Abstract
Objectives
To determine the cross-sectional and longitudinal associations of 25-hydroxyvitamin D (25(OH)D) levels with frailty status in older men.
Design
Prospective cohort study
Setting
Six U.S. community-based centers
Participants
1606 men aged ≥65 years
Measurements
25(OH)D (liquid chromatography-tandem mass spectroscopy) and frailty status (criteria similar to those used in the Cardiovascular Health Study) measured at baseline; frailty status assessment repeated an average of 4.6 years later. Frailty status classified as robust, intermediate stage, or frail at baseline; and robust, intermediate stage, frail, or dead at follow-up.
Results
After adjusting for multiple potential confounders, men with 25(OH)D levels <20.0 ng/mL had a 1.5-fold higher odds (multivariate odds ratio (MOR) 1.47, 95% confidence interval (CI) 1.07–2.02) of greater frailty status at baseline as compared with men with 25(OH)D levels ≥30.0 ng/mL (referent group), while frailty status was similar between men with 25(OH)D levels 20.0–29.9 ng/mL and those with 25(OH)D levels ≥30 ng/mL (MOR 1.02, 95% CI 0.78–1.32). However, among 1267 men not classified as frail at baseline, there was no association between lower baseline 25(OH)D level and odds of greater frailty status at the 4.6 year follow-up. Findings were unchanged when 25(OH)D was expressed in quartiles or as a continuous variable.
Conclusion
Lower levels of 25(OH)D (levels <20 ng/mL) among community dwelling older men were independently associated with greater evidence of frailty at baseline, but did not predict increased risk of greater frailty status at 4.6 years.
Keywords: 25-hydroxyvitamin D, frailty syndrome, elderly men
INTRODUCTION
Lower levels of circulating 25-hydroxyvitamin D (25(OH)D) and frailty (a term indicating multisystem impairment and expanding vulnerability) are increasingly prevalent with advancing age.1–3 Several prior studies have reported that older individuals classified as frail have a higher subsequent risk of adverse outcomes including disability, falls, fractures, and mortality.2–5 Components of frailty such as weakness and slowness are potential sequelae of vitamin D deficiency and lower 25(OH)D levels among older adults have been inconsistently associated with poorer physical performance and higher risks of falls, fractures and death.6,7
Previous cross-sectional studies have suggested that older adults with lower 25(OH)D levels are more likely to be classified as frail as compared with not frail.8,9 However, only one previous study10 expressed frailty status as a continuum ranging from robust to intermediate stage to frail and reported an association between lower 25(OH)D levels and greater frailty status among men, but not women. Among older adults classified as not frail at baseline, two studies9,11 have examined the association between 25(OH)D levels and odds of becoming frail during follow-up and reported inconsistent results.
To test the hypothesis that lower 25(OH)D levels at baseline were associated with greater prevalent frailty status, we measured 25(OH)D and assessed frailty status in a cohort of 1606 community-dwelling men aged ≥65 years enrolled in the Osteoporotic Fractures in Men (MrOS) study. To determine whether lower 25(OH)D levels at baseline were associated with an increased risk of greater frailty status at follow-up, 1267 men classified as non-frail (robust or intermediate) at baseline had frailty status reassessed an average of 4.6 years later.
METHODS
Participants
From March 2000 through April 2002, 5995 men who were ≥65 years were recruited for participation in the baseline examination of MrOS.12 Men were recruited from population based listings in six regions of the United States. Men with a history of bilateral hip replacement and men who were unable to walk without the assistance of another person were excluded.
Among the overall cohort of 5995 participants, a random sample comprised of 1606 men was selected for measurement of 25(OH)D. All 1606 men had adequate data for assessment of frailty status and were included in the cross-sectional analyses. Of these, 1476 men were classified as non-frail (robust or intermediate stage) at baseline and were eligible for the longitudinal analysis. After excluding 209 surviving men who did not provide enough data for adequate determination frailty status at the second examination an average of 4.6 years later, the final longitudinal cohort (n=1267) was comprised of 1128 men with repeat assessment of frailty status and 139 men who died prior to this follow-up examination.
Measurement of 25(OH)D
Fasting morning blood was collected, serum was prepared immediately after phlebotomy, and then was stored at −70°C. All samples remained frozen until assay in foil wrapped vials to prevent UV exposure. Measures for 25(OH)D2 (derived from ergocalciferol) and 25(OH)D3 (derived from cholecalciferol) were performed at the Mayo Clinic using liquid chromatography tandem mass spectroscopy (LC-MS/MS) as previously described.13 25(OH)D2 and 25(OH)D3 were quantified and summed for total 25(OH)D. The minimum detectable limit for 25(OH)D2 was 4 ng/mL and for 25(OH)D3 was 2 ng/mL. Duplicate pooled serum controls were included in every other assay run. Using the pooled serum, the inter-assay coefficient of variation (CV) was 4.4% and the intra-assay CV was 4.9%. Among the longitudinal cohort of 1267 men, 303 men had a repeat measurement of 25(OH)D at an interim timepoint (1.9 years after baseline) between baseline and follow-up examinations; the partial correlation coefficient (adjusted for season) between the two measures was 0.70 (p<0.001).
Other Measurements
Participants completed a questionnaire and were interviewed at the examinations. A selected medical history was obtained. A co-morbidity score was calculated for each participant and expressed as 0 (0–1 condition), 1 (2–3 conditions), or 2 (≥4 conditions). Physical activity was assessed using the Physical Activity Scale for the Elderly (PASE)14. The Medical Outcomes Study 12-item Short Form (SF-12)15 was completed by all participants. Cognitive function was assessed with the Teng Modified Mini-Mental State Exam (3MS).16 Tests of physical function included grip strength (using a hand-held Jamar dynamometer) and walk speed (time in seconds to walk 6 meters at usual pace expressed as m/sec). Body weight and height measurements were used to calculate a standard body mass index (BMI). Appendicular skeletal mass and total and percentage body fat were determined using dual-energy x-ray absorbtiometry (Hologic QDR4500W scanners, Hologic Inc., Bedford, MA) using standard protocols. Using baseline stored sera (n=1505 participants among the 1606 men in the cohort), serum creatinine was measured utilizing a variation of the Jaffe enzymatic method (inter-assay CV 5.3%) and total intact parathyroid hormone (PTH) was measured (n=1591) using an immunoradiometric assay (inter-assay CV 8.4%). Renal function was expressed as estimated glomerular filtration rate (eGFR) in mL/min/1.73m2 using a standardized serum creatinine based formula.17
Frailty
Frailty status at baseline was defined using the following criteria similar to those proposed by Fried and colleagues2 using data collected in the CHS study (Appendix):
Shrinking/Sarcopenia,4 identified by an appendicular lean mass (adjusted for height and total body fat) in the lowest quintile;
Weakness, identified by a grip strength in the lowest quintile stratified by BMI (quartiles);
Exhaustion, identified by an answer of “a little or none” to the question “How much of the time during the past four weeks did you have a lot of energy?” from the Medical Outcomes Study SF-12;
Slowness, identified by a walk speed in the lowest quintile stratified by standing height (median); and
Low physical activity, level as identified by a PASE score in the lowest quintile.
Men with none of the above components were considered to be robust, those with 1 or 2 components were considered to be in an intermediate stage, and those with ≥3 components were considered to be frail.
Frailty status at the follow-up examination was defined using criteria cut-points from the baseline examination. To jointly analyze the outcome of frailty status at the follow-up exam and mortality between baseline and the follow-up exam, 4 levels of frailty status were considered at the follow-up examination: robust, intermediate stage, frail, or dead (died between baseline and follow-up examination).
Statistical Analysis
Difference in baseline characteristics according to category of total 25(OH)D level were compared using analysis of variance for normally distributed continuous data, Kruskal-Wallis tests for skewed continuous data, and chi-square tests for categorical data. For the primary analyses, the predictor variable, 25(OH)D level, was expressed as categories based on vitamin D status defined as 25(OH)D level <20.0 ng/mL, 25(OH)D level 20.0–29.9 ng/mL, and 25(OH)D level ≥30.0 ng/mL.1 The cross-sectional association between 25(OH)D levels and the ordinal outcome of frailty status at baseline (robust, intermediate stage, frail) was examined using a proportional odds model and the assumption of proportionality was evaluated.18 Although the assumption of homogeneity of effect of the predictor across levels of the outcome was met for 25(OH)D, the assumption was violated for some of the covariates; therefore a partial proportional odds model was used. Since 25(OH)D met the assumption of proportionality, a single odds ratio (OR) summarizing the effect of the 25(OH)D over all levels of the outcome was calculated. Similarly, partial proportional odds models were used to determine the association between 25(OH)D levels at baseline and frailty status (robust, intermediate stage, frail, dead) at the follow-up examination. Since 25(OH)D level met the assumption of proportionality, a single OR relating the baseline 25(OH)D level to the ordinal follow-up frailty outcome was calculated.
Initial models were adjusted for age, race, clinic site, season of blood draw, and body mass index; then for multiple potential confounders (multivariable model). Factors previously associated with frailty status in the MrOS cohort or those related to 25(OH)D level at p≤0.10 were considered for inclusion in the multivariable model. Variables used to define each of the individual frailty components (such as physical activity) were not included in the covariate selection process. Covariates in the cross-sectional multivariable model included age, race, site, season, BMI, health status, education, living alone, smoking status, alcohol intake, co-morbidity score, and Teng 3MS score. To investigate the biological mechanism underlying the association between lower 25(OH)D levels and greater frailty status at baseline, cross-sectional multivariable models were further adjusted for eGFR and serum intact PTH. The longitudinal multivariable model was additionally adjusted for baseline frailty status (intermediate vs. robust).
We conducted sensitivity analyses expressing 25(OH)D level as quartiles (cut-points 19.9, 25.1, 29.8 ng/mL) and as a continuous variable. In a secondary analysis to evaluate for evidence of a linear cross-sectional association between 25(OH)D level and frailty status, a series of restricted cubic spline models were fit using knots specified at cut-points for quartiles and quintiles of 25(OH)D. These graphs were similar. Thus, a graph with knots specified as the 5th, 25th, 50th, 75th, and 95th percentile cut-points is presented.
Statistical analyses were completed using SAS v9.2 (SAS Inc., Cary, NC) and Stata v11.0 (Stata Corporation, College Station, TX).
RESULTS
Cross-Sectional Association between 25(OH)D Level and Frailty Status
Of the 1606 men in the cohort at baseline, 745 (46.4%) were classified as robust, 731 (45.5%) were in the intermediate stage, and 130 (8.1%) were classified as frail. Characteristics of participants by category of 25(OH)D level are shown in Table 1. The proportion of men classified as frail was 13.2% among 408 men with a 25(OH)D level <20 ng/mL, 6.9% among 803 men with a 25(OH)D level 20–29.9 ng/mL, and 5.3% among 395 men with a 25(OH)D level ≥30 ng/mL (p-trend ≤0.001). In addition, the prevalence of each of four individual frailty components (low activity level, exhaustion, weakness, and slowness) was highest among men with 25(OH)D levels <20 ng/mL (p-trend ≤0.001 for each component except exhaustion where p-trend 0.08). However, the prevalence of shrinking/sarcopenia was similar across categories of 25(OH)D level (p-trend 0.70).
Table 1.
Characteristics of 1606 Participants by Category of Serum 25(OH) D Level*
| Variable | Overall Cohort (n=1606) | Category of Serum 25(OH) D
|
|||
|---|---|---|---|---|---|
| <20.0 ng/mL (n=408) | 20.0–29.9 ng/mL (n=803) | ≥ 30.0 ng/mL (n=395) | p-value | ||
| Age, years, mean (SD) | 73.8 (5.9) | 74.6 (6.4) | 73.9 (5.8) | 72.7 (5.5) | <0.001 |
| Caucasian race, % | 89.7 | 82.1 | 91.7 | 93.7 | <0.001 |
| Excellent or good health status, % | 85.3 | 81.1 | 84.8 | 90.6 | <0.001 |
| College education, % | 75.2 | 71.6 | 75.0 | 79.5 | 0.03 |
| Lives alone, % | 13.4 | 20.1 | 11.2 | 10.9 | <0.001 |
| PASE score, mean (SD) | 146.7 (68.7) | 136.1 (72.4) | 147.6 (66.2) | 155.7 (68.5) | <0.001 |
| Current smoker, % | 3.7 | 5.4 | 3.5 | 2.3 | 0.06 |
| Alcohol intake, drinks/week, mean (SD) | 4.6 (7.4) | 4.8 (7.9) | 4.0 (6.7) | 5.5 (7.8) | <0.001 |
| Co-morbidity score, mean (SD) | 0.65 (0.69) | 0.72 (0.70) | 0.62 (0.68) | 0.65 (0.69) | 0.06 |
| Body mass index, kg/m2, mean (SD) | 27.4 (3.7) | 28.1 (4.2) | 27.3 (3.7) | 26.8 (3.1) | <0.001 |
| Teng 3MS score (range 0–100), mean (SD) | 93.2 (6.4) | 92.6 (6.9) | 93.2 (6.5) | 93.8 (5.6) | 0.01 |
| eGFR, mL/min/1.73m2, mean (SD) | 76.5 (18.3) | 79.0 (19.3) | 76.6 (18.2) | 73.8 (17.2) | 0.01 |
| Intact PTH, pg/mL, median (IQR) | 29.6 (23.6–38.5) | 33.6 (25.9–45.1) | 29.6 (23.8–37.5) | 26.7 (21.7–33.9) | <0.001 |
| Frailty criteria, % | |||||
| Low activity level | 20.0 | 26.7 | 17.8 | 17.5 | <0.001 |
| Exhaustion | 7.8 | 10.3 | 7.2 | 6.3 | 0.08 |
| Weakness | 18.9 | 27.2 | 16.4 | 15.2 | <0.001 |
| Shrinking/sarcopenia | 20.0 | 19.1 | 20.8 | 19.1 | 0.70 |
| Slowness | 20.0 | 27.2 | 18.7 | 14.9 | <0.001 |
| Frailty classification, % | <0.001 | ||||
| Robust | 46.4 | 39.0 | 47.6 | 51.7 | |
| Intermediate stage | 45.5 | 47.8 | 45.6 | 43.0 | |
| Frail | 8.1 | 13.2 | 6.9 | 5.3 | |
All characteristics measured at the baseline examination
Abbreviations: PASE, Physical Activity Scale for the Elderly; 3MS, Modified Mini-Mental State Examination; eGFR, estimated glomerular filtration rate; PTH, parathyroid hormone; IQR, interquartile range
After adjustment for age, race, site, season, and BMI (base model), men with 25(OH)D levels <20 ng/mL compared with those with levels ≥30 ng/mL had an increased odds of greater prevalent frailty status (OR 1.60, 95% CI 1.19–2.16), while the odds of greater frailty status did not differ between men with 25(OH)D level 20.0–29.9 ng/mL and those with levels ≥30 ng/mL.(OR 1.06, 95% CI 0.83–1.36) (Table 2). After further adjustment for multiple correlates of 25(OH)D level and frailty status, the association between lower 25(OH)D level (<20 ng/mL) and greater frailty status was slightly attenuated in magnitude, but remained significant (OR 1.47, 95% CI 1.07–2.02). In addition, the association remained unchanged after additional adjustment for eGFR and PTH levels (OR 1.51, 95% CI 1.09–2.08). Findings were consistent when 25(OH)D level was expressed in quartiles or as a continuous variable (Table 2).
Table 2.
Cross-Sectional and Longitudinal Association between Baseline 25(OH) D Level and Odds of Greater Frailty Status
| Analysis Type | Partial Proportional Odds Ratio (95% CI) of Greater Frailty by Level of 25(OH)D
|
|||||
|---|---|---|---|---|---|---|
| Category of 25(OH)D Level | Continuous, per 1 SD decrease (unit = −7.93) | |||||
| <20.0 ng/mL | 20.0–29.9 ng/mL | ≥30.0 ng/mL | P-trend | |||
| Cross-sectional | (n=408) | (n=803) | (n=395) | |||
| Base model* | 1.60 (1.19–2.16) | 1.06 (0.83–1.36) | 1.00 (referent) | 0.002 | 1.19 (1.07–1.33) | |
| Multivariate model† | 1.47 (1.07–2.02) | 1.02 (0.78–1.32) | 1.00 (referent) | 0.02 | 1.19 (1.06–1.32) | |
| Longitudinal | (n=274) | (n=657) | (n=336) | |||
| Base model‡ | 1.03 (0.73–1.45) | 0.99 (0.75–1.30) | 1.00 (referent) | 0.88 | 1.01 (0.90–1.14) | |
| Multivariate model§ | 1.03 (0.72–1.46) | 1.04 (0.78–1.38) | 1.00 (referent) | 0.88 | 1.03 (0.91–1.17) | |
| Quartile of 25(OHD) Level
|
||||||
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P-trend | ||
| Quartile of 25(OHD) Level
|
||||||
| Cross-sectional | (n=394) | (n=409) | (n=401) | (n=402) | ||
| Base model* | 1.57 (1.16–2.12) | 1.03 (0.78–1.37) | 1.05 (0.80–1.40) | 1.00 (referent) | 0.007 | |
| Multivariate model† | 1.44 (1.04–1.98) | 1.02 (0.75–1.38) | 0.97 (0.72–1.31) | 1.00 (referent) | 0.03 | |
| Longitudinal | (n=262) | (n=331) | (n=333) | (n=341) | ||
| Base model‡ | 1.03 (0.73–1.45) | 0.91 (0.66–1.24) | 1.05 (0.76–1.43) | 1.00 (referent) | 0.82 | |
| Multivariate model§ | 1.01 (0.71–1.45) | 0.95 (0.69–1.32) | 1.09 (0.80–1.51) | 1.00 (referent) | 0.84 | |
adjusted for age, race, site, season of blood draw, and body mass index
adjusted for age, race, site, season of blood draw, body mass index, self-reported health status, education, living alone, smoking status, alcohol intake, co-morbidity score, and Teng 3MS score
adjusted for age, race, site, season of blood draw, body mass index, and baseline frailty status
adjusted for age, race, site, season of blood draw, body mass index, self-reported health status, education, living alone, smoking status, alcohol intake, co-morbidity score, Teng 3MS score, and baseline frailty status
Quartile cutpoints: 19.9, 25.1, 29.8 ng/mL
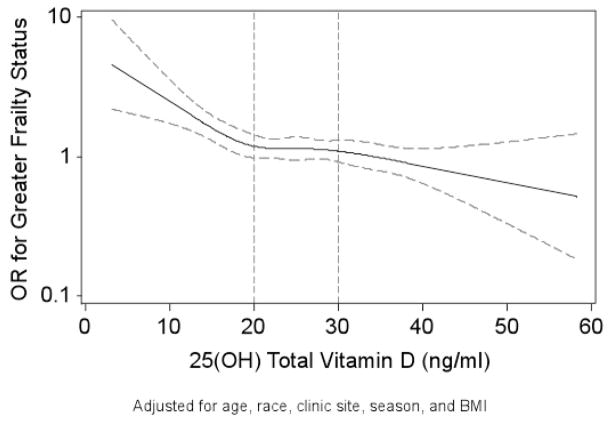
Analyses using restricted cubic spline models that suggested that the likelihood of greater frailty status at baseline increased as the baseline level of 25(OH)D fell below 20 ng/mL (Figure 1). The equality of slopes assumption failed for 25(OH)D <20 ng/mL vs. 20.0–29.9 ng/mL (p=0.04), but held for 25 (OH)D 20.0–29.9 vs. ≥30 ng/mL (p=0.92)
Figure 1.
Restricted Cubic Spline Plot of Odd Ratios for Greater Frailty Status at Baseline by Level of 25(OH)D
In base models, men with 25(OH)D levels <20 ng/mL compared with those with levels ≥30 ng/mL had a higher odds of the individual frailty component of weakness (OR 1.71, 95% CI 1.15–2.53), but associations between lower 25(OH)D level (<20 ng/mL) and other individual frailty components failed to reach significance (OR, 95% CI 1.36, 0.94–1.98 for low activity level; 1.39, 0.93–2.08 for slowness; 1.23, 0.83–1.84 for shrinking/sarcopenia; and 1.61, 0.92–2.83 for exhaustion).
Longitudinal Association between 25(OH)D Level and Frailty Status
A total of 1267 men classified as non-frail (robust or intermediate) at baseline comprised the longitudinal cohort. Compared with the longitudinal cohort, the 209 surviving men classified as non-frail at baseline who did not provide enough data for frailty status assessment at follow-up were, on average, older (75.1 vs. 73.2 years), more likely to report poor or fair health status (19.6 vs. 10.8%), and had slightly lower 25(OH)D levels (22.7 vs. 25.6 ng/mL) (p<0.001 for all comparisons).
At the follow-up examination an average of 4.6 years later, 509 men (40.2%) were classified as robust, 512 (40.4%) were in the intermediate stage, 107 (8.5%) were classified as frail, and 139 (11.0%) had died in the interim period. After adjustment for age, race, site, season, BMI and baseline frailty status, there was no evidence of an association between lower 25(OH)D levels at baseline and an increased odds of greater frailty status at the follow-up (Table 2). Compared with men in the referent group (25(OH)D levels ≥30.0 ng/mL), the odds of greater frailty status at follow-up was similar among men with levels <20 ng/mL (OR 1.03, 95% CI 0.73–1.45) and those with levels 20–29.9 ng/mL (OR 0.99, 95% CI 0.75–1.30). Results were unchanged when 25(OH)D was expressed as quartiles or as a continuous variable (Table 2) or when models were further adjusted for multiple potential confounders.
DISCUSSION
In this cohort of community-dwelling older men, low levels of 25(OH)D (levels <20 ng/mL) were independently associated with greater evidence of frailty at baseline, but did not predict increased risk of greater frailty status at 4.6 years.
These findings regarding the cross-sectional association between lower 25(OH)D levels and greater frailty status are generally consistent with previous studies, though most prior investigations defined frailty as a dichotomous outcome without an intermediate stage. The Longitudinal Aging Study Amsterdam (LASA) of 1321 men and women ≥65years 9 measured serum 25(OH)D using a competitive binding protein assay, defined frailty as the presence of three out of nine frailty indicators, and reported that the odds of being classified as frail (versus non-frail) was 1.7-fold higher among those with levels between 10 and 20 ng/mL and 2.6-fold higher among those with levels <10 ng/mL, compared with that among the referent group (serum 25(OH)D levels >20 ng/mL). A study using data from the Women’s Heath and Aging Studies8 measured serum 25(OH)D using a competitive binding protein assay, defined frailty using criteria similar to those used in the CHS index, and reported that the age-adjusted odds of being classified as frail (versus non-frail) was 1.7-fold higher among women with 25(OH)D levels in the lowest quartile (cut-point not reported) compared with that among women with levels in the upper three quartiles, but the association failed to reach significance after further adjustment. Finally, a study of older Italian adults10 measured serum 25(OH)D levels using a radioimmunoassay and defined frailty using a modified CHS index. After adjustment for multiple potential confounders, older men with low 25(OH)D levels (<20 ng/mL) were four times as likely to be classified as frail versus robust and twice as likely to be classified as intermediate stage versus robust. However, 25(OH)D levels were not associated with frailty status among women. Similar to results of this study, the association between low 25(OH)D levels and greater frailty status among men persisted despite further adjustment for renal function and PTH levels. In contrast to findings from this study, low 25(OH)D levels among men were not associated with the individual frailty component of weakness, but were associated with the individual components of low physical activity and slowness.
This study observed an independent association between lower 25(OH)D levels and greater frailty status in cross-sectional analyses. However among men classified as non-frail (robust or intermediate) at baseline, there was no evidence of an association between 25(OH)D levels at baseline and greater frailty status at follow-up 4.6 years later. The LASA study9 reported that among 885 non-frail participants at baseline, those with 25(OH)D levels <10 ng/mL, but not those with levels between 10–20 ng/mL, had an increased odds being classified as frail (versus non-frail) at the 3 year follow-up exam, compared with that among the referent group (serum 25(OH)D levels >20 ng/mL). An analysis of 463 women in the Women’s Health and Aging Study I11 classified as not-frail at baseline reported that the odds of becoming frail (versus non-frail) did not differ between women in the lowest quartile of 25(OH)D (cut-point not reported) as compared with that among women in the upper three quartiles.
Inconsistencies between the findings of studies examining the association between 25(OH)D level and frailty status may in part be explained by differences in study populations, methods to measure 25(OH)D, cut-points used to define 25(OH) status, definitions of frailty syndrome, or adequacy of adjustment for potential confounders. However, these factors do not explain the discrepant results found within this study between cross-sectional and longitudinal analyses. Since there was no evidence to support an association between lower 25(OH) levels and greater frailty status at follow-up, the observed cross-sectional association in this study may be due to the presence of residual confounding. On the other hand, we had limited power to detect a longitudinal association because of smaller sample size. In addition, surviving men not returning to clinic for repeat assessment of frailty status had slightly lower 25(OH)D levels and poorer health at baseline. Thus, it is possible that this missing data may have biased our longitudinal findings towards the null hypothesis of no association. Vitamin D supplementation has been proposed as a potential therapy for the prevention and treatment of frailty.19 However, the effect of vitamin D supplementation on incidence and progression of frailty, including among a target population defined by 25(OH)D status, can only be definitively addressed in a study utilizing a randomized trial design.
Strengths of this study include its prospective analysis; measurement of total 25(OH) using the gold standard LC-MS/MS method; validated definition of frailty status; and adjustment for multiple potential confounders. However, this study has several limitations. Participants were predominantly Caucasian community-dwelling healthy older men, and results may not apply to other populations. Power was insufficient to examine the association between severe vitamin D deficiency (e.g., 25(OH)D level <10 ng/mL) and frailty status. Analyses were adjusted for multiple factors, but the possibility of residual confounding cannot be eliminated. Finally, measurement of 25(OH)D was performed at the baseline examination only. Thus, it was not possible to examine whether decline in 25(OH)D levels was associated with greater incident frailty status. However, there was a strong correlation between 25(OH)D level at baseline and 25(OH)D level an average of 1.9 years later among a subset of men with two 25(OH)D measurements.
Lower levels of 25(OH)D (<20 ng/mL) at baseline were independently associated with greater evidence of frailty in older community-dwelling men, but were not associated with subsequent greater risk of frailty status at 4.6 years. Future research is warranted to address the directionality of this association.
Supplementary Material
Acknowledgments
Source of Funding: The Osteoporotic Fractures in Men (MrOS) Study is supported by National Institutes of Health funding. The following institutes provide support: the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Institute on Aging (NIA), the National Center for Research Resources (NCRR), and NIH Roadmap for Medical Research under the following grant numbers: U01 AR45580, U01 AR45614, U01 AR45632, U01 AR45647, U01 AR45654, U01 AR45583, U01 AG18197, U01-AG027810, and UL1 RR024140.
Sponsor’s Role:
The funding agencies had no direct role in the conduct of the study; the collection, management, analyses and interpretation of the data; or preparation or approval of the manuscript.
| Elements of Financial/Personal Conflicts | *KE Ensrud | TL Blackwell | JA Cauley | SR Cummings | ||||
|---|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Yes | No | Yes | No | |
| Employment or Affiliation | X | X | X | X | ||||
| Grants/Funds | X | X | X | X | ||||
| Honoraria | X | X | X | X | ||||
| Speaker Forum | X | X | X | X | ||||
| Consultant | X | X | X | X | ||||
| Stocks | X | X | X | X | ||||
| Royalties | X | X | X | X | ||||
| Expert Testimony | X | X | X | X | ||||
| Board Member | X | X | X | X | ||||
| Patents | X | X | X | X | ||||
| Personal Relationship | X | X | X | X | ||||
Drs. Ensrud, Cauley, and Cummings have received grant support from the NIH (and supporting agencies) grant as listed under Funding Sources on the title page
| Elements of Financial/Personal Conflicts | E Barrett-Connor | TL Dam | AR Hoffman | JM Shikany | ||||
|---|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Yes | No | Yes | No | |
| Employment or Affiliation | X | X | X | X | ||||
| Grants/Funds | X | X | X | X | ||||
| Honoraria | X | X | X | X | ||||
| Speaker Forum | X | X | X | X | ||||
| Consultant | X | X | X | X | ||||
| Stocks | X | X | X | X | ||||
| Royalties | X | X | X | X | ||||
| Expert Testimony | X | X | X | X | ||||
| Board Member | X | X | X | X | ||||
| Patents | X | X | X | X | ||||
| Personal Relationship | X | X | X | X | ||||
| Elements of Financial/Personal Conflicts | NE Lane | ML Stefanick | ES Orwoll | PM Cawthon | ||||
|---|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Yes | No | Yes | No | |
| Employment or Affiliation | X | X | X | X | ||||
| Grants/Funds | X | X | X | X | ||||
| Honoraria | X | X | X | X | ||||
| Speaker Forum | X | X | X | X | ||||
| Consultant | X | X | X | X | ||||
| Stocks | X | X | X | X | ||||
| Royalties | X | X | X | X | ||||
| Expert Testimony | X | X | X | X | ||||
| Board Member | X | X | X | X | ||||
| Patents | X | X | X | X | ||||
| Personal Relationship | X | X | X | X | ||||
Dr. Orwoll has received grant support from the NIH (and supporting agencies) grant as listed under Funding Sources on the title page
Footnotes
Author Contributions:
Kristine E. Ensrud, MD, MPH – study concept and design, acquisition of data, analysis and interpretation of data, preparation of manuscript
Terri L. Blackwell – analysis and interpretation of data, critical review of manuscript
Jane A. Cauley, DrPH – acquisition of data, analysis and interpretation of data, critical review of manuscript
Steven R. Cummings, MD – study concept and design, critical review of manuscript
Elizabeth Barrett-Connor, MD – analysis and interpretation of data, critical review of manuscript
Thuy-Tien Dam, MD – critical review of manuscript
Andrew R. Hoffman, MD – critical review of manuscript
James M. Shikany, DrPH – critical review of manuscript
Nancy E. Lane, MD – critical review of manuscript
Marcia L. Stefanick, PhD – critical review of manuscript
Eric S. Orwoll, MD – study concept and design, interpretation of data, critical review of manuscript
Peggy M. Cawthon, PhD – analysis and interpretation of data, critical review of manuscript
References
- 1.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 2.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 3.Rockwood K, Howlett SE, MacKnight C, et al. Prevalence, attributes, and outcomes of fitness and frailty in community-dwelling older adults: report from the Canadian study of health and aging. J Gerontol A Biol Sci Med Sci. 2004;59:1310–1317. doi: 10.1093/gerona/59.12.1310. [DOI] [PubMed] [Google Scholar]
- 4.Cawthon PM, Marshall LM, Michael Y, et al. Frailty in older men: prevalence, progression, and relationship with mortality. J Am Geriatr Soc. 2007;55:1216–1223. doi: 10.1111/j.1532-5415.2007.01259.x. [DOI] [PubMed] [Google Scholar]
- 5.Ensrud KE, Ewing SK, Taylor BC, et al. Frailty and risk of falls, fracture, and mortality in older women: the study of osteoporotic fractures. J Gerontol A Biol Sci Med Sci. 2007;62:744–751. doi: 10.1093/gerona/62.7.744. [DOI] [PubMed] [Google Scholar]
- 6.Effectiveness and safety of vitamin D in relation to bone health. Agency for Healthcare Research and Quality; Aug, 2007. [Accessed 4-19-2010]. http://www.ahrq.gov/clinic/tp/vitadtp.htm. [Google Scholar]
- 7.Vitamin D and calcium: a systematic review of health outcomes. Agency for Healthcare Research and Quality; Aug, 2009. [Accessed 4-19-2010]. http://www.ahrq.gov/clinic/tp/vitadcaltp.htm. [Google Scholar]
- 8.Michelon E, Blaum C, Semba RD, et al. Vitamin and carotenoid status in older women: associations with the frailty syndrome. J Gerontol A Biol Sci Med Sci. 2006;61:600–607. doi: 10.1093/gerona/61.6.600. [DOI] [PubMed] [Google Scholar]
- 9.Puts MT, Visser M, Twisk JW, et al. Endocrine and inflammatory markers as predictors of frailty. Clin Endocrinol (Oxf) 2005;63:403–411. doi: 10.1111/j.1365-2265.2005.02355.x. [DOI] [PubMed] [Google Scholar]
- 10.Shardell M, Hicks GE, Miller RR, et al. Association of low vitamin D levels with the frailty syndrome in men and women. J Gerontol A Biol Sci Med Sci. 2009;64:69–75. doi: 10.1093/gerona/gln007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Semba RD, Bartali B, Zhou J, et al. Low serum micronutrient concentrations predict frailty among older women living in the community. J Gerontol A Biol Sci Med Sci. 2006;61:594–599. doi: 10.1093/gerona/61.6.594. [DOI] [PubMed] [Google Scholar]
- 12.Orwoll E, Blank JB, Barrett-Connor E, et al. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study--a large observational study of the determinants of fracture in older men. Contemp Clin Trials. 2005;26:569–585. doi: 10.1016/j.cct.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 13.Ensrud KE, Taylor BC, Paudel ML, et al. Serum 25-hydroxyvitamin D levels and rate of hip bone loss in older men. J Clin Endocrinol Metab. 2009;94:2773–2780. doi: 10.1210/jc.2008-2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Washburn RA, Ficker JL. Physical Activity Scale for the Elderly (PASE): the relationship with activity measured by a portable accelerometer. J Sports Med Phys Fitness. 1999;39:336–340. [PubMed] [Google Scholar]
- 15.Ware JE, Kosinski M, Keller SD. SF-12: How to score the SF-12 Physical and Mental Health Summary Scores. 3. Lincoln, RI: QualityMetric, Inc; 1998. [Google Scholar]
- 16.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- 17.Levey AS, Coresh J, Greene T, et al. Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem. 2007;53:766–772. doi: 10.1373/clinchem.2006.077180. [DOI] [PubMed] [Google Scholar]
- 18.Scott SC, Goldberg MS, Mayo NE. Statistical assessment of ordinal outcomes in comparative studies. J Clin Epidemiol. 1997;50:45–55. doi: 10.1016/s0895-4356(96)00312-5. [DOI] [PubMed] [Google Scholar]
- 19.Cherniack EP, Florez HJ, Troen BR. Emerging therapies to treat frailty syndrome in the elderly. Altern Med Rev. 2007;12:246–258. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.