Abstract
Abscisic acid (ABA) plays a major role in plant development and adaptation to severe environmental conditions. ABA evokes cellular events to regulate stomatal apertures and thus contributes to the plant’s ability to respond to abiotic stresses. Reactive oxygen species (ROS) are produced in response to ABA and mediate ABA-induced stomatal closure. We have shown that two MAP kinases, MPK9 and MPK12, are highly and preferentially expressed in guard cells and function as positive regulators of ROS-mediated ABA signaling in guard cells. Cell biological and electrophysiological analyses demonstrated that MPK9 and MPK12 act downstream of ROS and cytosolic Ca2+ and upstream of anion channels in the guard cell ABA signaling cascade. Plant pathogens use stomata as the primary gateway to enter into their hosts, and previous studies have indicated crosstalk between ABA and defense signaling. Here we show that mpk9-1/12-1 double mutants are highly susceptible to Pseudomonas syringae DC3000 compared to WT plants. These results suggest that the regulation of stomatal apertures by MPK9 and MPK12 contributes to the first line of defense against pathogens.
Keywords: abscisic acid, biotic stress, crosstalk, defense, guard cell, MAP kinase
In response to environmental stress, the phytohormone abscisic acid (ABA) triggers a variety of adaptive mechanisms such as stomatal closure by modulating ion fluxes across membranes. ABA is perceived and transduced by the core receptor module composed of the ABA receptor PYR/PYL/RCAR and the clade A protein phosphatase 2Cs (PP2Cs), which inhibit SNF1-related protein kinase 2 (SnRK2).1,2 The binding of ABA to the receptor promotes the inactivation of PP2Cs, which in turn allows subsequent phosphorylation events by SnRK2s, possibly in concert with other kinases such as calcium-dependent protein kinases.3,4 Previous studies have identified a few SnRK2 target proteins including ABA responsive element binding transcription factors,5,6 SLAC1 anion channels7,8 and AtrbohF NADPH oxidase.9
One of the downstream cellular events mediated by ABA is the production of reactive oxygen species (ROS) that function as second messengers and mediate a variety of cellular responses.10 The ROS-dependent ABA signaling pathway leads to an elevation in cytosolic calcium concentration ([Ca2+]cyt) and the subsequent activation of anion channels in guard cells.11 Among the 10 different mechanisms by which ROS can be generated in plant cells,12 guard cell-expressed AtrbohF and AtrbohD NADPH oxidases have been shown to be responsible for ABA-triggered ROS production and subsequent ABA signaling.13 We have identified two MAP kinases, MPK9 and MPK12, which are preferentially and highly expressed in guard cells and function as positive regulators of ROS-mediated ABA signaling.14 Electrophysiological and cell biological studies of an mpk9–1/12 double mutant showed that MPK9 and MPK12 act downstream of [Ca2+]cyt and upstream of anion channels.14 We have also demonstrated that the MPK12 kinase activity is enhanced by both ABA and H2O2 treatment.
Stomata are entry gates for pathogens and the regulation of stomatal apertures by ABA plays a role in the FLS2-mediated immune response.15 Conserved pathogen-specific molecules called pathogen/microbe-associated molecular patterns (PAMPs) such as flagellin, lipopolysaccharide, oligogalacturonic acid, and chitosan can trigger stomatal closure or prevent light-induced stomatal opening.16,17 Arabidopsis recognizes the invasion through stomata by the pathogenic bacteria Pseudomonas syringae pv Tomato (Pst) DC3000 and activates ABA signaling cascades leading to stomatal closure to prevent further bacterial infection.15 Such bacterial-induced stomatal closure requires SnRK2 kinases, regulation of ion channels and/or activation of MAPKs.15,18,19 However, the phytotoxin coronatine produced by P.syringae is able to reopen stomata by suppressing PAMP-induced ABA signaling in order to facilitate further infection.15 Interestingly, an expression profiling analysis combined with a study using transgenic plants has suggested that once delivered inside the host cells, P. syringae hijacks the ABA signaling cascade to induce disease.20 These studies indicate complex crosstalk between ABA and defense signaling.
In order to investigate the possible contribution of MPK9 and MPK12 to stomatal closure in response to plant pathogens, we challenged mpk9–1/12–1 mutant plants with P. syringae DC3000 administered via spray-inoculation. mpk9–1/12-1 mutant plants were extremely susceptible to P. syringae and showed significantly more severe chlorosis and necrosis than wild-type plants (Fig. 1A). The multiplication of bacterial cells in mpk9–1/12–1 was about 40-fold higher than that in wild-type plants (Fig. 1B). This result indicates that the pathogen successfully invaded and grew inside the leaf of mpk9–1/12–1. Interestingly, when P. syringae DC3000 was infiltrated, mpk9–1/12–1 did not show any significant difference compared with wild-type plants (data not shown). Consistent with their inability to close stomata in response to ABA,14 the enhanced susceptibility of mpk9–1/12–1 to P. syringae DC3000 is likely due to a failure in the early steps of the immune response to bacterial infection, i.e the prevention of pathogen penetration inside the leaf by closing stomata. These results, taken together with those of our previous study, suggest that MPK9 and MPK12 are involved in both ABA- and pathogen-induced stomatal closure and that the ABA signaling cascade involving these two MAP kinases plays a crucial role in plant defense. Therefore, it would be interesting to test whether MPK9 and MPK12 are activated by P. syringae infection and whether their activation is dependent on FLS2. It would also be interesting to determine which MAPKK and MAPKKK activate MPK9 and MPK12 and whether different MAPKK and MAPKKK are utilized to activate MPK9 and MPK12 in response to ABA and P. syringae.
Figure 1.
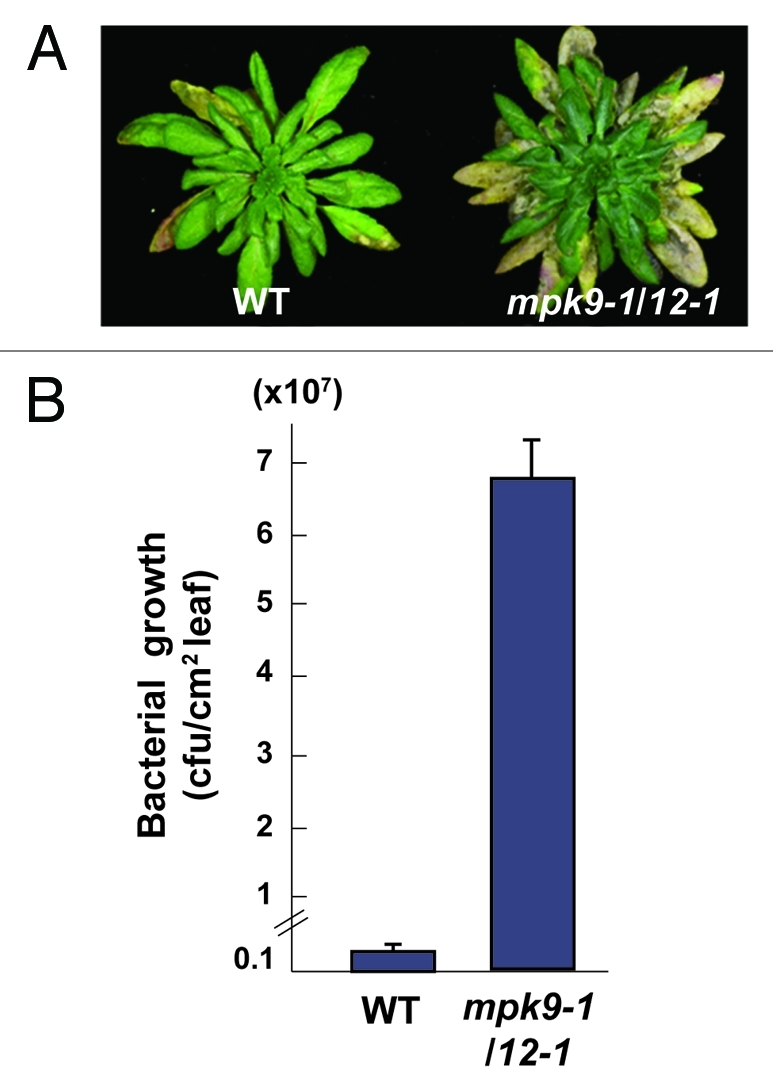
mpk9–1/12–1 double mutants are highly susceptible to P. syringae. (A) WT and mpk9–1/12–1 mutant plants 4 d after infection with P. syringae. (B) Five week WT and mpk9–1/12–1 mutant plants were spray-inoculated with P. syringae. Bacterial growth was assessed 4 d after infection. Error bars represent SEM n = 3 independent experiments.
We also examined whether MPK9 and MPK12 function in regulating gene expression related to ABA and defense signaling. We first compared expression of ABA-responsive genes between WT and mpk9–1/12–1 mutant plants. To test whether these mutations affect the expression of 12 ABA-responsive genes, we performed RT-PCR with RNA extracted from leaves of WT and mpk9–1/12–1 treated with 10 μM ABA for 1 h. We found no significant difference in ABA regulation of these genes between WT and mpk9–1/12–1 (data not shown). This is most likely due to the fact that MPK9 and MPK12 are highly and preferentially expressed in guard cells.14 Even if there had been differences in the expression of these ABA-inducible genes, it would not have been possible to visualize these differences because we examined changes in gene expression in all leaf cells, of which guard cells only make up a small fraction. The use of genes that are preferentially expressed in guard cells and also highly regulated by ABA or P. syringae could address this issue.
Acknowledgments
This work was supported by NRI grants from the USDA National Institute of Food and Agriculture (2004–35100–14909, 2007–35100–18377) to J.M.K.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/17933
References
- 1.Park SY, Fung P, Nishimura N, Jensen DR, Fujii H, Zhao Y, et al. Abscisic acid inhibits type 2C protein phosphatases via the PYR/PYL family of START proteins. Science. 2009;324:1068–71. doi: 10.1126/science.1173041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ma Y, Szostkiewicz I, Korte A, Moes D, Yang Y, Christmann A, et al. Regulators of PP2C phosphatase activity function as abscisic acid sensors. Science. 2009;324:1064–8. doi: 10.1126/science.1172408. [DOI] [PubMed] [Google Scholar]
- 3.Joshi-Saha A, Valon C, Leung J. Abscisic acid signal off the STARTing block. Mol Plant. 2011;4:562–80. doi: 10.1093/mp/ssr055. [DOI] [PubMed] [Google Scholar]
- 4.Wasilewska A, Vlad F, Sirichandra C, Redko Y, Jammes F, Valon C, et al. An update on abscisic acid signaling in plants and more. Mol Plant. 2008;1:198–217. doi: 10.1093/mp/ssm022. [DOI] [PubMed] [Google Scholar]
- 5.Furihata T, Maruyama K, Fujita Y, Umezawa T, Yoshida R, Shinozaki K, et al. Abscisic acid-dependent multisite phosphorylation regulates the activity of a transcription activator AREB1. Proc Natl Acad Sci USA. 2006;103:1988–93. doi: 10.1073/pnas.0505667103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yoshida T, Fujita Y, Sayama H, Kidokoro S, Maruyama K, Mizoi J, et al. AREB1, AREB2, and ABF3 are master transcription factors that cooperatively regulate ABRE-dependent ABA signaling involved in drought stress tolerance and require ABA for full activation. Plant J. 2010;61:672–85. doi: 10.1111/j.1365-313X.2009.04092.x. [DOI] [PubMed] [Google Scholar]
- 7.Geiger D, Scherzer S, Mumm P, Stange A, Marten I, Bauer H, et al. Activity of guard cell anion channel SLAC1 is controlled by drought-stress signaling kinase-phosphatase pair. Proc Natl Acad Sci USA. 2009;106:21425–30. doi: 10.1073/pnas.0912021106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee SC, Lan W, Buchanan BB, Luan S. A protein kinase-phosphatase pair interacts with an ion channel to regulate ABA signaling in plant guard cells. Proc Natl Acad Sci USA. 2009;106:21419–24. doi: 10.1073/pnas.0910601106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sirichandra C, Gu D, Hu HC, Davanture M, Lee S, Djaoui M, et al. Phosphorylation of the Arabidopsis AtrbohF NADPH oxidase by OST1 protein kinase. FEBS Lett. 2009;583:2982–6. doi: 10.1016/j.febslet.2009.08.033. [DOI] [PubMed] [Google Scholar]
- 10.Kwak JM, Nguyen V, Schroeder JI. The role of reactive oxygen species in hormonal responses. Plant Physiol. 2006;141:323–9. doi: 10.1104/pp.106.079004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kwak JM, Maser P, Schroeder J. I. The Clickable Guard Cell, Version II: Interactive model of guard cell signal transduction mechanisms and pathways. The Arabidopsis Book 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Apel K, Hirt H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol. 2004;55:373–99. doi: 10.1146/annurev.arplant.55.031903.141701. [DOI] [PubMed] [Google Scholar]
- 13.Kwak JM, Mori IC, Pei ZM, Leonhardt N, Torres MA, Dangl JL, et al. NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signalin in Arabidopsis. EMBO J. 2003;22:2623–33. doi: 10.1093/emboj/cdg277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jammes F, Song C, Shin D, Munemasa S, Takeda K, Gu D, et al. MAP kinases MPK9 and MPK12 are preferentially expressed in guard cells and positively regulate ROS-mediated ABA signaling. Proc Natl Acad Sci USA. 2009;106:20520–5. doi: 10.1073/pnas.0907205106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Melotto M, Underwood W, Koczan J, Nomura K, He SY. Plant stomata function in innate immunity against bacterial invasion. Cell. 2006;126:969–80. doi: 10.1016/j.cell.2006.06.054. [DOI] [PubMed] [Google Scholar]
- 16.Zeng W, Melotto M, He SY. Plant stomata: a checkpoint of host immunity and pathogen virulence. Curr Opin Biotechnol. 2010;21:599–603. doi: 10.1016/j.copbio.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee S, Choi H, Suh S, Doo IS, Oh KY, Choi EJ, et al. Oligogalacturonic acid and chitosan reduce stomatal aperture by inducing the evolution of reactive oxygen species from guard cells of tomato and Commelina communis. Plant Physiol. 1999;121:147–52. doi: 10.1104/pp.121.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang W, He SY, Assmann SM. The plant innate immunity response in stomatal guard cells invokes G-protein-dependent ion channel regulation. Plant J. 2008;56:984–96. doi: 10.1111/j.1365-313X.2008.03657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Asai T, Tena G, Plotnikova J, Willmann MR, Chiu WL, Gomez-Gomez L, et al. MAP kinase signalling cascade in Arabidopsis innate immunity. Nature. 2002;415:977–83. doi: 10.1038/415977a. [DOI] [PubMed] [Google Scholar]
- 20.de Torres-Zabala M, Truman W, Bennett MH, Lafforgue G, Mansfield JW, Rodriguez Egea P, et al. Pseudomonas syringae pv. tomato hijacks the Arabidopsis abscisic acid signalling pathway to cause disease. EMBO J. 2007;26:1434–43. doi: 10.1038/sj.emboj.7601575. [DOI] [PMC free article] [PubMed] [Google Scholar]


