Abstract
Objective
The goal of this study is to describe the complications and toxicities for post-operative radiation therapy in Fanconi anemia patients.
Design
Cohort study.
Setting
Patients treated at community and tertiary care hospitals throughout the United States.
Patients
Fanconi anemia patients enrolled in the International Fanconi Anemia Registry (IFAR) who developed head and neck squamous cell carcinoma and received post-operative radiation.
Main Outcome Measures
Demographics of Fanconi anemia patients, radiation doses, and radiation toxicities.
Results
12 Fanconi anemia patients (7 male, 5 female) were identified. Patients developed cancers at a mean age of 35.5 years (range 20 to 48). Sites of primary cancer were oral cavity (8/12), larynx (2/12), pharynx (1/12), and unknown (1/12). Median radiation dose was 5590 cGy (range: 2500 to 7020). The most common toxicities were mucositis (9/12), dysphagia (8/12), and pancytopenia (6/12). Other complications included esophageal stenosis, laryngeal edema, and wound breakdown. Radiotherapy could not be completed in 5/12 cases. Overall 8/12 patients died, 4 during the course of radiation. The post-operative disease-free survival time ranged from 0 to 55 months.
Conclusions
Fanconi anemia patients have a high rate of complications to radiation therapy. Common radiation toxicities, particularly mucositis, are especially prevalent and difficult to manage in this population. Pancytopenia is common and may lead to further complications, particularly bleeding and infection. Overall survival is poor. Further study of FA patients’ response to radiation should be attempted in order to establish appropriate doses to balance treating disease while limiting toxicity.
INTRODUCTION
Fanconi anemia (FA) is a rare recessive disorder (1–2:100,000 births)1 caused by mutations in one of at least 14 known genes in the FA pathway2,3. Genes in this pathway are involved in interstrand cross-link and double-strand break DNA repair4. FA is characterized clinically by aplastic anemia/ pancytopenia, congenital malformations (short stature, hypoplastic thumbs, café-au-lait spots, cardiac and renal anomalies), sensitivity to DNA cross-linking agents and increased risk of malignancy1. Leukemias are the most common cancers in FA patients, but patients are also at a significantly increased risk of developing solid cancers, with a 28% cumulative incidence of solid cancers by the age of 405. In particular, head and neck squamous cell carcinomas (HNSCC) are significantly more common in FA patients6, with some reports calculating a several hundred-fold risk increase7. As bone marrow transplants, leukemia therapies and other recent advances have prolonged life in these patients8, an increasing number are developing HNSCC.
Standard treatment for HNSCC incorporates surgery in combination with radiation and chemotherapy, depending on the tumor characteristics. Radiation therapy is known to potentially induce numerous side-effects and complications in the general population, including mucositis, dysphagia, taste changes and fatigue. Rarer complications of osteonecrosis, fibrosis, and esophageal stenosis exist as well.
In FA patients, sensitivity to chemotherapy agents (particularly cisplatin and mitomycin C) is well-known1, and is thus commonly avoided in post-operative treatment for HNSCC. Adjuvant radiation therapy is recommended to treat patients with high stage tumors (stage III–IV). However, post-operative sensitivity to radiation therapy in FA patients is not as well characterized. While some case reports mention radiation toxicity and complications9–11, others do not document toxicities or suggest mild toxicity12–14. Due to evolving treatments in radiation techniques and uncertainty in identifying safe doses in an FA population, it is important to document radiation dose levels, toxicities, and outcomes from FA patients who have received radiation therapy. Given their sensitivity to DNA damaging processes, understanding how FA patients respond to radiation is important in guiding their therapy.
METHODS
Fanconi Anemia Registry
The International Fanconi Anemia Registry (IFAR) was instituted in 1982 as a repository to collect clinical and genetic information from FA patients throughout the world. It currently has enrolled approximately 1200 families. The registry collects medical and other pertinent information from these patients by contacting them or surviving family members on a regular basis.
Patient Data Collection
Overall, we identified and collected information on 12 FA patients with HNSCC who received post-operative radiation. We obtained patient or family consent when appropriate and obtained all available histories, medical, surgical and radiation records from their respective treating hospitals. The diagnosis of FA was made by diepoxybutane breakage test, as previously described15. FA complementation groups were documented when possible.
Statistical Analysis
Kaplan-Meier was used to calculate disease-free survival and overall survival. Disease-free survival was calculated in months post-operation without primary disease or recurrence of disease. Overall survival time was calculated in months from date of surgery until death, or to date in surviving patients.
RESULTS
Patient Demographic Information
Overall we studied five female and seven male patients who developed HNSCC and received post-operative radiation therapy (Table 1). Seven patients were in complementation group FA-A, one in FA-C, one in FA-J, and one in FA-P. Two are currently untyped. The median age for development of HNSCC was 36.3 years, the mean was 35.5 years, (range: 20.9 to 48.5 years). All 12 cancers were stage IV. Sites of cancers included the oral cavity (eight cases), larynx (two cases), pharynx (one case), and unknown primary (one case). All patients underwent initial surgery, including lymph node dissection, and received post-operative radiation therapy. Patients’ records were screened for environmental risk factors known to enhance the risk of HNSCC: tobacco and previous bone marrow transplant (BMT), of which two had received previous BMTs and four had history of smoking (Table 1). In addition, five patients suffered from local or regional recurrence of cancer.
Table 1.
Fanconi Anemia Patient Demographics
| Patient | Gender | Onset Age |
FA Group | Ethnic Group |
Environmental Factors |
Primary Site | T/N/M (Stage) |
|---|---|---|---|---|---|---|---|
| 1 | M | 48.5 | J | C | Oral Cav (Retromolar trigone) | T4N2bM0 (IV) | |
| 2 | F | 30.2 | A | ME | Oral Cav (Buccal Gingiva) | T4N0M0 (IV) | |
| 3 | M | 44.9 | A | C | Tobacco | Oral Cav (Alveolus) | T3N2bM0 (IV) |
| 4 | F | 37.8 | A | AA | Oral Cav (Alveolus) | T4N1M0 (IV) | |
| 5 | F | 26.7 | C | AA | BMT | Oral Cav (Alveolus) | T4N2cM0 (IV) |
| 6 | M | 28.1 | Untyped | C | Tobacco | Oropharynx | T3N2bM0 (IV) |
| 7 | F | 42.0 | A | H | Oral Cav (Mandible) | T4N2bM0 (IV) | |
| 8 | F | 40.9 | A | H | Tobacco | Unknown | TxN2bM0 (IV) |
| 9 | M | 29.8 | A | C | Larynx | T1N2bM0 (IV) | |
| 10 | M | 20.9 | P | C | Tobacco | Oral Cav (Tongue) | T4N2cM0 (IV) |
| 11 | M | 34.7 | A | C | Oral Cav (Tongue) | T4N1M0 (IV) | |
| 12 | M | 42.0 | Untyped | C | BMT | Larynx | T?N2bM0 (IV) |
Abbreviations: M = male, F = female, FA = Fanconi anemia, T = tumor, N = node, M = metastasis, C = Caucasian, H = Hispanic, ME = Middle-Eastern, AA = African-American, BMT = bone marrow transplant
Radiation Doses and Toxicities
The total radiation dose ranged from 2500 cGy to 7020 cGy (Table 2), with a median dose of 5590 cGy. Dose per fraction ranged from 170 cGy to 200 cGy. Number of fractions ranged from 20 to 39. Total treatment days ranged from 31 to 70 days.
Table 2.
Post-operative Radiation Doses
| Patient | Total Dose (cGy) | Dose per fraction / number of fractions / treatment days |
|---|---|---|
| 4000 | 200 / 20 / 33d | |
| 2 | 2500 | |
| 3 | 6100 | 55d |
| 4 | 5600 | |
| 5 | unfinished | |
| 6 | 5100 + chemo | 52d |
| 7 | 6460 | 170 / 30 / 70d |
| 8 | 6180 | 200 / 30 / 39d |
| 9 | unknown | |
| 10 | 7020 + chemo | 180 / 39 / 50d |
| 11 | 4240 + chemo | 200 / 20 / 28d |
| 12 | 5580 | 180 / 22 / 31d |
Abbreviations: cGy = centigray, d = days, chemo = chemotherapy
The most prevalent complications during radiation treatment were high-grade (grade 3 or above) mucositis (9/12), dysphagia (8/12), and hematologic abnormalities (6/12). Other complications included asystole/ cardiac arrest, wound site breakdown, fibrosis, local edema, sepsis, tracheal stenosis, and radiation pneumonitis (Table 3). For five patients, radiation therapy needed to be prematurely halted or interrupted due to toxicities associated with the treatment, primarily due to mucositis.
Table 3.
Post-operative Radiation Complications
| Patient | High- grade mucositis |
Dysphagia | Cytopenia | Premature termination/ interruption of RT |
Other Complications | Status / Disease-free interval (mo) / Post-op survival (mo) |
|---|---|---|---|---|---|---|
| X | X | X | X | Sepsis | D / 0 / 2 | |
| 2 | X | Recurrence, sepsis | D / 2 / 2 | |||
| 3 | X | X | Graft site breakdown, mandibular hardware removal, recurrence | D / 16 / 54 | ||
| 4 | X | X | X | Hemorrhage, pleural thickening, sepsis | D / 55 / 55 | |
| 5 | X | X | Dyspnea, asystole/ cardiac arrest | D / 2 / 2 | ||
| 6 | X | X | X | Tracheal stenosis, radiation pneumonitis, recurrent pneumonia, recurrence | D / 4 / 12 | |
| 7 | X | X | A / 21+ / 21+ | |||
| 8 | X | X | Hemorrhage, trismus, fibrosis, esophageal stenosis, oral dryness | A / 25+ / 25+ | ||
| 9 | X | X | Recurrence | D / 5 / 77 | ||
| 10 | X | X | Dermatitis, sepsis | D / 10 / 10 | ||
| 11 | X | X | X | X | Wound breakdown, dermatitis, hemorrhage | A / 15+ / 15+ |
| 12 | X | X | Laryngeal edema, fibrosis, esophageal stenosis, recurrence | A / 33 / 129+ | ||
| Totals | 9/12 (75%) | 8/12 (67%) | 6/12 (50%) | 5/12 (42%) | / 15.7 / 33/7 (avg) / (avg) |
Abbreviations: A = Alive, D = Deceased, RT = radiotherapy, mo = months, avg = average
Disease-free survival ranged from 0 to 55 months (Fig. 1A). Overall survival time ranged from 2 months to 129 months (Fig. 1B). Overall, 8 patients are deceased, 4 from complications while receiving radiation therapy. Three patients died from sepsis. One died from cardiac arrest during the course of radiation therapy. Radiation to the head and neck is not known to cause cardiac arrest, and this patient likely had other underlying medical issues.
Figure 1.
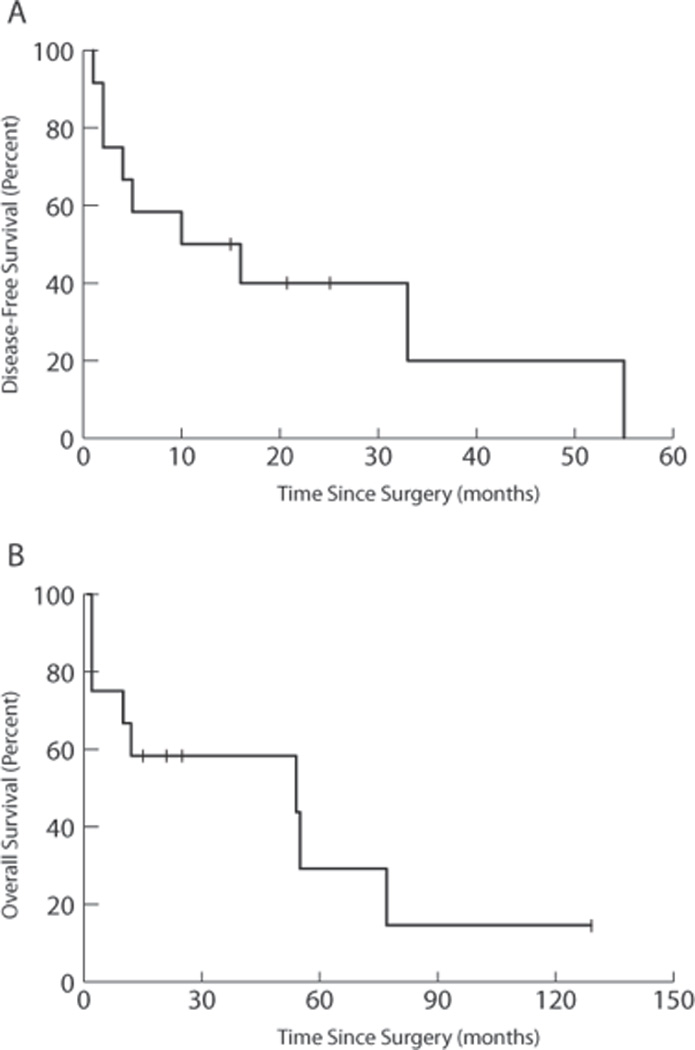
Kaplan-Meier survival curves for Disease-free Survival (A) and Overall Survival (B) for FA patients with HNSCC. Survival time is measured in months from date of surgery.
DISCUSSION
FA patients, given their predisposition to sensitivity toward DNA-damaging processes like radiation, may have increased toxicities. Case reports to date have had differing accounts of radiation sensitivity for HNSCC in FA patients. We sought to study such patients in our IFAR cohort to gain a better sense of FA patient response to radiation and to provide more insight into treatment-related toxicities.
Overall, FA patients developed HNSCC at a very young age compared to the general population, with a mean of 35.5 years in our population versus 63 years in a general population16. In addition, HNSCC developed often without history of tobacco, a common cause of HNSCC in a general population (4/12 in our population versus 75–85% in general HNSCC17). These findings suggest an increased screening and awareness of HNSCC should be maintained in this population starting at a young age. Reports in literature document development of HNSCC at ages as young as 13 (average 28) in FA patients18. Conversely, patients who develop HNSCC at young ages may be appropriate candidates for screening for FA.
A history of BMT is thought to increase the risk of developing subsequent solid malignancies, particularly HNSCC18,19. In this cohort, two patients had a prior history of BMT, suggesting that although this may be a risk, many patients without BMTs develop HNSCC. Nevertheless, particularly rigorous screening for HNSCC should be performed in FA patients who have a history of BMT.
Tumors were most commonly located in the oral cavity (8/12). This is consistent with reports in the literature that FA patients are especially prone to develop HNSCC of the in the oral cavity.
The overall mortality was high in these patients (8/12), with a mean overall survival of 33.7 months. Disease-free survival was poor, with a mean disease-free interval of 15.7 months. Patients suffered complications even at low doses of radiation, and no minimal safe dose of radiation was seen in this cohort, as complications arose in one patient at 2500 cGy. Compared to a target minimum dose of 5760 cGy for post-operative patients20, FA patients in this cohort received a median dose of 5590 cGy and mean dose of 5278 cGy. This decreased average dose was due to the intolerance of these FA patients to the toxicity of the treatment, requiring termination or interruption of therapy (5/12). Since FA patient tumors carry FA mutations, the tumors themselves may be more sensitive to radiation, and consideration of lower doses of radiation to achieve cure may be appropriate.
Radiation treatment for head and neck cancers is associated with numerous complications in general populations, with the most common being mucositis, dysphagia, dry mouth, and taste changes. High-grade mucositis is reported to range from 34% to 57% in the general population21. Mucositis was seen in 9/12 (75%) patients in our cohort (Table 3), suggesting an enhanced risk in FA patients. In addition, pancytopenia, which is a rare complication in the general population, was experienced at high rates (6/12) in our FA population. The pancytopenia is of particular concern in dealing with post-operative complications in these patients as it can lead to bleeding complications, fatigue, poor wound healing, and infection. Due to the underlying stem-cell problems in these patients, time to recovery of normal blood counts may also be delayed. In this cohort, three patients developed sepsis, one had recurrent pneumonia, and two bleeding complications. Blood counts should be monitored closely during radiation therapy to avoid low WBC levels, thus decreasing risk for infectious complications.
The addition of chemotherapy to radiation in three patients in this cohort resulted in significant complications (including interruption or cessation of therapy in two), suggesting special care may need to be taken in considering chemotherapy in addition to radiation in FA patients.
It is important to incorporate post-operative radiation therapy in stage III and IV HNSCC, as it has been shown to improve cancer-specific and overall 5-year survival rates22. Radiation therapy in FA patients can be successful, and was completed in 7/12 cases in this study. Based on this case series, however, it is important to be aware of the complications that may present in FA patients receiving post-operative radiation therapy, particularly hematologic abnormalities and high-grade mucositis. We recommend frequent monitoring of hematologic counts during radiation therapy. In particular, careful monitoring of WBC counts is needed to avoid potential infections and sepsis. Close monitoring of mucositis should be performed as well as severe mucositis can limit completion of radiation therapy. For patients with significant complications, temporarily suspending therapy may be needed to avoid worsening of the complications and to allow for recovery. In developing radiation treatment plans, longer courses at lower daily doses (150–180 cGy per fraction) may be considered in order to decrease risk of developing significant toxicities.
Although our study is limited to 12 FA patients, these cases suggest an enhanced sensitivity to post-operative radiation therapy for HNSCC in FA patients. Future reports and studies in FA patients should be collected to determine if particular FA groups are more prone to radiation complications, and if particular dose levels or radiation treatment parameters cause more complications. Uncovering minimal safe doses and identifying risk factors for radiation toxicity can provide valuable information in treating these patients. In future cases, it will be crucial to adjust radiation therapy to balance the risks of undertreating the cancer versus the complications of radiation.
ACKNOWLEDGMENTS
We are grateful to the patients and their families for their participation in this study. This project was supported by Grant Award Number UL1RR024143 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) and NIH Roadmap for Medical Research, and its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. A.S. is supported by the Burroughs Wellcome Fund Career Award for Medical Scientists and is a Rita Allen Foundation and an Irma T. Hirschl scholar.
Footnotes
This study was presented at the annual meeting of the American Head and Neck Society, Chicago, IL, April 27-28, 2011.
REFERENCES
- 1.Auerbach AD, Buchwald M, Jeonje H. Scriver CR, Sly WS, Childs B, et al. The Metabolic and Molecular Bases of Inherited Disease. 8th. Vol 1. New York, NY: McGraw- Hill; 2001. Fanconi anemia; pp. 753–768. [Google Scholar]
- 2.Levitus M, Joenje H, de Winter JP. The Fanconi anemia pathway of genomic maintenance. Cell Oncol. 2006;28:3–29. doi: 10.1155/2006/974975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim Y, Lach F, Desetty R, Hanenberg H, Auerbach AD, Smogorzewska A. Mutations of the SLX4 gene in Fanconi anemia. Nat Genet. 2011;43:142–146. doi: 10.1038/ng.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Knipscheer P, Raschle M, Smogorzewska A, et al. The Fanconi anemia pathway promotes replication-dependent DNA interstrand cross-link repair. Science. 2009;326:1698–1701. doi: 10.1126/science.1182372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kutler DI, Singh B, Satagopan J, et al. A 20-year perspective on the International Fanconi Anemia Registry (IFAR) Blood. 2003;101:1249–1256. doi: 10.1182/blood-2002-07-2170. [DOI] [PubMed] [Google Scholar]
- 6.Kutler DI, Auerbach AD, Satagopan J, et al. High incidence of head and neck squamous cell carcinoma in patients with Fanconi anemia. Arch Otolaryngol Head Neck Surg. 2003;129:106–112. doi: 10.1001/archotol.129.1.106. [DOI] [PubMed] [Google Scholar]
- 7.Rosenberg PS, Alter BP, Ebell W. Cancer risk in Fanconi anemia: findings from the German Fanconi Anemia Registry. Haematologica. 2008;93:511–517. doi: 10.3324/haematol.12234. [DOI] [PubMed] [Google Scholar]
- 8.Alter BP. Inherited bone marrow failure syndromes. In: Nathan DG, Orkin SH, Ginsburg D, Look AT, editors. Nathan and Oski’s Hematology of Infancy and Childhood. 6th ed. Vol 1. Philadelphia, PA: Saunders; 2003. pp. 280–365. 2003: 280–365. [Google Scholar]
- 9.Bremer M, Schindler D, Gross M, Dork T, Morlot S, Karstens JH. Fanconi's anemia and clinical radiosensitivity report on two adult patients with locally advanced solid tumors treated by radiotherapy. Stahlenther Onkol. 2003;179:748–753. doi: 10.1007/s00066-003-1099-8. [DOI] [PubMed] [Google Scholar]
- 10.Marcou Y, D'Andrea A, Jeggo PA, Plowman PN. Normal cellular radiosensitivity in an adult Fanconi anaemia patient with marked clinical radiosensitivity. Radiother Oncol. 2001;60:75–79. doi: 10.1016/s0167-8140(01)00370-x. [DOI] [PubMed] [Google Scholar]
- 11.Lustig JP, Lugassy G, Neder A, Sigler E. Head and neck carcinoma in Fanconi's anaemia - report of a case and review of the literature. Oral Oncol Eur J Cancer. 1995;31B:68–72. doi: 10.1016/0964-1955(94)00044-5. [DOI] [PubMed] [Google Scholar]
- 12.Budrukkar A, Shahid T, Murthy V, et al. Squamous cell carcinoma of base of tongue in a patient with Fanconi's anemia treated with radiation therapy: case report and review of literature. Head Neck. 2010;32:1422–1427. doi: 10.1002/hed.21211. [DOI] [PubMed] [Google Scholar]
- 13.Snow DG, Campbell JB, Smallman LA. Fanconi's anaemia and post-cricoid carcinoma. J Larungol Otol. 1991;105:125–127. doi: 10.1017/s0022215100115130. [DOI] [PubMed] [Google Scholar]
- 14.Vaitiekaitis AS, Grau WH. Squamous cell carcinoma of the mandible in Fanconi anemia: report of case. J Oral Surg. 1980;38:372–373. [PubMed] [Google Scholar]
- 15.Auerbach AD. Fanconi anemia diagnosis and the diepoxybutane (DEB) test. Exp Hematol. 1993;21:731–733. [PubMed] [Google Scholar]
- 16.Ries LAG, Eisner MP, Kosary CL, et al. SEER Cancer Statistics Review: 1975–2002. Bethesda, MD: National Cancer Institute; 2005. [Google Scholar]
- 17.Hashibe M, Brennan P, Benhamou S, et al. Alcohol drinking in never users of tobacco, cigarette smoking in never drinkers, and the risk of head and neck cancer: pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. J Natl Cancer Inst. 2007;99:777–789. doi: 10.1093/jnci/djk179. [DOI] [PubMed] [Google Scholar]
- 18.Alter BP. Cancer in Fanconi anemia: 1927–2001. Cancer. 2003;97:425–440. doi: 10.1002/cncr.11046. [DOI] [PubMed] [Google Scholar]
- 19.Guardiola P, Socie G, Li X, et al. Acute graft-versus-host disease in patients with Fanconi anemia or acquired aplastic anemia undergoing bone marrow transplantation from HLA-identical sibling donors: risk factors and influence on outcome. Blood. 2004;103:73–77. doi: 10.1182/blood-2003-06-2146. [DOI] [PubMed] [Google Scholar]
- 20.Peters LJ, Goepfert H, Ang KK, et al. Evaluation of the dose for postoperative radiation therapy of head and neck cancer: first report of a prospective randomized trial. Int J Radiat Oncol Biol Phys. 1993;26:3–11. doi: 10.1016/0360-3016(93)90167-t. [DOI] [PubMed] [Google Scholar]
- 21.Trotti A, Bellm LA, Epstein JB, et al. Mucositis incidence, severity and associated outcomes in patients with head and neck cancer receiving radiotherapy with or without chemotherapy: a systematic literature review. Radiother Oncol. 2003;66:253–262. doi: 10.1016/s0167-8140(02)00404-8. [DOI] [PubMed] [Google Scholar]
- 22.Lavaf A, Genden EM, Cesaretti JA, Packer S, Kao J. Adjuvant radiotherapy improves overall survival for patients with lymph node-positive head and neck squamous cell carcinoma. Cancer. 2008;112:535–543. doi: 10.1002/cncr.23206. [DOI] [PubMed] [Google Scholar]


