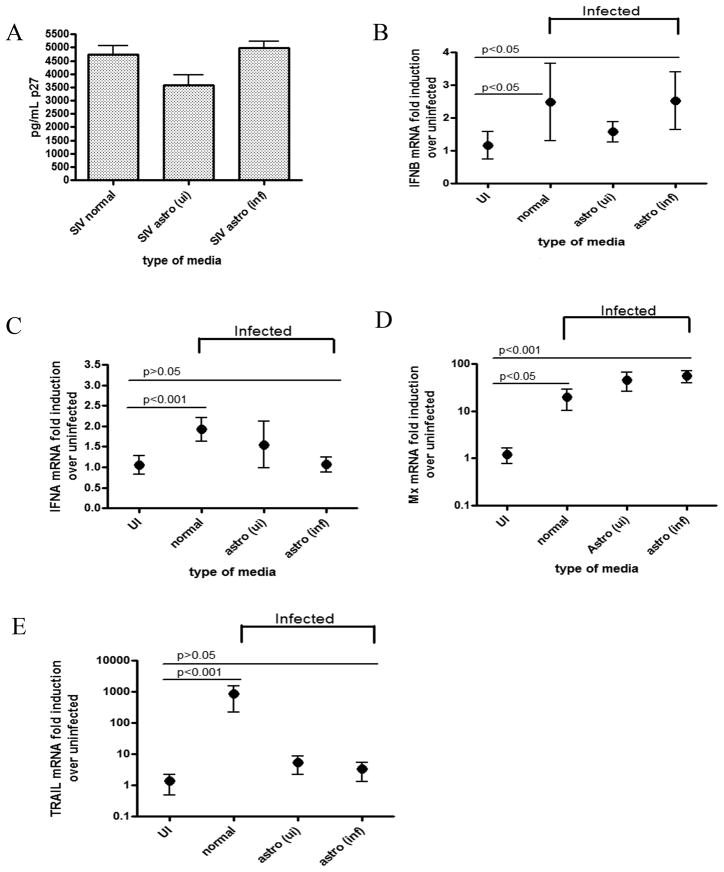
We found that type I IFN signaling is upregulated in SIV-infected macrophages in vitro, while SIV infected astrocytes exhibit the opposite effect. In brain tissue, however, there is an intermediate response. It has been reported that proteins released by astrocytes have anti-inflammatory effects on stimulated macrophages. Because IFNα is a more potent inflammatory cytokine in the CNS than IFNβ, we determined if soluble factors released by astrocytes could specifically downregulate IFNα in SIV infected macrophages while maintaining the IFNβ response. We opted to perform a series of supernatant exchange experiments instead of coculturing the cells for two reasons: The two primary cell types were from different donor macaques, and obtaining mixed primary glial cultures directly from adult primate brain is extremely costly and complicated. Therefore, we studied the effects of astrocytes on macrophages by doing a series of astrocyte supernatant exchange experiments. Supernatants were collected from either uninfected or SIV infected astrocytes and cleared of virus by ultracentrifugation. Gag p27 ELISAs were done to ensure no infectious virus was present (data not shown). All macrophages were infected with SIV in normal macrophage media, and then cultured with one of three different media conditions: normal macrophage media, uninfected astrocyte supernatant, or SIV-infected astrocyte supernatant. Cells were then harvested at 48 hours p.i., and mRNA expression of IFNs α and β, MxA and TRAIL were measured.
In cells cultured in normal media and infected astrocyte supernatants, IFNβ mRNA is significantly upregulated upon SIV infection compared to uninfected controls (p<0.05 for both conditions) (Figure 3B). Normal macrophage media, as well as SIV infected astrocyte supernatant, yielded a 2.5 fold induction of IFNβ mRNA, while culturing with uninfected astrocyte supernatants yielded a 1.5 fold induction, although not significantly (p>0.05). IFNα mRNA expression was significantly upregulated (p<0.001) in SIV infected macrophages cultured with normal macrophage media (Figure 3C). IFNα expression in macrophages cultured with uninfected astrocyte supernatants also was increased above uninfected although not significantly (p>0.05). In contrast, SIV infected macrophages cultured with infected astrocyte supernatant show no induction of IFNα mRNA as compared to uninfected controls (p>0.05) (Figure 3C). Furthermore, the induction pattern of downstream ISGs showed that MxA RNA was significantly upregulated in all media conditions (p<0.05 for normal media, p<0.001 for both astrocyte conditions), with the highest fold inductions (over 50 fold) in macrophages cultured with either type of astrocyte supernatant (Figure 3D). TRAIL mRNA levels were significantly upregulated almost 800 fold in cells cultured in normal macrophage media (p<0.001), but SIV infected macrophages cultured with infected or uninfected astrocyte supernatant show no significant up regulation (p>0.05 for both) (Figure 3E). (Figure 3E). Taken together, SIV infected macrophages cultured with infected astrocyte supernatants most closely resembles the expression pattern in brain, where IFNα and TRAIL are suppressed, and IFNβ and MxA are significantly induced. These results indicate that SIV infected astrocytes secrete soluble factor(s) that, when cultured with SIV infected macrophages, lead to a strong suppression of IFNα and TRAIL mRNA, while not suppressing IFNβ and MxA.
Figure 3. Astrocytes release soluble factors that specifically suppress IFNα induction in SIV infected macrophages.
Primary pigtailed MDM were infected with SIV and then cultured with either normal macrophage media (normal), uninfected astrocyte supernatants (astro ui), or infected astrocyte supernatants (astro inf). Cells were harvested at 48 hours p.i. (A) Levels of p27 were measured by ELISA in the supernatants. (B–E) RNA was isolated from cells and qRT-PCR analysis was done for (B) IFNβ, (C) IFNα, (D) MxA, and (E) TRAIL. Values are expressed as a fold induction over the respective uninfected controls. All values are normalized to 18s rRNA. Graphs represent means of 3 independent experiments (each done with duplicate technical replicates) with 95% confidence interval. One-way ANOVA with Bonferroni post test for multiple comparisons was performed for mRNA comparisons. Significance (p<0.05) or lack thereof is shown for pairwise comparisons discussed in the text.
Astrocytes release a higher concentration of CCL2 than macrophages which can selectively suppress IFNα in response to SIV infection
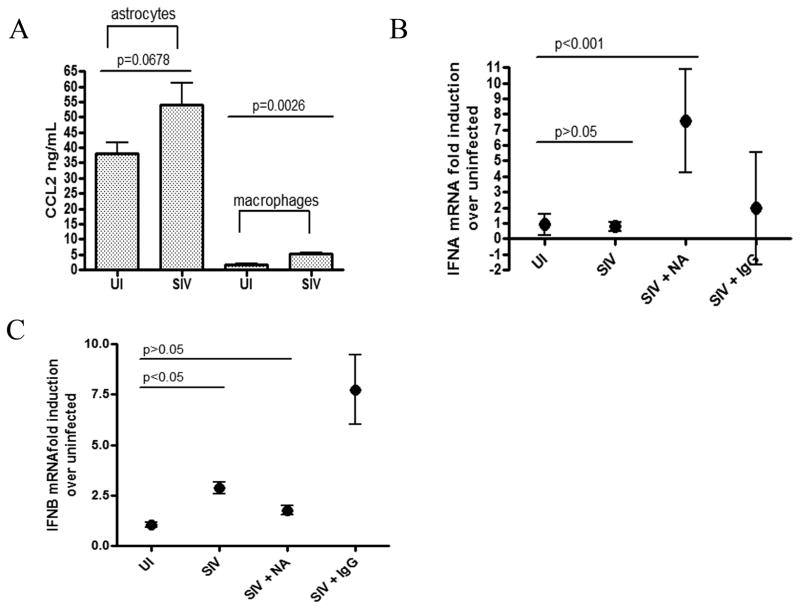
In order to identify the soluble factors in the infected astrocyte supernatants that lead to differential type I IFN expression in SIV infected macrophages, we examined levels of CCL2, which is one of the major chemokines released by activated astrocytes in brain in response to HIV infection (42). We have previously shown in our macaque model that activated astrocytes in brain are associated with an upregulation of CCL2 protein (43). Although we have shown this in vivo, we wanted to confirm it in our astrocytes in vitro. CCL2 protein was measured by ELISA in the supernatants of uninfected and SIV infected astrocytes between days 15 and 20 p.i. We found that CCL2 was upregulated by 55% upon SIV infection with a trend towards significance (p=0.0678) (Figure 4A). CCL2 was also measured in uninfected and infected macrophage supernatants, and, although macrophages did upregulate CCL2 upon SIV infection (p=0.0026), both infected and uninfected macrophages produced at least 10 times less CCL2 than astrocytes (Figure 4A), suggesting that culturing macrophages with astrocyte supernatants creates an entirely different immunological environment than macrophages are exposed to when cultured alone.
Figure 4. Astrocytes release a higher concentration of CCL2 than macrophages which can selectively suppress IFNA in response to SIV infection.
(A) Levels of CCL2 were measured in the supernatants of uninfected (ui) and SIV infected (SIV) primary astrocytes and primary MDM. Graphs represent mean levels in supernatants from at least 3 different experiments with standard error. An unpaired t test was performed to compare levels of CCL2 in uninfected and SIV infected supernatants for both astrocytes and macrophages. (B–C) SIV infected astrocyte supernatants were incubated with either a neutralizing antibody to CCL2 (NA) or a control goat IgG. SIV infected MDM were then cultured with either infected astrocyte supernatants (SIV), infected astrocyte supernatants with CCL2 neutralizing antibody (SIV + NA), or infected astrocyte supernatants with control IgG (SIV + IgG). Cells were harvested 48 hours p.i. and RNA was isolated. qRT-PCR analysis was done for (B) IFNα and (C) IFNβ. Graphs represent means of 2 independent experiments (each done with duplicate technical replicates) with 95% confidence interval. One-way ANOVA with Bonferroni post test was performed. Significance (p<0.05) or lack thereof is shown for comparisons discussed in the text.
To determine if CCL2 was responsible for differential IFN regulation, supernatants from SIV infected astrocytes were incubated in the presence or absence of a neutralizing antibody to CCL2. Primary SIV-infected MDMs were then cultured with either the CCL2 neutralized or non-neutralized astrocyte supernatant. In macrophages cultured with infected supernatants without the CCL2 neutralizing antibody, there was no significant induction of IFNα mRNA upon SIV infection (p>0.05), whereas, when cultured with supernatants containing the neutralizing antibody to CCL2, IFNα mRNA was significantly upregulated 7 fold (p<0.001) upon SIV infection (Figure 4B). This was not seen in the presence of a nonspecific IgG. IFNβ mRNA was up-regulated in both media conditions, but only significantly in media without neutralizing antibody (Figure 4C). These data suggest that CCL2 production by astrocytes is responsible for the selective suppression of IFNα mRNA in SIV infected macrophages.
Recombinant CCL2 differentially regulates the IFN response
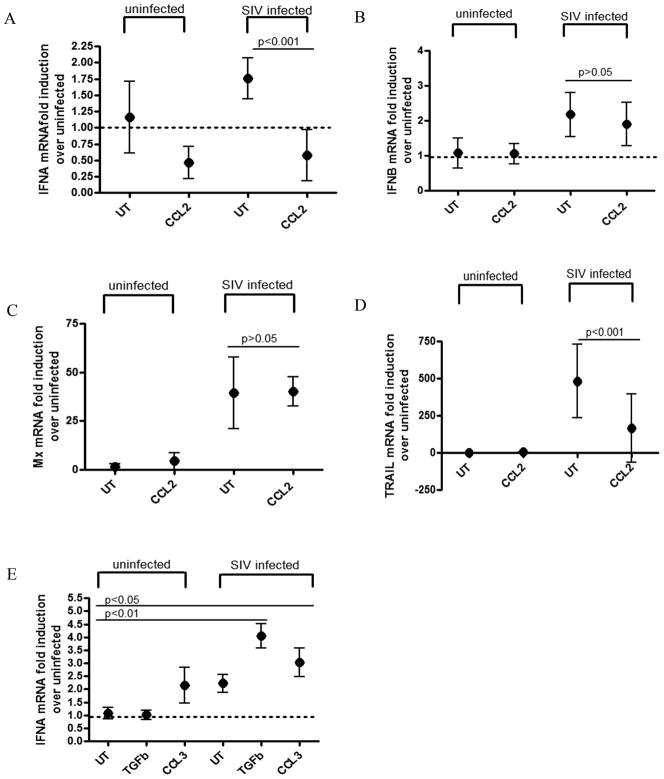
We have shown that CCL2 is highly secreted by astrocytes upon SIV infection, and that this chemokine selectively down-modulates IFNα mRNA, since its neutralization restores IFNα expression upon SIV infection. To examine whether CCL2 alone would reproduce this effect, recombinant CCL2 was added to macrophages in culture to mimic the astrocyte supernatant exchange experiments. Primary macaque MDMs were infected with SIV for 6 hours and then incubated in the presence or absence of recombinant CCL2 (100ng/mL) in normal macrophage media. Treating SIV infected macrophages with CCL2 significantly down regulated both IFNα and TRAIL mRNA expression (p<0.001 for both genes) (Figure 5A, 5D). However, neither IFNβ nor MxA mRNA induction in SIV infected macrophages were affected by treatment with CCL2 (p>0.05 for both genes) (Figure 5B, 5C). In order to test the specificity of the CCL2 effect, primary macrophages were also treated with CCL3, which is another chemokine, and TGFβ (10ng/mL and 0.2ng/mL respectively), which is a cytokine that astrocytes are known to produce (37). Infection of cells treated with either of these proteins resulted in significant up regulation of IFNα (p<0.01 for TGFβ, p<0.05 for CCL3) (Figure 5E). These data indicate that CCL2 is able to specifically down modulate mRNA expression of IFNα and TRAIL in SIV infected macrophages, while having no effect on the mRNA expression of IFNβ and Mx. Therefore, CCL2 causes a non-canonical IFN signaling cascade in SIV infected macrophages, where IFNα and certain ISGs (ie TRAIL) are selectively suppressed transcriptionally.
Figure 5. Recombinant CCL2 differentially regulates the interferon response.
(A–D) Primary MDM were infected with SIV and then either treated with CCL2 (CCL2) or left untreated (UT). Cells were harvested at 48 hours p.i. and RNA was isolated. qRT-PCR analysis was done for (A) IFNα, (B) IFNβ, (C) MxA, and (D) TRAIL. Data represents means of 3 independent experiments (each done with 2 technical replicates) with 95% confidence interval. (E) Primary MDM were infected with SIV and then treated with either TGFβ or CCL3 (0.2 ng/mL and 10ng/mL respectively), or left untreated (UT). Cells were harvested at 48 hours p.i. and RNA was isolated. qRT-PCR analysis was done for IFNα. Data represents means of 2 independent experiments (each done with 2 technical replicates) with 95% confidence interval. Two-way ANOVA with Bonferroni post test for multiple comparisons was performed. Significance (p<0.05) or lack thereof is shown for comparisons discussed in the text.
CCL2 induced IFNα suppression occurs through CCR2 signaling
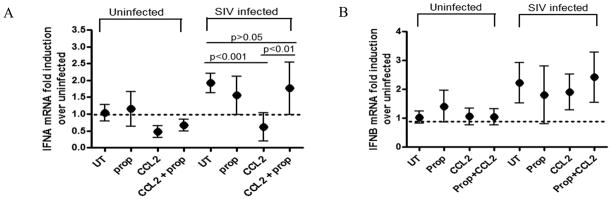
To examine whether CCL2 exerts its effect through its cognate receptor, CCR2, a small molecule inhibitor of CCR2, propagermanium (100uM), was used. Propagermanium is an organic small molecule inhibitor that specifically blocks CCR2 without stimulating downstream signaling (44). CCL2-treated and untreated macrophages were infected in the presence or absence of inhibitor. Cells were harvested 48 hours p.i. and qRT-PCR analysis was performed on RNA for IFNs α and β. Blocking the CCR2 receptor in the presence of CCL2 was able to restore IFNα induction levels in SIV infected macrophages (p<0.01), while neither the presence of CCL2 nor the blocking of CCR2 affected mRNA levels of IFN β (Figures 6A and 6B). These data indicate that CCL2 mediates the selective suppression of IFNα in SIV infected macrophages via a CCR2 dependant mechanism.
Figure 6. CCL2 induced IFNα suppression occurs through CCR2 signaling.
(A–B) SIV infected primary MDM were treated with either propagermanium (prop), CCL2 (CCL2), CCL2 + propagermanium (CCL2 + prop), or left untreated (UT). Cells were harvested 48 hours p.i. and RNA was isolated. qRT-PCR analysis was done for (A) IFNα or (B) IFNβ. Data represents means of 3 independent experiments (each done with 2 technical replicates) with 95% confidence interval. One-way ANOVA with Bonferroni post test for multiple comparisons was performed on the SIV infected group, and selected pairwise comparisons are shown. Results were considered significant with p<0.05.
Discussion
In this report we identified the cellular pathway that leads to the non-canonical type I IFN signaling pattern seen during acute SIV infection in the macaque brain. We show for the first time that SIV infected macrophages cultured with supernatants from SIV infected astrocytes specifically suppress IFNα and TRAIL mRNA while maintaining an induction of IFNβ and MxA. This effect was due to the high concentration of CCL2 that infected astrocytes produce, since neutralizing CCL2 in the infected astrocyte supernatants ameliorated this selective suppression of IFNα and TRAIL. The addition of recombinant CCL2 to SIV infected macrophages reproduced this selective suppression, and this was mediated through the CCR2 receptor. This is a novel and important discovery which shows how CCR2 signaling can communicate with the type I IFN pathway to alter the canonical IFN response.
The complete suppression of the type I IFN response in SIV infected astrocytes is very striking. Because astrocytes are able to induce the IFN response in polyIC and IFNβ treated cells, this suppression of signaling in astrocytes is specific to SIV infection. We report that there is a deficiency in IRF7 mRNA and protein induction upon SIV infection which may be responsible for the inability to produce IFNs α and β. The exact intracellular sensor that recognizes HIV and SIV in astrocytes is currently unknown, although one group has suggested the mannose receptor as playing a role (45). Therefore, more work is needed to determine which upstream innate intracellular regulators are being affected during infection that leads to lack of IRF7 activation. It is already known that astrocytes contain restrictive factors that severely impair the kinetics of SIV and HIV replication (39, 40). It is therefore possible that these restrictive factors may somehow prevent activation of the innate interferon response by interfering with the production of viral intermediates that would otherwise be immediately recognized by pattern recognition receptors.
While macrophages do up regulate CCL2 protein upon SIV infection, the amount of CCL2 is 10 times less than the amount produced by infected astrocytes. This suggests that immunological signaling is affected by not only the type of soluble factors that are present in a particular microenvironment, but also the concentration. The suppressive effects that high concentrations of CCL2 have on IFNα mRNA that we see in vitro are consistent with what we see in vivo in our macaque model. During acute SIV infection, there is about 10 times more CCL2 protein production in the CSF than there is in the plasma (43). Furthermore, there is an almost 100 fold induction of CCL2 mRNA in brain (46), whereas there is no significant induction in peripheral tissues such as the lung (data unpublished). We have previously found that suppression of IFNα mRNA occurs in the brain during acute infection, but not lung (2). Thus, the CNS, which contains a high concentration of CCL2, exhibits differential type I IFN regulation, while tissues in the periphery such as the lung, which are not exposed to as much CCL2, do not. These results provide valuable insight into how the compartmentalization of different tissues and cell types results in an entirely different immunological response.
Why this differential regulation of the IFN signaling pathway occurs is most likely due to type of tissue in which infection occurs. Astrocytes are cells that are exclusively found in the brain and are potent producers of CCL2 upon HIV infection (42, 47). Macrophages and microglia are strong producers of type I IFNs in response to viral infection in the CNS, but each interferon has very different effects. IFNα has known neurotoxic and neuroinflammatory effects. Not only is it associated with HIV dementia and genetic diseases such as Aicardi-Goutierres syndrome and Cree encephalitis which are characterized by neurodegeneration and ongoing inflammatory processes, but Hepatitis C patients on IFNα therapy have been known to suffer from depression, seizures and EEG changes (4–6, 8, 10). IFNβ, however, has been associated with the secretion of anti-inflammatory cytokines and neurotropic growth factors (9, 48). Therefore, the high level of CCL2 production by astrocytes in the CNS may be an evolutionary mechanism that limits the production of proteins that lead to neuronal death, such as IFNα and TRAIL, while allowing the induction of cytokines that can provide protection against pathogens without the destructive effects, such as IFNβ and MxA. This balance is extremely important in the CNS, since it is an organ composed of non-renewable neurons. However, in the periphery, where cell turnover is particularly high and apoptosis can serve a protective purpose rather than a permanent loss of non-renewable cells, this fine tuned balance between inflammatory versus anti-inflammatory cytokine production is not as vital.
The immunomodulatory and protective effects of CCL2 on cells in the CNS have been well characterized. CCL2 has been reported to protect neurons and astrocytes from HIV-tat and NMDA induced apoptosis (30, 49). Furthermore, the treatment of microglia and neurons with CCL2 does not activate an inflammatory response, nor does it induce apoptosis, indicating that this chemokine does not inflict deleterious inflammatory effects (50). In addition, CCR2 deficiency in microglia has been reported to accelerate disease progression in a mouse model of Alzheimer’s disease (51). Our findings that CCL2 suppresses signaling pathways leading to IFNα, which is a known inflammatory modulator in the CNS, coincides with evidence in the literature of its neuroprotective role.
While the finding that CCL2 plays a role in the differential regulation of type I IFNs is novel, cross talk between IFN and CCL2 signaling pathways has been established. Mice lacking the IFN receptor have an impaired ability to produce CCL2 in the liver in response to CMV, as well as Listeria infection (52, 53), suggesting that IFN signaling is necessary for CCL2 expression. Conversely, in mice coinfected with influenza virus and pneumococcal pneumonia, increased type I IFN production leads to decreased CCL2 gene expression (54). This has also been supported by microarray analysis on mouse splenocytes showing that IFNα treatment leads to decreased CCL2 gene expression (55). Furthermore, studies using the CCR2 inhibitor, propagermanium, have shown that blocking this pathway in mice increases the production of type I IFNs in response to influenza infection (44, 56). Additionally, both NK cells and macrophages have increased IFN production after treatment with propagermanium (57, 58). It is probable that the type of relationship between cytokines and chemokines largely depends on the cell type, pathogenic stimulus and the presence of other cytokines and chemokines in that particular microenvironment. These studies, as well as ours, demonstrate that these signaling networks are not mutually exclusive and show constant interaction.
Examining the CCR2 and IFN signaling pathways is required to obtain the mechanism behind their interactions. Binding of CCL2 to CCR2 leads to a conformational change in the receptor/ligand complex, which enables binding of heterotrimeric (αβγ) G-proteins to certain receptor residues (59). This conformational change is also attributed to the activation of receptor-associated Janus kinases, resulting in receptor phosphorylation (60). Once bound, the α subunit disassociates from the complex and different effecter pathways, including calcium mobilization, cell migration and increased cAMP levels, are activated. IFN signaling is initiated upon cellular pathogen sensing via pattern recognition receptors, and subsequent transcription of IFNβ, which is secreted and binds to the IFN receptor, where it activates the JAK/STAT pathway. This leads to the induction of hundreds of antiviral genes, as well as the production of IFNα. Alternative pathways activated by IFNβ, such as MAPK and PI3K, have also been shown to activate antiviral genes in a JAK/STAT independent way (3). IFNα is secreted and binds to the IFN receptor, activating a positive feedback loop and amplifying the IFN response (1). It is possible that CCR2 signaling produces certain inhibitory factors which may suppress any of the JAK/STAT signaling modulators downstream of the IFN receptor, leading to suppression of IFNα and a subset of ISGs.
While these results are no doubt of paramount importance to studying the neuroimmunological effects of viral infection, they also provide valuable and novel information about basic immunological regulatory networks. Studying the interactions between cytokines and chemokines in different immunological environments provides valuable information about the pathogenesis of tissue specific infections and will ultimately aid in the discovery of new treatments.
Acknowledgments
We would like to thank the Retrovirus Lab for their thought provoking ideas and interesting discussions, as well as Brandon Bullock for help in preparing cell cultures. We also thank Dr. Patrick Tarwater and Dr. Kenneth Witwer for their help with statistical analyses.
Footnotes
This work was supported by grants from the NIH to JEC (NS047984, NS055648 and MH070306).
References
- 1.Kawai T, Akira S. Antiviral signaling through pattern recognition receptors. J Biochem. 2007;141:137–145. doi: 10.1093/jb/mvm032. [DOI] [PubMed] [Google Scholar]
- 2.Alammar L, Gama L, Clements JE. Simian immunodeficiency virusinfection in the brain and lung leads to differential type I IFN signaling during acute infection. J Immunol. 2011;186:4008–4018. doi: 10.4049/jimmunol.1003757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rani MR, Ransohoff RM. Alternative and accessory pathways in the regulation of IFN-beta-mediated gene expression. J Interferon Cytokine Res. 2005;25:788–798. doi: 10.1089/jir.2005.25.788. [DOI] [PubMed] [Google Scholar]
- 4.van Heteren JT, Rozenberg F, Aronica E, Troost D, Lebon P, Kuijpers TW. Astrocytes produce interferon-alpha and CXCL10, but not IL-6 or CXCL8, in Aicardi-Goutieres syndrome. Glia. 2008;56:568–578. doi: 10.1002/glia.20639. [DOI] [PubMed] [Google Scholar]
- 5.Crow YJ, Black DN, Ali M, Bond J, Jackson AP, Lefson M, Michaud J, Roberts E, Stephenson JB, Woods CG, Lebon P. Cree encephalitis is allelic with Aicardi-Goutieres syndrome: implications for the pathogenesis of disorders of interferon alpha metabolism. J Med Genet. 2003;40:183–187. doi: 10.1136/jmg.40.3.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schaefer M, Engelbrecht MA, Gut O, Fiebich BL, Bauer J, Schmidt F, Grunze H, Lieb K. Interferon alpha (IFNalpha) and psychiatric syndromes: a review. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:731–746. doi: 10.1016/s0278-5846(01)00324-4. [DOI] [PubMed] [Google Scholar]
- 7.Ban M, Goris A, Lorentzen AR, Baker A, Mihalova T, Ingram G, Booth DR, Heard RN, Stewart GJ, Bogaert E, Dubois B, Harbo HF, Celius EG, Spurkland A, Strange R, Hawkins C, Robertson NP, Dudbridge F, Wason J, De Jager PL, Hafler D, Rioux JD, Ivinson AJ, McCauley JL, Pericak-Vance M, Oksenberg JR, Hauser SL, Sexton D, Haines J, Sawcer S, Compston A. Replication analysis identifies TYK2 as a multiple sclerosis susceptibility factor. Eur J Hum Genet. 2009;17:1309–1313. doi: 10.1038/ejhg.2009.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rho MB, Wesselingh S, Glass JD, McArthur JC, Choi S, Griffin J, Tyor WR. A potential role for interferon-alpha in the pathogenesis of HIV-associated dementia. Brain Behav Immun. 1995;9:366–377. doi: 10.1006/brbi.1995.1034. [DOI] [PubMed] [Google Scholar]
- 9.Chabot S, V, Yong W. Interferon beta-1b increases interleukin-10 in a model of T cell-microglia interaction: relevance to MS. Neurology. 2000;55:1497–1505. doi: 10.1212/wnl.55.10.1497. [DOI] [PubMed] [Google Scholar]
- 10.Paul S, Ricour C, Sommereyns C, Sorgeloos F, Michiels T. Type I interferon response in the central nervous system. Biochimie. 2007;89:770–778. doi: 10.1016/j.biochi.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 11.Davis LE, Hjelle BL, Miller VE, Palmer DL, Llewellyn AL, Merlin TL, Young SA, Mills RG, Wachsman W, Wiley CA. Early viral brain invasion in iatrogenic human immunodeficiency virus infection. Neurology. 1992;42:1736–1739. doi: 10.1212/wnl.42.9.1736. [DOI] [PubMed] [Google Scholar]
- 12.An SF, Giometto B, Scaravilli F. HIV-1 DNA in brains in AIDS and pre-AIDS: correlation with the stage of disease. Ann Neurol. 1996;40:611–617. doi: 10.1002/ana.410400411. [DOI] [PubMed] [Google Scholar]
- 13.Overholser ED, Coleman GD, Bennett JL, Casaday RJ, Zink MC, Barber SA, Clements JE. Expression of simian immunodeficiency virus (SIV) nef in astrocytes during acute and terminal infection and requirement of nef for optimal replication of neurovirulent SIV in vitro. J Virol. 2003;77:6855–6866. doi: 10.1128/JVI.77.12.6855-6866.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buckner CM, Calderon TM, Willams DW, Belbin TJ, Berman JW. Characterization of monocyte maturation/differentiation that facilitates their transmigration across the blood-brain barrier and infection by HIV: implications for NeuroAIDS. Cell Immunol. 2011;267:109–123. doi: 10.1016/j.cellimm.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Persidsky Y, Stins M, Way D, Witte MH, Weinand M, Kim KS, Bock P, Gendelman HE, Fiala M. A model for monocyte migration through the blood-brain barrier during HIV-1 encephalitis. J Immunol. 1997;158:3499–3510. [PubMed] [Google Scholar]
- 16.Nottet HS, Persidsky Y, Sasseville VG, Nukuna AN, Bock P, Zhai QH, Sharer LR, McComb RD, Swindells S, Soderland C, Gendelman HE. Mechanisms for the transendothelial migration of HIV-1-infected monocytes into brain. J Immunol. 1996;156:1284–1295. [PubMed] [Google Scholar]
- 17.van’t Wout AB, Ran LJ, Kuiken CL, Kootstra NA, Pals ST, Schuitemaker H. Analysis of the temporal relationship between human immunodeficiency virus type 1 quasispecies in sequential blood samples and various organs obtained at autopsy. J Virol. 1998;72:488–496. doi: 10.1128/jvi.72.1.488-496.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mankowski JL, Carter DL, Spelman JP, Nealen ML, Maughan KR, Kirstein LM, Didier PJ, Adams RJ, Murphey-Corb M, Zink MC. Pathogenesis of simian immunodeficiency virus pneumonia: an immunopathological response to virus. Am J Pathol. 1998;153:1123–1130. doi: 10.1016/S0002-9440(10)65656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koenig S, Gendelman HE, Orenstein JM, Dal Canto MC, Pezeshkpour GH, Yungbluth M, Janotta F, Aksamit A, Martin MA, Fauci AS. Detection of AIDS virus in macrophages in braintissue from AIDS patients with encephalopathy. Science. 1986;233:1089–1093. doi: 10.1126/science.3016903. [DOI] [PubMed] [Google Scholar]
- 20.Wiley CA, Schrier RD, Nelson JA, Lampert PW, Oldstone MB. Cellular localization of human immunodeficiency virus infection within the brains of acquired immune deficiency syndrome patients. Proc Natl Acad Sci U S A. 1986;83:7089–7093. doi: 10.1073/pnas.83.18.7089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barber SA, Gama L, Dudaronek JM, Voelker T, Tarwater PM, Clements JE. Mechanism for the establishment of transcriptional HIV latency in the brain in a simian immunodeficiency virus-macaque model. J Infect Dis. 2006;193:963–970. doi: 10.1086/500983. [DOI] [PubMed] [Google Scholar]
- 22.Dudaronek JM, Barber SA, Clements JE. CUGBP1 is required for IFNbeta-mediated induction of dominant-negative CEBPbeta and suppression of SIV replication in macrophages. J Immunol. 2007;179:7262–7269. doi: 10.4049/jimmunol.179.11.7262. [DOI] [PubMed] [Google Scholar]
- 23.Giulian D, Vaca K, Noonan CA. Secretion of neurotoxins by mononuclear phagocytes infected with HIV-1. Science. 1990;250:1593–1596. doi: 10.1126/science.2148832. [DOI] [PubMed] [Google Scholar]
- 24.Kaul M, Zheng J, Okamoto S, Gendelman HE, Lipton SA. HIV-1 infection and AIDS: consequences for the central nervous system. Cell Death Differ. 2005;12(Suppl 1):878–892. doi: 10.1038/sj.cdd.4401623. [DOI] [PubMed] [Google Scholar]
- 25.Bsibsi M, Ravid R, Gveric D, van Noort JM. Broad expression of Toll-like receptors in the human central nervous system. J Neuropath Exp Neur. 2002;61:1013–1021. doi: 10.1093/jnen/61.11.1013. [DOI] [PubMed] [Google Scholar]
- 26.Farina C, Krumbholz M, Giese T, Hartmann G, Aloisi F, Meinl E. Preferential expression and function of Toll-like receptor 3 in human astrocytes. J Neuroimmunol. 2005;159:12–19. doi: 10.1016/j.jneuroim.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 27.Barres BA. The mystery andmagic of glia: a perspective on their roles in health and disease. Neuron. 2008;60:430–440. doi: 10.1016/j.neuron.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 28.Ranki A, Nyberg M, Ovod V, Haltia M, Elovaara I, Raininko R, Haapasalo H, Krohn K. Abundant expression of HIV Nef and Rev proteins in brain astrocytes in vivo is associated with dementia. AIDS. 1995;9:1001–1008. doi: 10.1097/00002030-199509000-00004. [DOI] [PubMed] [Google Scholar]
- 29.Soulas C, Conerly C, Kim WK, Burdo TH, Alvarez X, Lackner AA, Williams KC. Recently infiltrating MAC387(+) monocytes/macrophages a third macrophage population involved in SIV and HIV encephalitic lesion formation. Am J Pathol. 2011;178:2121–2135. doi: 10.1016/j.ajpath.2011.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eugenin EA, D’Aversa TG, Lopez L, Calderon TM, Berman JW. MCP-1 (CCL2) protects human neurons and astrocytes from NMDA or HIV-tat-induced apoptosis. J Neurochem. 2003;85:1299–1311. doi: 10.1046/j.1471-4159.2003.01775.x. [DOI] [PubMed] [Google Scholar]
- 31.Meda L, Cassatella MA, Szendrei GI, Otvos L, Jr, Baron P, Villalba M, Ferrari D, Rossi F. Activation of microglial cells by beta-amyloid protein and interferon-gamma. Nature. 1995;374:647–650. doi: 10.1038/374647a0. [DOI] [PubMed] [Google Scholar]
- 32.Matsumoto H, Kumon Y, Watanabe H, Ohnishi T, Shudou M, Ii C, Takahashi H, Imai Y, Tanaka J. Antibodies to CD11b, CD68, and lectin label neutrophils rather than microglia in traumatic and ischemic brain lesions. J Neurosci Res. 2007;85:994–1009. doi: 10.1002/jnr.21198. [DOI] [PubMed] [Google Scholar]
- 33.Vincent VA, Tilders FJ, Van Dam AM. Inhibition of endotoxin-induced nitric oxide synthase production in microglial cells by the presence of astroglial cells: a role for transforming growth factor beta. Glia. 1997;19:190–198. doi: 10.1002/(sici)1098-1136(199703)19:3<190::aid-glia2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 34.Aloisi F, Borsellino G, Care A, Testa U, Gallo P, Russo G, Peschle C, Levi G. Cytokine regulation of astrocyte function: in-vitro studies using cells from the human brain. Int J Dev Neurosci. 1995;13:265–274. doi: 10.1016/0736-5748(94)00071-a. [DOI] [PubMed] [Google Scholar]
- 35.Min KJ, Yang MS, Kim SU, Jou I, Joe EH. Astrocytes induce hemeoxygenase-1 expression in microglia: a feasible mechanism for preventing excessive brain inflammation. J Neurosci. 2006;26:1880–1887. doi: 10.1523/JNEUROSCI.3696-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim JH, Min KJ, Seol W, Jou I, Joe EH. Astrocytes in injury states rapidly produce anti-inflammatory factors and attenuate microglial inflammatory responses. J Neurochem. 2010;115:1161–1171. doi: 10.1111/j.1471-4159.2010.07004.x. [DOI] [PubMed] [Google Scholar]
- 37.Nottet HS, Jett M, Flanagan CR, Zhai QH, Persidsky Y, Rizzino A, Bernton EW, Genis P, Baldwin T, Schwartz J, et al. A regulatory role for astrocytes in HIV-1 encephalitis. An overexpression of eicosanoids, platelet-activating factor, and tumor necrosis factor-alpha by activated HIV-1-infected monocytes is attenuated by primary human astrocytes. J Immunol. 1995;154:3567–3581. [PubMed] [Google Scholar]
- 38.Co JG, Witwer KW, Gama L, Zink MC, Clements JE. Induction of Innate Immune Responses by SIV In Vivo and In Vitro: Differential Expression and Function of RIG-I and MDA5. J Infect Dis. 2011;204:1104–1114. doi: 10.1093/infdis/jir469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Overholser ED, Babas T, Zink MC, Barber SA, Clements JE. CD4-independent entry and replication of simian immunodeficiency virus in primary rhesus macaque astrocytes are regulated by the transmembrane protein. J Virol. 2005;79:4944–4951. doi: 10.1128/JVI.79.8.4944-4951.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sabri F, Tresoldi E, Di Stefano M, Polo S, Monaco MC, Verani A, Fiore JR, Lusso P, Major E, Chiodi F, Scarlatti G. Nonproductive human immunodeficiency virus type 1 infection of human fetal astrocytes: independence from CD4 and major chemokine receptors. Virology. 1999;264:370–384. doi: 10.1006/viro.1999.9998. [DOI] [PubMed] [Google Scholar]
- 41.Honda K, Takaoka A, Taniguchi T. Type I interferon [corrected] gene induction by the interferon regulatory factor family of transcription factors. Immunity. 2006;25:349–360. doi: 10.1016/j.immuni.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 42.Conant K, Garzino-Demo A, Nath A, McArthur JC, Halliday W, Power C, Gallo RC, Major EO. Induction of monocyte chemoattractant protein-1 in HIV-1 Tat-stimulated astrocytes and elevation in AIDS dementia. Proc Natl Acad Sci U S A. 1998;95:3117–3121. doi: 10.1073/pnas.95.6.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zink MC, Coleman GD, Mankowski JL, Adams RJ, Tarwater PM, Fox K, Clements JE. Increased macrophage chemoattractant protein-1 in cerebrospinal fluid precedes and predicts simian immunodeficiency virus encephalitis. J Infect Dis. 2001;184:1015–1021. doi: 10.1086/323478. [DOI] [PubMed] [Google Scholar]
- 44.Yokochi S, Hashimoto H, Ishiwata Y, Shimokawa H, Haino M, Terashima Y, Matsushima K. An anti-inflammatory drug, propagermanium, may target GPI-anchored proteins associated with an MCP-1 receptor, CCR2. J Interferon Cytokine Res. 2001;21:389–398. doi: 10.1089/107999001750277862. [DOI] [PubMed] [Google Scholar]
- 45.Liu Y, Liu H, Kim BO, Gattone VH, Li J, Nath A, Blum J, He JJ. CD4-independent infection of astrocytes by human immunodeficiency virus type 1: requirement for the human mannose receptor. J Virol. 2004;78:4120–4133. doi: 10.1128/JVI.78.8.4120-4133.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Witwer KW, Gama L, Li M, Bartizal CM, Queen SE, Varrone JJ, Brice AK, Graham DR, Tarwater PM, Mankowski JL, Zink MC, Clements JE. Coordinated regulation of SIV replication and immune responses in the CNS. PLoS One. 2009;4:e8129. doi: 10.1371/journal.pone.0008129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brack-Werner R. Astrocytes: HIV cellular reservoirs and important participants in neuropathogenesis. AIDS. 1999;13:1–22. doi: 10.1097/00002030-199901140-00003. [DOI] [PubMed] [Google Scholar]
- 48.Boutros T, Croze E, Yong VW. Interferon-beta is a potent promoter of nerve growth factor production by astrocytes. J Neurochem. 1997;69:939–946. doi: 10.1046/j.1471-4159.1997.69030939.x. [DOI] [PubMed] [Google Scholar]
- 49.Bruno V, Copani A, Besong G, Scoto G, Nicoletti F. Neuroprotective activity of chemokines against N-methyl-D-aspartate or beta-amyloid-induced toxicity in culture. Eur J Pharmacol. 2000;399:117–121. doi: 10.1016/s0014-2999(00)00367-8. [DOI] [PubMed] [Google Scholar]
- 50.Hinojosa AE, Garcia-Bueno B, Leza JC, Madrigal JL. CCL2/MCP-1 modulation of microglial activation and proliferation. J Neuroinflammation. 2011;8:77. doi: 10.1186/1742-2094-8-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.El Khoury J, Toft M, Hickman SE, Means TK, Terada K, Geula C, Luster AD. Ccr2 deficiency impairs microglial accumulation and accelerates progression of Alzheimer-like disease. Nat Med. 2007;13:432–438. doi: 10.1038/nm1555. [DOI] [PubMed] [Google Scholar]
- 52.Hokeness KL, Kuziel WA, Biron CA, Salazar-Mather TP. Monocyte chemoattractant protein-1 and CCR2 interactions are required for IFN-alpha/beta-induced inflammatory responses and antiviral defense in liver. J Immunol. 2005;174:1549–1556. doi: 10.4049/jimmunol.174.3.1549. [DOI] [PubMed] [Google Scholar]
- 53.Jia T, Leiner I, Dorothee G, Brandl K, Pamer EG. MyD88 and Type I interferon receptor-mediated chemokine induction and monocyte recruitment during Listeria monocytogenes infection. J Immunol. 2009;183:1271–1278. doi: 10.4049/jimmunol.0900460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nakamura S, Davis KM, Weiser JN. Synergistic stimulation of type I interferons during influenza virus coinfection promotes Streptococcus pneumoniae colonization in mice. J Clin Invest. 2011;121:3657–3665. doi: 10.1172/JCI57762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zimmerer JM, Lesinski GB, Radmacher MD, Ruppert A, Carson WE., 3rd STAT1-dependent and STAT1-independent gene expression in murine immune cells following stimulation with interferon-alpha. Cancer Immunol Immunother. 2007;56:1845–1852. doi: 10.1007/s00262-007-0329-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ishiwata Y, Yokochi S, Suzuki E, Michishita H, Tashita A, Asano K, Mitani T, Kurono M. Effects of proxigermanium on interferon production and 2′,5′-oligoadenylate synthetase activity in the lung of influenza virus-infected mice and in virus-infected human peripheral blood mononuclear cell cultures. Arzneimittelforschung. 1990;40:896–899. [PubMed] [Google Scholar]
- 57.Munakata T, Arai S, Kuwano K, Furukawa M, Tomita Y. Induction of interferon production by natural killer cells by organogermanium compound, Ge132. J Interferon Res. 1987;7:69–76. doi: 10.1089/jir.1987.7.69. [DOI] [PubMed] [Google Scholar]
- 58.Aso H, Suzuki F, Yamaguchi T, Hayashi Y, Ebina T, Ishida N. Induction of interferon and activation of NK cells and macrophages in miceby oral administration of Ge-132, an organic germanium compound. Microbiol Immunol. 1985;29:65–74. doi: 10.1111/j.1348-0421.1985.tb00803.x. [DOI] [PubMed] [Google Scholar]
- 59.Sun Y, McGarrigle D, Huang XY. When a G protein-coupled receptor does not couple to a G protein. Mol Biosyst. 2007;3:849–854. doi: 10.1039/b706343a. [DOI] [PubMed] [Google Scholar]
- 60.Mellado M, Rodriguez-Frade JM, Aragay A, del Real G, Martin AM, Vila-Coro AJ, Serrano A, Mayor F, Jr, Martinez AC. The chemokine monocyte chemotactic protein 1 triggers Janus kinase 2 activation and tyrosine phosphorylation of the CCR2B receptor. J Immunol. 1998;161:805–813. [PubMed] [Google Scholar]