Abstract
To reduce connective tissue IL-6 level stimulated by LPS, it is essential to control IL-6 expression in both mononuclear cells and fibroblasts. However, it is unclear whether the regulatory mechanisms for both cells are similar or not. In this study, we found that signaling pathways mediating LPS-stimulated IL-6 in mononuclear U937 cells and fibroblasts were different. Furthermore, our studies showed that while LPS activated AP-1 and NFκB in U937 cells, it only activated NFκB in fibroblasts. Analysis of nuclear AP-1 subunits showed that LPS stimulated c-Fos, Fra-1 and Jun D activities in U937 cells, but not fibroblasts. The lack of ERK involvement in LPS-stimulated IL-6 in fibroblasts was further supported by the observations that simvastatin, which is known to target ERK-AP-1, failed to inhibit LPS-stimulated IL-6 by fibroblasts. Finally, we showed that targeting NFκB pathway was highly effective in inhibition of LPS-stimulated IL-6 in coculture of U937 cells and fibroblasts.
Keywords: Inflammation, LPS, Cytokine, Gene expression, Signal transduction, Transcription
1. Introduction
A prompt and strong reaction of our immune system is crucial for defending our body against bacterial infection and infection-associated tissue injury. However, chronic and excessive host inflammatory response and/or inadequate resolution of inflammation cause many chronic diseases [1–3]. Studies have shown that elevated level of inflammatory cytokines such as interleukin (IL)-6 in periodontal tissue in response to lipopolysaccharide (LPS), a component of the outer membrane of Gram-negative bacteria, plays an important role in periodontal disease, which is characterized by chronic inflammation and tissue destruction including alveolar bone loss [4].
IL-6 is a key inflammatory cytokine involved in many diseases via its multiple functions [5–7]. For tissue destruction, IL-6 increases matrix degradation by stimulating matrix metalloproteinase (MMP) expression and promotes bone loss by increasing osteoclastogenesis and reducing osteoblast activity [8]. The important role of IL-6 in inflammation is indicated by its prompt and high responsiveness to inflammatory stimuli as its expression rises rapidly and robustly and reaches the peak in a few hours [9]. Clinical trials have shown that blockade of IL-6 action by IL-6 receptor specific monoclonal antibody tocilizumab is highly efficient for controlling not only the symptoms of rheumatoid arthritis, but also inflammatory bone loss [10], further indicating a critical role of IL-6 in inflammation and inflammation-related diseases.
IL-6 is expressed and released by a variety of cell types including not only immune cells such as monocytes, macrophages, and lymphocytes, but also connective tissue cells such as fibroblasts [6]. Our recent study using a co-culture system has shown that both mononuclear U937 cells and fibroblasts contribute to a markedly elevated IL-6 level in response to LPS, and IL-6 in turn exerts a synergistic effect with LPS on MMP-1 expression by U937 cells [11], suggesting that IL-6 serves as a mediator for crosstalk between fibroblasts and mononuclear cells for inflammation. Given that a large number of fibroblasts are present in the connective tissue, it is possible that LPS stimulates more IL-6 release from fibroblasts than mononuclear cells. Therefore, it is important to seek a common target in both mononuclear cells and fibroblasts to reduce the total amount of IL-6 in the inflamed connective tissue.
In this study, we demonstrated that the signaling mechanism regulating IL-6 expression by LPS in gingival fibroblasts was different from that in U937 mononuclear cells. Based on these findings, we further explored the molecular mechanisms underlying the difference in the transcriptional activation of IL-6 gene by LPS in U937 cells and gingival fibroblast. Finally, our study indicates that the nuclear factor kappa B (NFκB) signaling pathway is a common target for controlling LPS-stimulated IL-6 expression by both gingival fibroblasts and U937 cells.
2. Materials and Methods
2.1. Cell culture
Human U937 mononuclear phagocytes [12] and human gingival fibroblasts were purchased from American Type Culture Collection (Manassas, VA). The cells were cultured in a 5% CO2 atmosphere in RPMI 1640 medium (GIBCO, Invitrogen Cop. Carlsbad, CA) containing 10% fetal calf serum, 1% MEM non-essential amino acid solution, and 0.6 g/100 ml of HEPES either independently or in Corning Transwell plates (Sigma, St. Louis, MO) that have 2-compartments separated by a polycarbonate membrane with 0.4 µm pores. Fibroblasts were grown to 80% confluence in the lower compartment and U937 cells were grown (0.5 × 106 cells/ml) in the upper compartment. For cell treatment, LPS from E. coli and simvastatin (Sigma, St. Louis, MO) were used. The LPS was highly purified by phenol extraction and gel filtration chromatography, and was cell culture tested.
2.2. ELISA
IL-6 and MMP-1 in conditioned medium was quantified using sandwich enzyme-liked immunosorbent assay (ELISA) kits according to the protocol provided by the manufacturer (R&D System, Minneapolis, MN).
2.3. Real-time polymerase chain reaction (PCR)
Total RNA was isolated from cells using the RNeasy minikit (Qiagen, Santa Clarita, CA). First-strand complementary DNA (cDNA) was synthesized with the iScript™ cDNA synthesis kit (Bio-Rad Laboratories, Hercules, CA) using 20 µl of reaction mixture containing 0.5 µg of total RNA, 4 µl of 5× iScript reaction mixture, and 1 µl of iScript reverse transcriptase. The complete reaction was cycled for 5 minutes at 25 °C, 30 minutes at 42 °C and 5 minutes at 85°C using a PTC-200 DNA Engine (MJ Research, Waltham, MA). The reverse transcription reaction mixture was then diluted 1:10 with nuclease-free water and used for PCR amplification in the presence of the primers. The Beacon designer software (PREMIER Biosoft International, Palo Alto, CA) was used for primer designing (IL-6: 5’ primer sequence, AACAACCTGAACCTTCCAAAGATG; 3’ primer sequence, TCAAACTCCAAAAGACCAGTGATG. MMP-1: 5’ primer sequence, CTGGGAAGCCATCACTTACCTTGC; 3’ primer sequence, GTTTCTAGAGTCGCTGG GAAGCTG). Primers were synthesized by Integrated DNA Technologies, Inc. (Coralville, IA) and real-time PCR was performed in duplicate using 25 µl of reaction mixture containing 1.0 µl of RT mixture, 0.2 µM of both primers, and 12.5 µl of iQ™ SYBR Green Supermix (Bio-Rad Laboratories). Real-time PCR was run in the iCycler™ real-time detection system (Bio-Rad Laboratories) with a two-step method. The hot-start enzyme was activated (95°C for 3 min) and cDNA was then amplified for 40 cycles consisting of denaturation at 95°C for 10 sec and annealing/extension at 53°C for 45 sec. A melt-curve assay was then performed (55°C for 1 min and then temperature was increased by 0.5°C every 10 sec) to detect the formation of primer-derived trimmers and dimmers. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as a control (5’ primer sequence, GAATTTGGCTACAGCAACAGGGTG; 3’ primer sequence, TCTCTTCCTCTTGTGCTCTTGCTG). Data were analyzed with the iCycler iQ™ software. The average starting quantity (SQ) of fluorescence units was used for analysis. Quantification was calculated using the SQ of targeted cDNA relative to that of GAPDH cDNA in the same sample.
2.4. Treatment of cells with the inhibitors of signaling pathways
U937 cells or gingival fibroblasts were treated with 100 ng/ml of LPS in the absence or presence of 10 or 25 µM of PD98059, SP600125, SB203580, 1 or 5 µM of Bay117085, 0.25, 1, or 5 µM of parthenolide (Calbiochem/EMD Biosciences, Inc., San Diego, CA) for 24 h. After the treatment, IL-6 in medium was quantified using ELISA
2.5. Extraction of nuclear proteins
Nuclear protein was extracted using NE-PER™ nuclear and cytoplasmic extraction kit (Pierce, Rockfold, IL). The concentration of protein was determined using a protein assay kit (Bio-Rad, Hercules, CA).
2.6. Immunoblotting
After treatment, total cellular proteins were extracted using a protein extraction kit (Millipore, Billerica, MA) by following the instruction provided by the manufacturer. Twenty-five µg of protein from each sample was electrophoresed in a 10% polyacrylamide gel. After transferring proteins to a polyvinylidene fluoride (PVDF) membrane, immunoblotting was performed using antibodies against phosphorylated extracellular signal-regulated kinase (ERK), phosphorylated c-Jun N-terminal kinase (JNK), phosphorylated p38 mitogen activated protein kinase (MAPK), inhibitor of NFκB (IκB) or GAPDH (New England BioLabs, Inc., Ipswich, MA). The nuclear protein was used to detect nuclear NFkB p50 and p65 using anti-p50 or antip65 antibodies (New England BioLabs, Inc.). The proteins were visualized by incubating the membrane with chemiluminescence reagent (NEN Life Science Products, Boston, MA) for 1 min and exposing it to x-ray film for 30–60 seconds.
2.7. RNA interference
Gingival fibroblasts were transiently transfected with 200 nM of NFκB p65 siRNA (5´-UCACACAGUAGGAAGAUCUCAUCCC-3´) or scrambled siRNA control (Invitrogen Corp., Carlsbad, CA) using Lipofectamine RNAi MAX reagent (Invitrogen Corp.) by following the manufacturer's instructions. Forty-eight hours later, transfected cells were treated with or without 100 ng/ml of LPS for 24 h.
2.8. AP-1 and NFκB reporter and activity assays
Gingival fibroblasts or U937 cells in 12-well plates with RPMI 1640 medium containing 10% FBS were transfected with 0.5 µg of AP-1 or NFκB promoter-firefly/renilla luciferase (40:1) reporter constructs (SuperArray Bioscience Corp.) using FuGENE HD transfection reagent (Promega Corp. Madison, WI) for 18–20 h. The cells were then treated with fresh medium containing 100 ng/ml of LPS for 24 h. After the treatment, the cells were rinsed with cold PBS and lysed with PLB Passive Lysis Buffer (Promega Corp.). The lysate was centrifuged at 15,000 g for 5 min at 4°C and the supernatant harvested. Both firefly and renilla luciferase levels were measured in a luminometer using the dual-luciferase reporter assay system according to the instruction from the manufacturer (Promega Corp.). The firefly luciferase levels were normalized to the renilla luciferase levels.
Five µg of nuclear protein of each sample was applied to the assay for AP-1 and NFκB activity using the TransAM kits produced by Active Motif (Carlsbad, California) according to the protocol provided by the manufacturer. These kits contain a 96-stripwell plate to which the consensus-binding site oligonucleotides were immobilized. The nuclear extract is added to each well and the transcription factor of interest binds specifically to the immobilized oligonucleotides. The TransAM assays are up to 100 times more sensitive than gel mobility shift assay and capable of detecting transcription factors with specific antibodies.
2.9. Statistic analysis
Data were presented as mean ± SD. ANOVA was performed to determine the statistical significance of cytokine expression among different experimental groups. A value of P< 0.05 was considered significant.
3. Results
3.1. The kinetics of LPS-stimulated IL-6 mRNA expression and protein secretion by U937 mononuclear cells and gingival fibroblasts
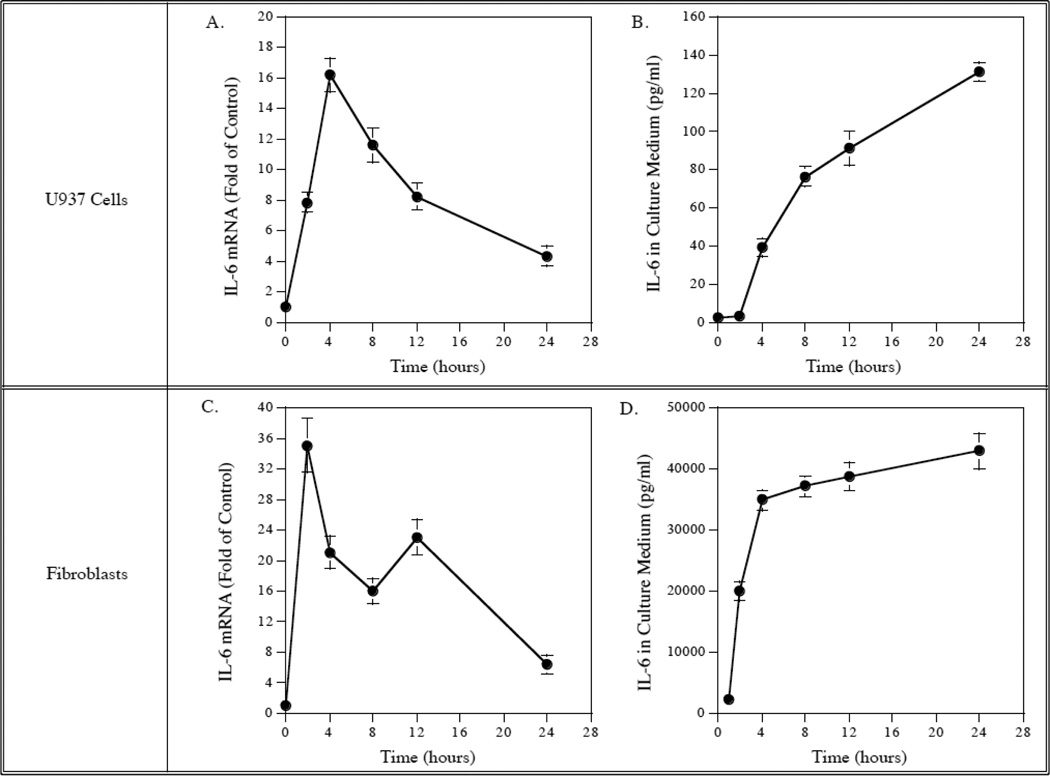
We first compared the kinetics of LPS-stimulated IL-6 mRNA expression and protein secretion between U937 mononuclear cells and gingival fibroblasts. Results showed that IL-6 mRNA expression was stimulated by LPS in a rapid and transient fashion in both U937 cells and gingival fibroblasts and reached the peak at 4 h in U937 cells and 2h in gingival fibroblasts (Fig. 1, A and C). In consistence with the kinetics of IL-6 mRNA expression, IL-6 protein released in culture medium was also increased rapidly and then plateaued in both U937 cells and gingival fibroblasts (Fig. 1, B and D). These data showed that U937 cells and gingival fibroblasts had a similar kinetics of LPS-stimulated IL-6 expression and protein secretion. These data also indicate that the LPS stimulates IL-6 protein secretion by increasing IL-6 mRNA expression.
Figure 1.
Kinetics of IL-6 mRNA expression and protein secretion in response to LPS by U937 cells and gingival fibroblasts. U937 cells (A and B) or gingival fibroblasts (C and D) were treated with 100 ng/ml of LPS for different times as indicated and culture medium was collected and RNA was isolated at each time point for quantification of IL-6 mRNA using real-time PCR (A and C) and IL-6 protein using ELISA (B and D). The data presented are from one of three independent experiments with similar results.
3.2. Different effects of pharmacological inhibitors of signaling pathways on LPS-stimulated IL-6 secretion by U937 cells and gingival fibroblasts
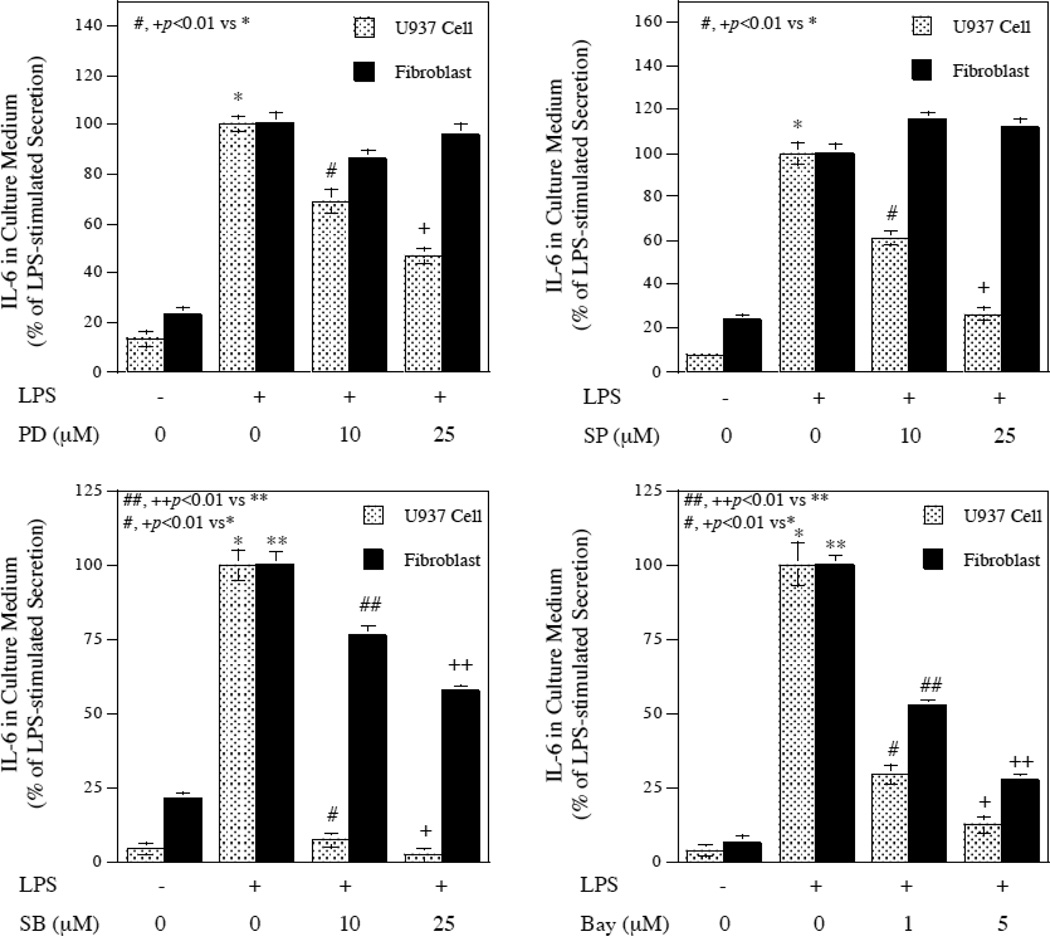
To determine whether the signaling pathways involved in LPS-stimulated IL-6 expression between U937 cells and gingival fibroblasts are similar or not, we used pharmacological inhibitors of signaling pathways. The concentrations of these inhibitors applied to these studies have been shown previously to inhibit gene expression in both U937 cells and gingival fibroblasts [11, 13]. Results showed that PD98059, SP600125, SB203580, and Bay117085, the specific inhibitors for ERK, JNK, p38 MAPK, and NFκB pathways, respectively, significantly inhibited LPS-stimulated IL-6 secretion from U937 cells (Fig. 2). Interestingly, PD98059 and SP600125 failed to inhibit LPS-stimulated IL-6 secretion from gingival fibroblasts (Fig. 2). These results suggest that the signaling mechanisms for LPS-stimulated IL-6 expression by U937 cells and gingival fibroblasts are different.
Figure 2.
Different effects of pharmacological inhibitors on LPS-stimulated IL-6 secretion between U937 cells and gingival fibroblasts. U937 cells or gingival fibroblasts were treated with or without 100 ng/ml of LPS in the absence or presence of different concentrations of PD98059, SP600125, SB203580, or Bay117085 as indicated for 24 h. After the treatment, IL-6 in culture medium was quantified using ELISA. The data presented are from one of two independent experiments with similar results.
3.3. LPS stimulated ERK, JNK, and p38 MAPK and NFκB activities in both U937 cells and gingival fibroblasts
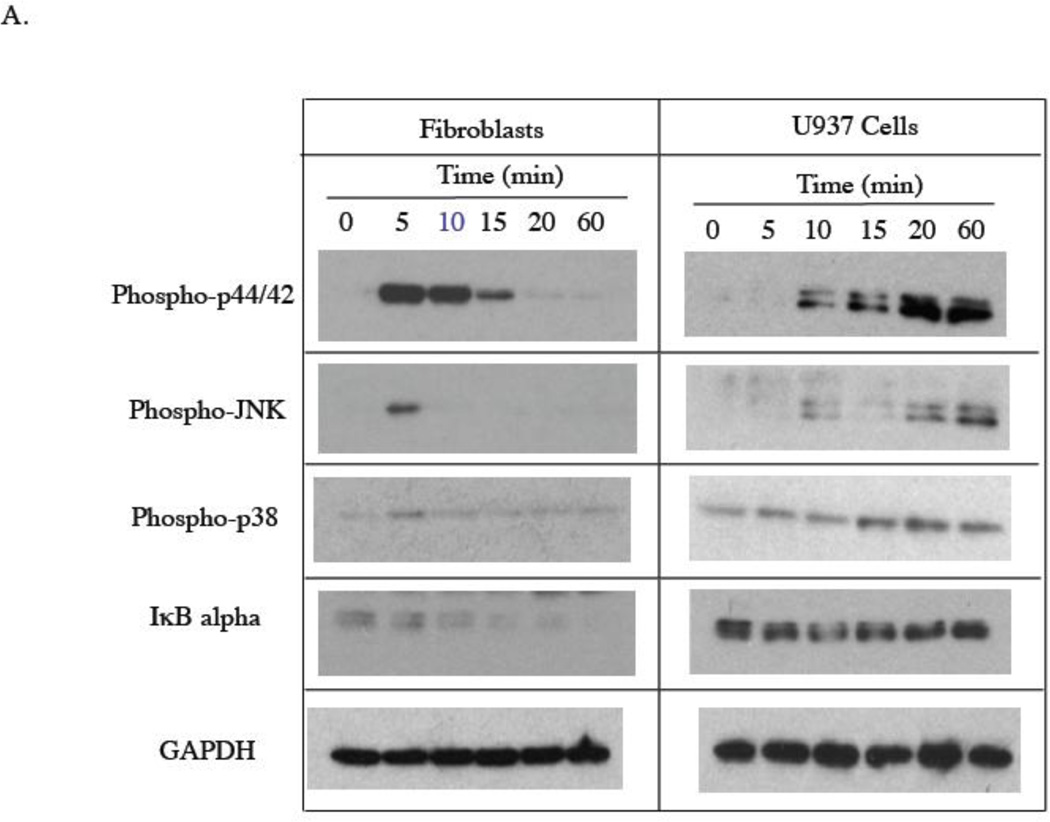
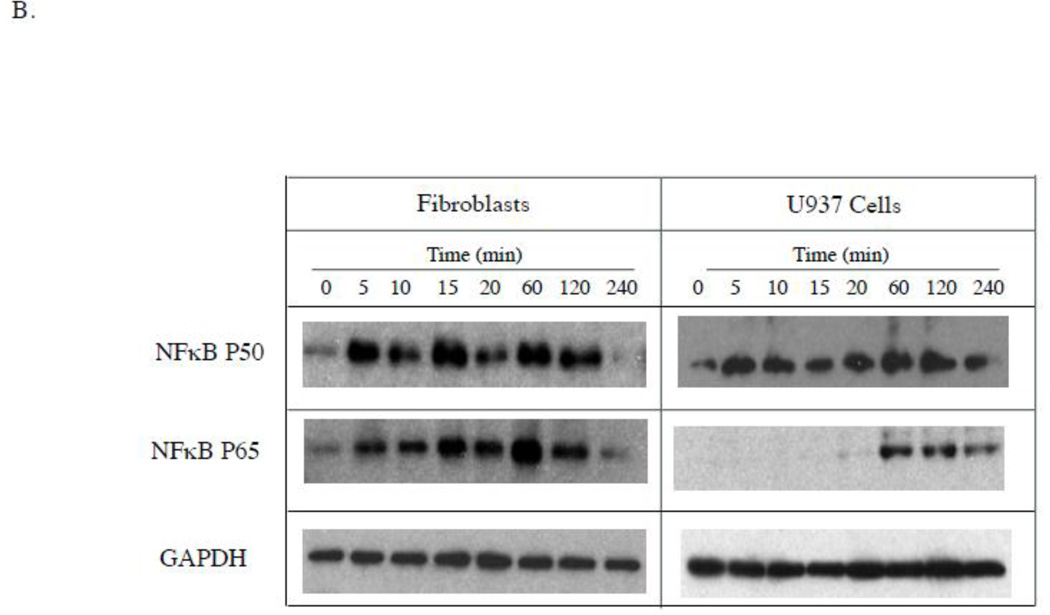
There are two possibilities that may lead to the ineffectiveness of PD98059 and SP600125 in the inhibition of LPS-stimulated IL-6 secretion by gingival fibroblasts: (1) LPS simply does not activate ERK and JNK pathways; (2) LPS activates ERK and JNK pathways, but the activation of these pathways does not lead to an increase in IL-6 transcriptional activation. To exclude the first possibility, we determined if LPS induced phosphorylation of ERK and JNK in gingival fibroblasts. Results showed that LPS was capable of inducing the phosphorylation of ERK, JNK and p38 MAPK in both U937 cells and gingival fibroblasts (Fig. 3A). Furthermore, LPS treatment of gingival fibroblasts led to a time-dependent decrease in cytosol IκB alpha (Fig. 3A) and an increase in nuclear NFκB P50 and P65 (Fig. 3B). In U937 cells, LPS treatment induced a brief reduction of cytosol IκB alpha at 10 min (Fig. 3A) and an increase in nuclear NFκB p50 and p65 (Fig. 3B), suggesting that LPS stimulates NFκB activity in gingival fibroblasts and U937 cells. Taken together, these data indicate that LPS is capable of stimulating ERK, JNK, p38 MAPK and NFκB activities in both gingival fibroblasts and U937 cells.
Figure 3.
Stimulation of signaling pathways by LPS in gingival fibroblasts and U937 cells. A. Gingival fibroblasts or U937 cells were treated with 100 ng/ml of LPS for different times as indicated. At each time point, cellular phosphorylated p44/p42 (phospho-p44/42), JNK (phosphor-JNK), p38 (phosphor-p38) as well as IκB alpha and GAPDH were detected using immunoblotting as described in Experimental Procedure. B. Gingival fibroblasts or U937 cells were treated with 100 ng/ml of LPS for different times as indicated. At each time point, nuclear NFκB p50 and NFκB p65, and cellular GAPDH were detected using immunoblotting as described in Experimental Procedure. The graphs presented are from one of two independent experiments with similar results.
3.4. LPS stimulated NFκB, but not AP-1 activity in gingival fibroblasts
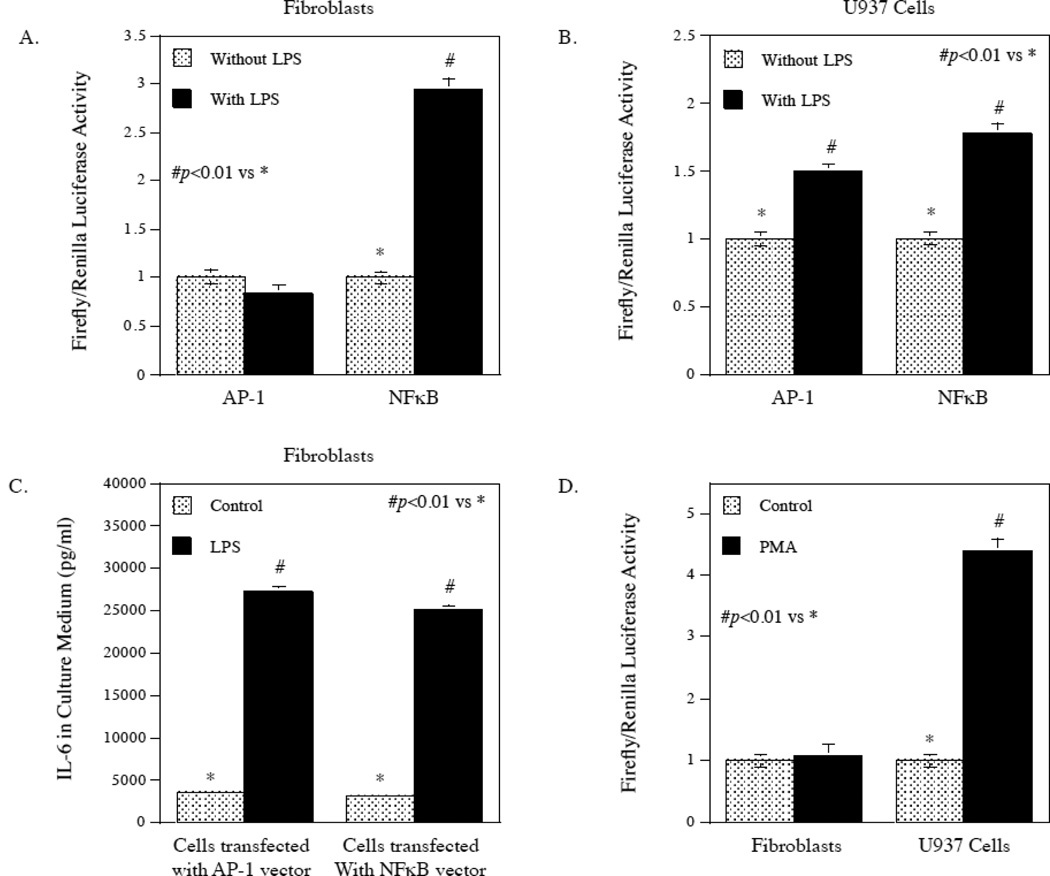
Since it is known that activation of ERK and JNK pathways leads to activation of transcription factor AP-1 [14, 15], we further determined the effect of LPS on AP-1 transcription activity using an AP-1-driven luciferase reporter activation assay. Interestingly, LPS did not increase AP-1 activity but significantly stimulated NFκB in gingival fibroblasts (Fig. 4A). In contrast, LPS increased both AP-1 and NFκB activities in U937 cells (Fig. 4B). As control, we determined if LPS stimulated IL-6 expression by gingival fibroblasts in the above experiment shown in Fig. 4A. Results showed that LPS stimulated IL-6 secretion (Fig. 4C). To confirm the difference in AP-1 activation between gingival fibroblasts and U937 cells, cells were treated with phorbol-12-myristate-13-acetate (PMA), a compound known to activate AP-1 activity in U937 cells previously [16]. Results confirmed that PMA stimulated AP-1 activity in U937 cells as reported, but it did not stimulate AP-1 activity in gingival fibroblasts (Fig. 4D). These findings showed that LPS had different effects on AP-1 activity in U937 cells and gingival fibroblasts, but had similar actions on NFκB activity.
Figure 4.
Different effects of LPS on AP-1 transcriptional activation in gingival fibroblasts and U937 cells. A and B. Gingival fibroblasts (A) or U937 cells (B) were transfected with the AP-1 or NFκB-responsive luciferase constructs encoding the Firefly luciferase reporter gene for 24 h. After the transfection, cells were treated with or without 100 ng/ml of LPS for 24 h and Firefly luciferase activity was then determined and normalized to Renilla luciferase activity. C. The stimulation of IL-6 secretion by LPS from gingival fibroblasts transfected with AP-1 or NFκB-responsive luciferase constructs. The culture medium from the above experiment (A) was collected for IL-6 quantification using ELISA. D. The effect of PMA on AP-1 transcriptional activity in gingival fibroblasts and U937 cells. Gingival fibroblasts or U937 cells were transfected with AP-1-responsive luciferase constructs for 24 h and then treated with or without 10 ng/ml of PMA for 24 h. Firefly luciferase activity was determined after the treatment and normalized to Renilla luciferase activity. The data presented are representative of 3 independent experiments with similar results.
3.5. LPS did not stimulate AP-1 subunits in gingival fibroblasts
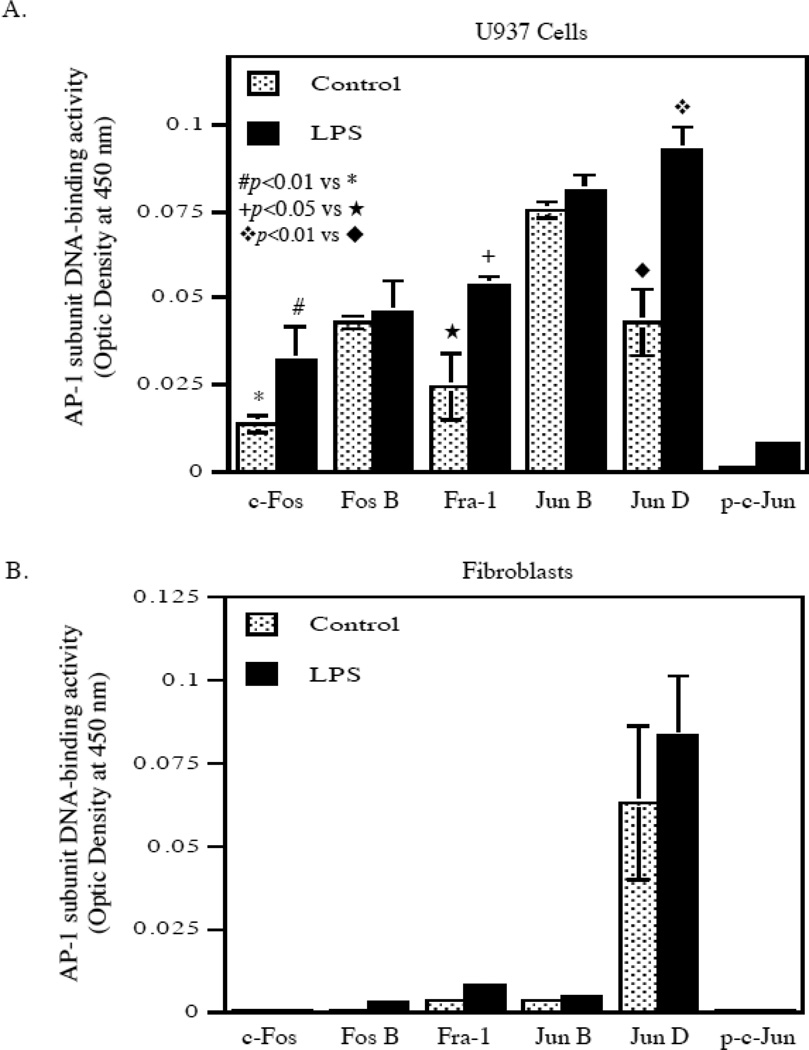
To confirm that LPS did not stimulate AP-1 transcriptional activity, we took another approach by applying an ELISA-type DNA-binding activity assay system in which the oligonucleotides containing AP-1 consensus sequence were immobilized on the surface of wells. After incubation of the wells with nuclear extract to allow AP-1 to bind the oligonucleotides, the bound AP-1 subunits were identified by specific antibodies. Results showed that in U937 cells, LPS stimulated the DNA binding activities of c-Fos, Fra-1, and Jun D, but not Fos B, Jun B and phosphorylated c-Jun (p-c-Jun) (Fig. 5A). In contrast, the baseline levels of bound AP-1 subunits c-Fos, Fos B, Fra-1, Jun B and p-c-Jun in fibroblasts were very low and LPS did not increase the DNA binding activity of any AP-1 subunit (Fig. 5B). Although the baseline activity of Jun D was higher, LPS did not stimulate it significantly.
Figure 5.
The effect of LPS on nuclear activities of AP-1 subunits in U937 cells and gingival fibroblasts. U937 cells (A) or gingival fibroblasts (B) were treated with or without 100 ng/ml of LPS for 2 h. After the treatment, the nuclear proteins were isolated from cells and subjected to a transcription factor activity assay system in which AP-1-binding element-containing oligonucleotides were immobilized on the surface of wells. After incubating the wells with nuclear proteins, the AP-1 subunits bound to the oligonucleotides were identified by specific antibodies. The bound levels of c-Fos, Fos B, Fra-1, Jun B, Jun D and phosphorylated c-Jun (p-c-Jun) were determined. The data presented are representative of two experiments with similar results.
3.6. Simvastatin failed to inhibit LPS-stimulated IL-6 expression in gingival fibroblasts, although it inhibited LPS-stimulated IL-6 expression by U937 mononuclear cells
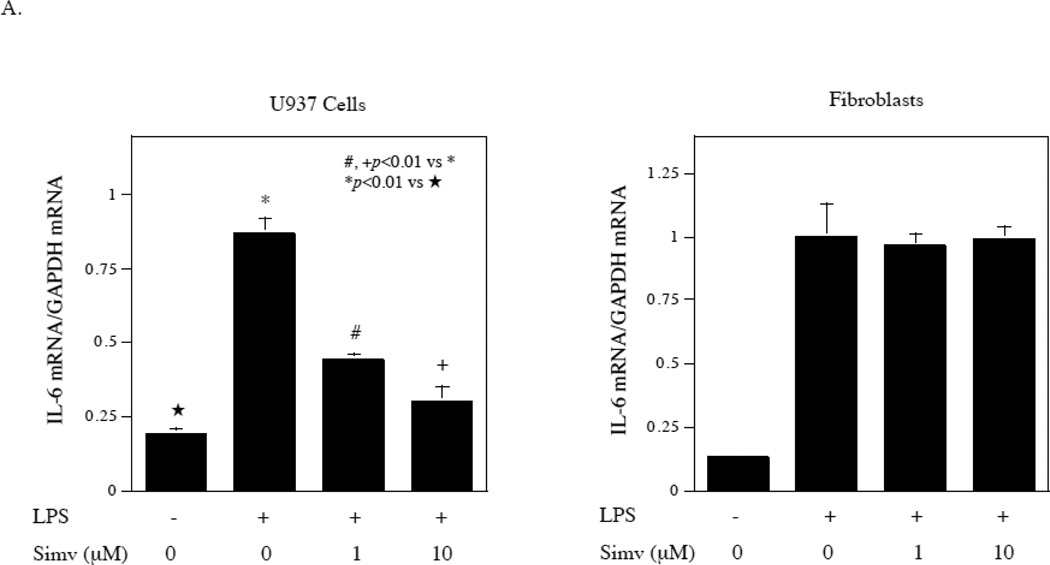
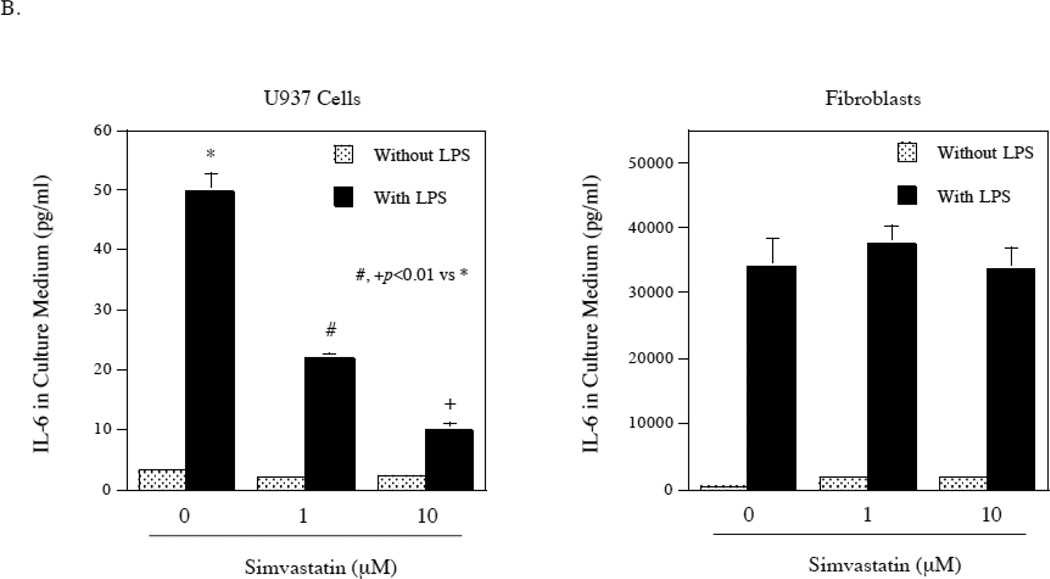
Our previous study [17] and reports from another laboratory [18] have shown that simvastatin inhibited protein isoprenylation-mediated ERK signaling activation in U937 cells and fibroblasts and thus inhibited ERK pathway/AP-1-mediated gene expression. Since the above studies showed that the ERK/AP-1 pathway was not involved in LPS-stimulated IL-6 expression by gingival fibroblasts (Fig. 2), it is expected that simvastatin would not inhibit LPS-stimulated IL-6 expression in gingival fibroblasts. Indeed, results showed that while simvastatin effectively inhibited LPS-stimulated IL-6 mRNA expression by U937 cells, it had no effect on LPS-stimulated IL-6 mRNA expression by gingival fibroblasts (Fig. 6A). We also determine the effect of simvastatin on LPS-stimulated IL-6 secretion by U937 cells and gingival fibroblasts. Similar to the regulation of IL-6 mRNA expression, while simvastatin effectively inhibited LPS-stimulated IL-6 secretion by U937 cells, it did not inhibit it by gingival fibroblasts (Fig. 6B). Simvastatin at 10 µM inhibited LPS-stimulated IL-6 secretion from U937 cells by 80%, but had no effect on LPS-stimulated IL-6 secretion from gingival fibroblasts.
Figure 6.
Lack of inhibition by simvastatin on LPS-stimulated IL-6 secretion by gingival fibroblasts. A. The effect of simvastatin (Simv) on LPS-stimulated IL-6 mRNA expression by U937 cells and gingival fibroblasts. U937 cells and gingival fibroblasts were treated with 100 ng/ml of LPS in the absence or presence of 1 or 10 µM of simvastatin for 4 h for U937 cells and 2 h for fibroblasts and IL-6 mRNA was quantified using real-time PCR. B. The effect of simvastatin on LPS-stimulated IL-6 secretion by U937 cells and gingival fibroblasts. U937 cells or gingival fibroblasts were treated with or without 100 ng/ml of LPS in the absence or presence of 1 or 10 µM of simvastatin for 24 h. After the treatment, IL-6 in culture medium was quantified using ELISA. The data (mean ± SD) presented are representative of three independent experiments with similar results.
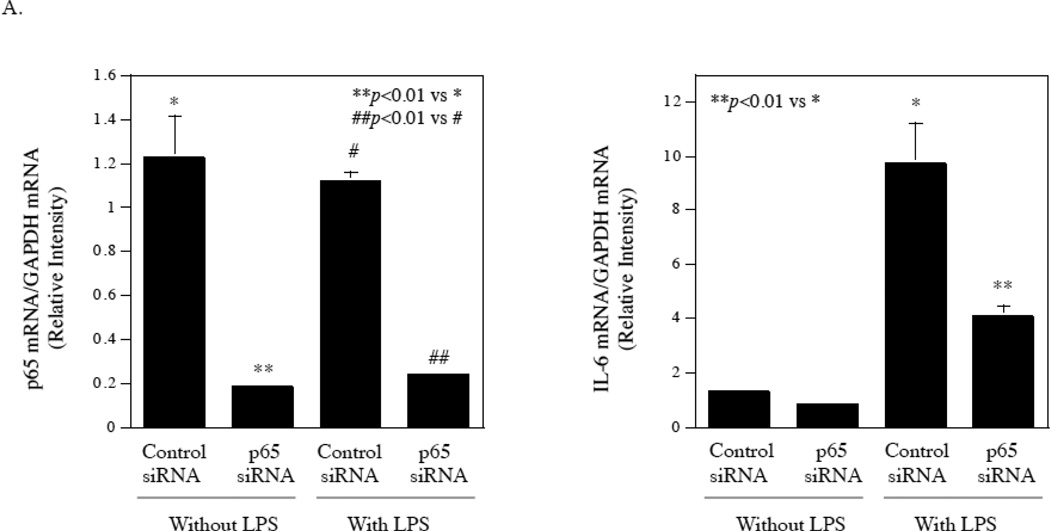
3.7. Interference of NFκB p65 expression or inhibition of NFκB signaling pathway effectively suppressed LPS-stimulated IL-6 expression by gingival fibroblasts
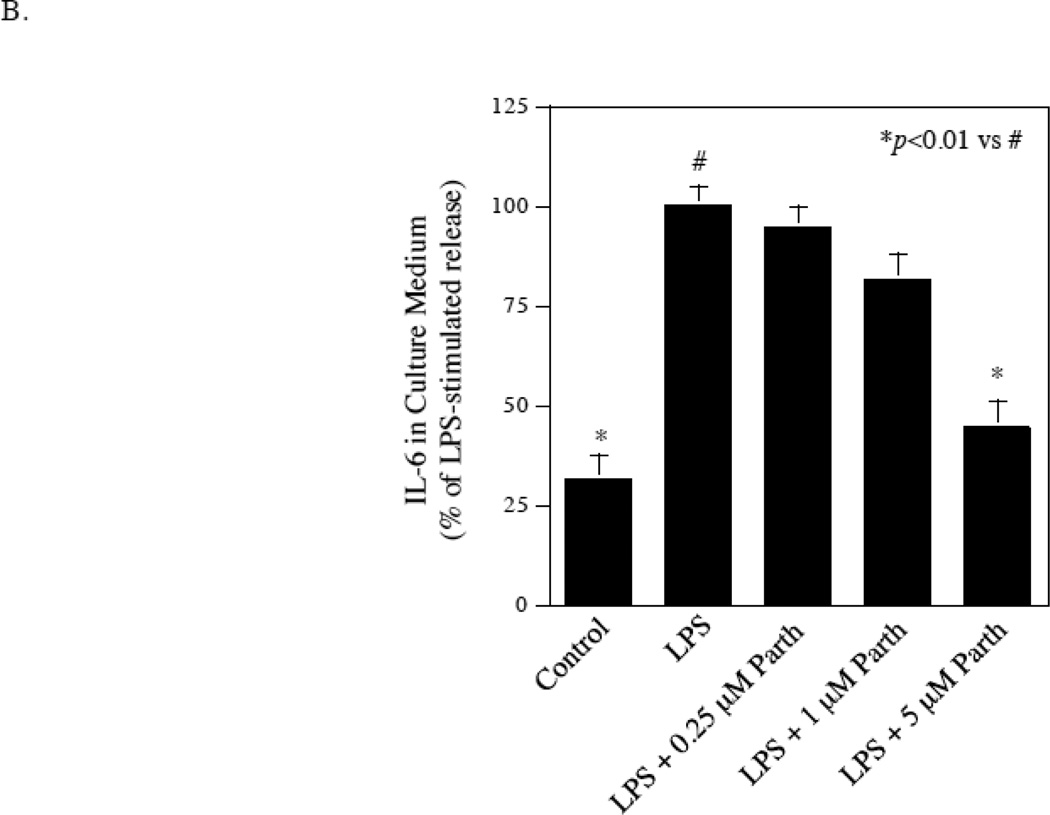
The study presented in Fig. 2 showed that the inhibitor of NFκB signaling pathway effectively inhibited LPS-stimulated IL-6 secretion by gingival fibroblasts. To confirm this observation, we employed RNA interference technique to knockdown p65, which is an important subunit of NFκB [19, 20]. Results showed that the transfection of gingival fibroblasts with p65 siRNA knocked the p65 expression down by 80% in gingival fibroblasts with or without LPS treatment and the knockdown of p65 in turn led to an inhibition of IL-6 mRNA expression by 40% in control cells and 60% in cells treated with LPS (Fig. 7A). Furthermore, treatment of gingival fibroblasts with parthenolide, another NFκB signaling pathway inhibitor [21], inhibited LPS-stimulated IL-6 secretion in a concentration-dependent manner (Fig. 7B). Taken together, these data showed that NFκB is a potential target for inhibition of LPS-stimulated IL-6 expression by gingival fibroblasts.
Figure 7.
Inhibition of LPS-stimulated IL-6 secretion from fibroblasts by knocking down NFκB p65 or pharmacological inhibitor. A. Gingival fibroblasts were transfected with p65 siRNA or control siRNA for 48 h and then treated with or without 100 ng/ml of LPS for 24 h. After the treatment, p65 and IL-6 mRNA were quantified using real-time PCR and normalized to GAPDH mRNA. B. Inhibition of LPS-stimulated IL-6 secretion from gingival fibroblasts by NFκB inhibitor parthenolide. Gingival fibroblasts were treated with 100 ng/ml of LPS in the absence or presence of different concentrations of parthenolide as indicated for 24 h. After the treatment, IL-6 in culture medium was quantified using ELISA. The data (mean ± SD) presented are representative of two independent experiments with similar results.
3.8. Targeting NFκB pathway in coculture of gingival fibroblasts and U937 cells inhibited LPS-stimulated IL-6 expression
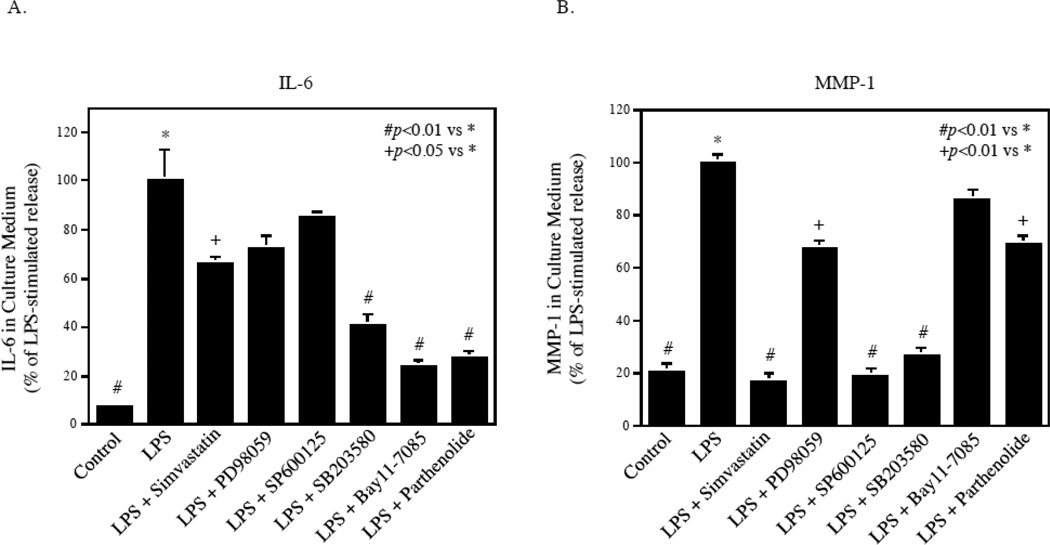
To demonstrate that the NFκB pathway is a potential common target for inhibition of LPS-stimulated IL-6 expression by both fibroblasts and U937 cells, we cocultured gingival fibroblasts and U937 cells and treated them with LPS in the absence or presence of simvastatin or different pharmacological inhibitors. As expected, results showed that simvastatin inhibited LPS-stimulated IL-6 secretion by only 35%, and PD98059 and SP600125 had no significant inhibition (Fig. 8A). In contrast, SB203580, Bay 11-7085 and parthenolide suppressed LPS-stimulated IL-6 secretion by 55%, 75%, and 70%, respectively. These findings indicate that targeting NFκB pathway is highly effective in inhibition of LPS-stimulated IL-6 secretion by the coculture of gingival fibroblasts and U937 cells. Interestingly, LPS-stimulated MMP-1 secretion by the coculture of gingival fibroblasts and U937 cells was effectively inhibited by simvastatin, SP600125 and SB203580, but not Bay11-7085 and parthenolide (Fig. 8B), suggesting that different signaling pathways are involved in controlling IL-6 and MMP-1 gene expression by cocultured gingival fibroblasts and U937 cells.
Figure 8.
The effect of simvastatin and different pharmacological inhibitors on LPS-stimulated IL-6 secretion by coculture of gingival fibroblasts and U937 cells. Gingival fibroblasts and U937 cells were co-cultured in the Transwell system and treated with or without 100 ng/ml of LPS in the absence or presence of 10 µM of simvastatin, 10 µM of PD98059, 10 µM of SP600125, 10 µM of SB203580, 5 µM of Bay117085 or 5 µM of parthenolide for 24 h. After the treatment, IL-6 (A) and MMP-1 (B) in culture medium were quantified using ELISA.
4. Discussion
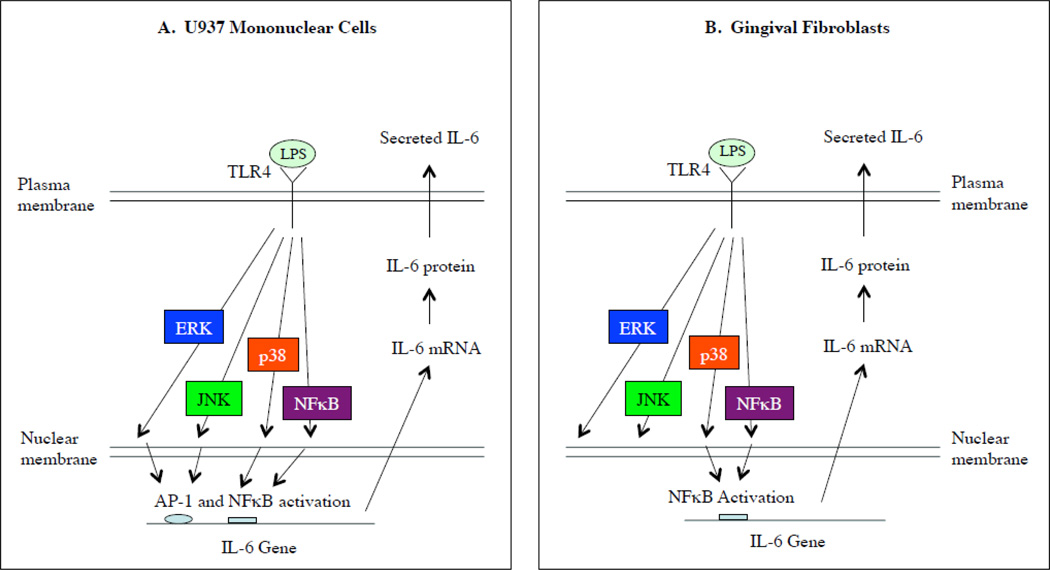
As illustrated in Fig. 9, our present study explored for the first time the mechanisms underlying the difference in the regulation of IL-6 expression by LPS between fibroblasts and mononuclear cells. The major difference in controlling IL-6 expression by LPS in gingival fibroblasts from that in U937 mononuclear cells is a lack of coupling between the activation of ERK/JNK pathways by LPS and the activation of AP-1 transcriptional activity in gingival fibroblasts. Therefore, the inhibitors for the ERK pathway (PD98059 and simvastatin) and JNK pathway (SP600125) failed to inhibit LPS-stimulated IL-6 expression by gingival fibroblasts.
Figure 9.
Illustration of different signaling mechanisms regulating IL-6 expression by LPS in U937 mononuclear cells and gingival fibroblasts. A. In U937 cells, LPS stimulates IL-6 expression via ERK, JNK, p38 and NFκB pathways and subsequent AP-1 and NFκB activation. B. In gingival fibroblasts, although LPS stimulates ERK, JNK, p38, NFκB pathways, it only activates NFκB. Only p38 and NFκB pathways are involved in LPS-stimulated IL-6 expression in gingival fibroblasts.
Eugui et al. reported previously that antioxidants inhibited IL-1β-stimulated IL-6 expression in monocytes, but not in fibroblasts [22], suggesting that different regulatory mechanisms were involved in IL-6 expression between monocytes and fibroblasts. However, no further investigation was done to explore the mechanisms. With respect to the activation of transcription factors by LPS in fibroblasts, there is no dispute in the notion that LPS activates NFκB and NFκB-mediated inflammatory gene expression [23–26]. Contrarily, there have been conflicting reports on the effect of LPS on AP-1 activation in fibroblasts. Matsushita et al. reported that while LPS activated AP-1 in human pulp cells, it had no effect in fibroblasts [27]. In contrast, Wang et al. reported that LPS activated both NFκB and AP-1 in fibroblasts [25]. While the cause of these conflicting observations is unknown, our present study, using both well-controlled luciferase reporter system and transcription factor activity assay, demonstrated that LPS was unable to activate AP-1 activity in gingival fibroblasts. Furthermore, our study explored the potential molecular mechanisms underlying the lack of AP-1 activation by LPS in gingival fibroblasts.
Previous studies have shown that the ERK pathway is coupled to AP-1 activation through two molecular mechanisms [28, 29]. First, a critical threshold level of ERK-1 and/or ERK2 activates Fra-1 and activated Fra-1 is necessary to complete the signaling cascade leading to AP-1 activation [28]. Interestingly, our present study showed that LPS failed to stimulate Fra-1 in gingival fibroblasts (Fig. 5B), although it increased Fra-1 activity in U937 cells (Fig. 5A). Second, it is known that ERK induces and activates c-Fos for AP-1 activation [29]. Our present study showed that LPS failed to activate c-Fos in gingival fibroblasts (Fig. 5B), but did stimulate it in U937 cells (Fig. 5A). In addition to Fra-1 and c-Fos, our data also showed that LPS stimulated Jun D in U937 cells, but not gingival fibroblasts, despite both control U937 cells and gingival fibroblasts had Jun D DNA-binding activity (Fig. 5A and B). Jun D has been shown to play an important role in AP-1 activity [30, 31]. Thus, it is likely that lack of Fra-1, c-Fos and Jun D activation by LPS is the molecular mechanisms by which LPS failed to activate AP-1 in gingival fibroblasts. To understand why LPS failed to stimulate Fra-1 and c-Fos, it is noteworthy that the baseline activities of nuclear AP-1 subunits such as c-Fos and Fra-1 were found to be extremely low in fibroblasts as compared to U937 cells (Fig. 5B). Although the baseline activity of Jun D was relatively high in gingival fibroblasts and LPS seemed to stimulate it, the increase was not statistically significant. Thus, it is possible that the low baseline activities of nuclear Fra-1 and c-Fos in fibroblasts may preclude the activation by LPS of Fra-1 and c-Fos.
We have demonstrated previously that while LPS activates ERK, JNK, p38 MAPK and NFκB signaling pathways, simvastatin specifically targets LPS-stimulated ERK activation by inhibiting protein isoprenylation-mediated ERK activation in U937 mononuclear cells [17, 32]. Since the ERK signaling pathway is required for LPS-stimulated IL-6 expression by U937 cells as shown by Fig. 2, simvastatin is expected to inhibit LPS-stimulated IL-6 expression by U937 cells, which was confirmed by our present study (Fig. 6A and B). In contrast, simvastatin failed to inhibit LPS-stimulated IL-6 expression by gingival fibroblasts (Fig. 6A and B) because the ERK pathway was not required for LPS-stimulated IL-6 expression (Fig. 2). The finding on simvastatin’s action on LPS-stimulated IL-6 expression by gingival fibroblast strongly supports the notion that the signaling mechanism regulating IL-6 expression by LPS in gingival fibroblasts is different from that in U937 mononuclear cells.
After exploring the difference in the regulatory mechanism controlling LPS-stimulated IL-6 expression between U937 mononuclear cells and gingival fibroblasts, we found that the NFκB pathway played an essential role in the regulation of IL-6 expression by LPS in both types of cells. Our data showed that both Bay117085 and parthenolide, the specific inhibitors of NFκB pathway, exerted marked inhibition on LPS-stimulated IL-6 production by coculture of U937 cells and gingival fibroblasts (Fig. 8A). Thus, our study suggests that the NFκB pathway is a common signaling pathway mediating IL-6 upregulation by LPS in U937 mononuclear cells and gingival fibroblasts and hence a potential target for reducing IL-6 expression in connective tissue contracted with infection by Gram-negative bacteria.
IL-6 is a pleiotropic cytokine involved in the acute phase response, immunity, hematopoiesis, and inflammation [5, 6]. In addition to its role in infectious disease such as periodontal disease [33, 34], IL-6 is also a marker for cardiovascular disease [35, 36], which is considered as an inflammatory disease and a major complication of diabetes. Studies have well documented that the plasma levels of IL-6 and C-reactive protein are strong independent predictors of risk of future cardiovascular events, both in patients with a history of coronary heart disease and in apparently healthy subjects [35]. Furthermore, IL-6 plays an essential role in many other chronic inflammatory diseases such as rheumatoid arthritis, systemic-onset juvenile chronic arthritis, osteoporosis, psoriasis, and autoimmune diseases such as antigen-induced arthritis and experimental allergic encephalomyelitis [5]. Numerous studies have demonstrated that IL-6 is not just a marker for inflammation-associated diseases; it is a major player involved in the initiation and progression of the diseases [35, 37, 38]. Thus, reduction of IL-6 overproduction in inflammation-related diseases is a potentially effective therapeutic approach.
Given that both fibroblasts and monocytes release IL-6 in inflamed connective tissue, targeting monocyte-derived IL-6 alone is apparently not sufficient to reduce IL-6 level. It is important to target the pathways responsible for IL-6 upregulation in both monocytes and fibroblasts. Toward this goal, the present study showed that the NFκB pathway is a common target for both monocytes and fibroblasts to control IL-6 level.
Highlights.
We found the following differences between mononuclear cells and fibroblasts:
Different signaling pathways for LPS-stimulated IL-6 expression.
Different transcriptional regulation for LPS-stimulated IL-6 expression.
We found that NFκB is the common target for reducing IL-6 in both types of cells.
Acknowledgements
This work was supported by the Biomedical Laboratory Research and Development Program of the Department of Veterans Affairs and by NIH grant DE16353 (to Y.H.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Iacopino AM. Periodontitis and diabetes interrelationships: role of inflammation. Annals of periodontology / the American Academy of Periodontology. 2001;6:125–137. doi: 10.1902/annals.2001.6.1.125. [DOI] [PubMed] [Google Scholar]
- 2.Nichols TC, Fischer TH, Deliargyris EN, Baldwin AS., Jr Role of nuclear factor-kappa B (NF-kappa B) in inflammation, periodontitis, and atherogenesis. Annals of periodontology / the American Academy of Periodontology. 2001;6:20–29. doi: 10.1902/annals.2001.6.1.20. [DOI] [PubMed] [Google Scholar]
- 3.Ridker PM, Silvertown JD. Inflammation, C-reactive protein, and atherothrombosis. Journal of periodontology. 2008;79:1544–1551. doi: 10.1902/jop.2008.080249. [DOI] [PubMed] [Google Scholar]
- 4.Page RC. The role of inflammatory mediators in the pathogenesis of periodontal disease. Journal of periodontal research. 1991;26:230–242. doi: 10.1111/j.1600-0765.1991.tb01649.x. [DOI] [PubMed] [Google Scholar]
- 5.Ishihara K, Hirano T. IL-6 in autoimmune disease and chronic inflammatory proliferative disease. Cytokine Growth Factor Rev. 2002;13:357–368. doi: 10.1016/s1359-6101(02)00027-8. [DOI] [PubMed] [Google Scholar]
- 6.Kishimoto T. Interleukin-6: discovery of a pleiotropic cytokine. Arthritis Res Ther. 2006;8(Suppl 2):S2. doi: 10.1186/ar1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tanaka T, Narazaki M, Kishimoto T. Therapeutic Targeting of the Interleukin-6 Receptor. Annual review of pharmacology and toxicology. 2011 doi: 10.1146/annurev-pharmtox-010611-134715. [DOI] [PubMed] [Google Scholar]
- 8.Li Y, Samuvel DJ, Sundararaj KP, Lopes-Virella MF, Huang Y. IL-6 and high glucose synergistically upregulate MMP-1 expression by U937 mononuclear phagocytes via ERK1/2 and JNK pathways and c-Jun. Journal of cellular biochemistry. 2010;110:248–259. doi: 10.1002/jcb.22532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patil C, Zhu X, Rossa C, Jr, Kim YJ, Kirkwood KL. p38 MAPK regulates IL-1beta induced IL-6 expression through mRNA stability in osteoblasts. Immunological investigations. 2004;33:213–233. doi: 10.1081/imm-120034231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blanchard F, Duplomb L, Baud'huin M, Brounais B. The dual role of IL-6-type cytokines on bone remodeling and bone tumors. Cytokine & growth factor reviews. 2009;20:19–28. doi: 10.1016/j.cytogfr.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 11.Sundararaj KP, Samuvel DJ, Li Y, Sanders JJ, Lopes-Virella MF, Huang Y. Interleukin-6 released from fibroblasts is essential for up-regulation of matrix metalloproteinase-1 expression by U937 macrophages in coculture: cross-talking between fibroblasts and U937 macrophages exposed to high glucose. The Journal of biological chemistry. 2009;284:13714–13724. doi: 10.1074/jbc.M806573200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sundstrom C, Nilsson K. Establishment and characterization of a human histiocytic lymphoma cell line (U-937) Int J Cancer. 1976;17:565–577. doi: 10.1002/ijc.2910170504. [DOI] [PubMed] [Google Scholar]
- 13.Samuvel DJ, Sundararaj KP, Li Y, Lopes-Virella MF, Huang Y. Adipocyte-mononuclear cell interaction, Toll-like receptor 4 activation, and high glucose synergistically up-regulate osteopontin expression via an interleukin 6-mediated mechanism. The Journal of biological chemistry. 2010;285:3916–3927. doi: 10.1074/jbc.M109.033951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu Y, Cheng L, Hochleitner BW, Xu Q. Activation of mitogen-activated protein kinases (ERK/JNK) and AP-1 transcription factor in rat carotid arteries after balloon injury. Arteriosclerosis, thrombosis, and vascular biology. 1997;17:2808–2816. doi: 10.1161/01.atv.17.11.2808. [DOI] [PubMed] [Google Scholar]
- 15.Jin YJ, Park I, Hong IK, Byun HJ, Choi J, Kim YM, Lee H. Fibronectin and vitronectin induce AP-1-mediated matrix metalloproteinase-9 expression through integrin alpha(5)beta(1)/alpha(v)beta(3)-dependent Akt, ERK and JNK signaling pathways in human umbilical vein endothelial cells. Cellular signalling. 2011;23:125–134. doi: 10.1016/j.cellsig.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 16.Shin YH, Son KN, Lee GW, Kwon BS, Kim J. Transcriptional regulation of human CC chemokine CCL15 gene by NF-kappaB and AP-1 elements in PMA-stimulated U937 monocytoid cells. Biochimica et biophysica acta. 2005;1732:38–42. doi: 10.1016/j.bbaexp.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 17.Sundararaj KP, Samuvel DJ, Li Y, Nareika A, Slate EH, Sanders JJ, Lopes-Virella MF, Huang Y. Simvastatin suppresses LPS-induced MMP-1 expression in U937 mononuclear cells by inhibiting protein isoprenylation-mediated ERK activation. Journal of leukocyte biology. 2008;84:1120–1129. doi: 10.1189/jlb.0108064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He YP, Zhao LY, Zheng QS, Liu SW, Zhao XY, Lu XL, Niu XL, Li X. Involvement of ERK and AKT signaling in the growth effect of arginine vasopressin on adult rat cardiac fibroblast and the modulation by simvastatin. Molecular and cellular biochemistry. 2008;317:33–41. doi: 10.1007/s11010-008-9802-9. [DOI] [PubMed] [Google Scholar]
- 19.Fazal F, Bijli KM, Minhajuddin M, Rein T, Finkelstein JN, Rahman A. Essential role of cofilin-1 in regulating thrombin-induced RelA/p65 nuclear translocation and intercellular adhesion molecule 1 (ICAM-1) expression in endothelial cells. The Journal of biological chemistry. 2009;284:21047–21056. doi: 10.1074/jbc.M109.016444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zerfaoui M, Errami Y, Naura AS, Suzuki Y, Kim H, Ju J, Liu T, Hans CP, Kim JG, Abd Elmageed ZY, Koochekpour S, Catling A, Boulares AH. Poly(ADP-ribose) polymerase-1 is a determining factor in Crm1-mediated nuclear export and retention of p65 NF-kappa B upon TLR4 stimulation. Journal of immunology (Baltimore, Md. : 1950) 2010;185:1894–1902. doi: 10.4049/jimmunol.1000646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghashghaeinia M, Toulany M, Saki M, Bobbala D, Fehrenbacher B, Rupec R, Rodemann HP, Ghoreschi K, Rocken M, Schaller M, Lang F, Wieder T. The NFkB pathway inhibitors Bay 11-7082 and parthenolide induce programmed cell death in anucleated Erythrocytes. Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology. 2011;27:45–54. doi: 10.1159/000325204. [DOI] [PubMed] [Google Scholar]
- 22.Eugui EM, DeLustro B, Rouhafza S, Ilnicka M, Lee SW, Wilhelm R, Allison AC. Some antioxidants inhibit, in a co-ordinate fashion, the production of tumor necrosis factor-alpha, IL-beta, and IL-6 by human peripheral blood mononuclear cells. Int Immunol. 1994;6:409–422. doi: 10.1093/intimm/6.3.409. [DOI] [PubMed] [Google Scholar]
- 23.Gutierrez-Venegas G, Kawasaki-Cardenas P, Arroyo-Cruz SR, Maldonado-Frias S. Luteolin inhibits lipopolysaccharide actions on human gingival fibroblasts. European journal of pharmacology. 2006;541:95–105. doi: 10.1016/j.ejphar.2006.03.069. [DOI] [PubMed] [Google Scholar]
- 24.Kobayashi H, Yoshida R, Kanada Y, Fukuda Y, Yagyu T, Inagaki K, Kondo T, Kurita N, Suzuki M, Kanayama N, Terao T. Suppression of lipopolysaccharide-induced cytokine production of gingival fibroblasts by a soybean, Kunitz trypsin inhibitor. Journal of periodontal research. 2005;40:461–468. doi: 10.1111/j.1600-0765.2005.00824.x. [DOI] [PubMed] [Google Scholar]
- 25.Wang PL, Azuma Y, Shinohara M, Ohura K. Toll-like receptor 4-mediated signal pathway induced by Porphyromonas gingivalis lipopolysaccharide in human gingival fibroblasts. Biochemical and biophysical research communications. 2000;273:1161–1167. doi: 10.1006/bbrc.2000.3060. [DOI] [PubMed] [Google Scholar]
- 26.Wang PL, Ohura K. Porphyromonas gingivalis lipopolysaccharide signaling in gingival fibroblasts-CD14 and Toll-like receptors. Critical reviews in oral biology and medicine : an official publication of the American Association of Oral Biologists. 2002;13:132–142. doi: 10.1177/154411130201300204. [DOI] [PubMed] [Google Scholar]
- 27.Matsushita K, Motani R, Sakuta T, Nagaoka S, Matsuyama T, Abeyama K, Maruyama I, Takada H, Torii M. Lipopolysaccharide enhances the production of vascular endothelial growth factor by human pulp cells in culture. Infection and immunity. 1999;67:1633–1639. doi: 10.1128/iai.67.4.1633-1639.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dhar A, Young MR, Colburn NH. The role of AP-1, NF-kappaB and ROS/NOS in skin carcinogenesis: the JB6 model is predictive. Molecular and cellular biochemistry. 2002;234–235:185–193. [PubMed] [Google Scholar]
- 29.Lee ZH, Kim HH. Signal transduction by receptor activator of nuclear factor kappa B in osteoclasts. Biochemical and biophysical research communications. 2003;305:211–214. doi: 10.1016/s0006-291x(03)00695-8. [DOI] [PubMed] [Google Scholar]
- 30.Hamada H, Sawyer RT, Kittle LA, Newman LS. Beryllium-stimulation does not activate transcription factors in a mouse hybrid macrophage cell line. Toxicology. 2000;143:249–261. doi: 10.1016/s0300-483x(99)00183-3. [DOI] [PubMed] [Google Scholar]
- 31.Medicherla R, Leers-Sucheta S, Luo Y, Azhar S. Impaired activation of AP-1 and altered expression of constituent proteins in rat adrenal during ageing. Mechanisms of ageing and development. 2001;122:1169–1186. doi: 10.1016/s0047-6374(01)00258-5. [DOI] [PubMed] [Google Scholar]
- 32.Nareika A, Maldonado A, He L, Game BA, Slate EH, Sanders JJ, London SD, Lopes-Virella MF, Huang Y. High glucose-boosted inflammatory responses to lipopolysaccharide are suppressed by statin. Journal of periodontal research. 2007;42:31–38. doi: 10.1111/j.1600-0765.2006.00911.x. [DOI] [PubMed] [Google Scholar]
- 33.Rusconi F, Parizzi F, Garlaschi L, Assael BM, Sironi M, Ghezzi P, Mantovani A. Interleukin 6 activity in infants and children with bacterial meningitis. The Collaborative Study on Meningitis. Pediatr Infect Dis J. 1991;10:117–121. doi: 10.1097/00006454-199102000-00008. [DOI] [PubMed] [Google Scholar]
- 34.Geivelis M, Turner DW, Pederson ED, Lamberts BL. Measurements of interleukin-6 in gingival crevicular fluid from adults with destructive periodontal disease. J Periodontol. 1993;64:980–983. doi: 10.1902/jop.1993.64.10.980. [DOI] [PubMed] [Google Scholar]
- 35.Rattazzi M, Puato M, Faggin E, Bertipaglia B, Zambon A, Pauletto P. C-reactive protein and interleukin-6 in vascular disease: culprits or passive bystanders? J Hypertens. 2003;21:1787–1803. doi: 10.1097/00004872-200310000-00002. [DOI] [PubMed] [Google Scholar]
- 36.Kristiansen OP, Mandrup-Poulsen T. Interleukin-6 and diabetes: the good, the bad, or the indifferent? Diabetes. 2005;54(Suppl 2):S114–S124. doi: 10.2337/diabetes.54.suppl_2.s114. [DOI] [PubMed] [Google Scholar]
- 37.Moutsopoulos NM, Madianos PN. Low-grade inflammation in chronic infectious diseases: paradigm of periodontal infections. Ann N Y Acad Sci. 2006;1088:251–264. doi: 10.1196/annals.1366.032. [DOI] [PubMed] [Google Scholar]
- 38.Lowe GD. The relationship between infection, inflammation, and cardiovascular disease: an overview. Ann Periodontol. 2001;6:1–8. doi: 10.1902/annals.2001.6.1.1. [DOI] [PubMed] [Google Scholar]