Abstract
Glycerol-3-phosphate (G3P), a conserved three-carbon sugar, is an obligatory component of energy-producing reactions including glycolysis and glycerolipid biosynthesis. G3P can be derived via the glycerol kinase-mediated phosphorylation of glycerol or G3P dehydrogenase (G3Pdh)-mediated reduction of dihydroxyacetone phosphate. Previously, we showed G3P levels contribute to basal resistance against the hemibiotrophic pathogen, Colletotrichum higginsianum. Inoculation of Arabidopsis with C. higginsianum correlated with an increase in G3P levels and a concomitant decrease in glycerol levels in the host. Plants impaired in GLY1 encoded G3Pdh accumulated reduced levels of G3P after pathogen inoculation and showed enhanced susceptibility to C. higginsianum. Recently, we showed that G3P is also a potent inducer of systemic acquired resistance (SAR) in plants. SAR is initiated after a localized infection and confers whole-plant immunity to secondary infections. SAR involves generation of a signal at the site of primary infection, which travels throughout the plants and alerts the un-infected distal portions of the plant against secondary infections. Plants unable to synthesize G3P are defective in SAR and exogenous G3P complements this defect. Exogenous G3P also induces SAR in the absence of a primary pathogen. Radioactive tracer experiments show that a G3P derivative is translocated to distal tissues and this requires the lipid transfer protein, DIR1. Conversely, G3P is required for the translocation of DIR1 to distal tissues. Together, these observations suggest that the cooperative interaction of DIR1 and G3P mediates the induction of SAR in plants.
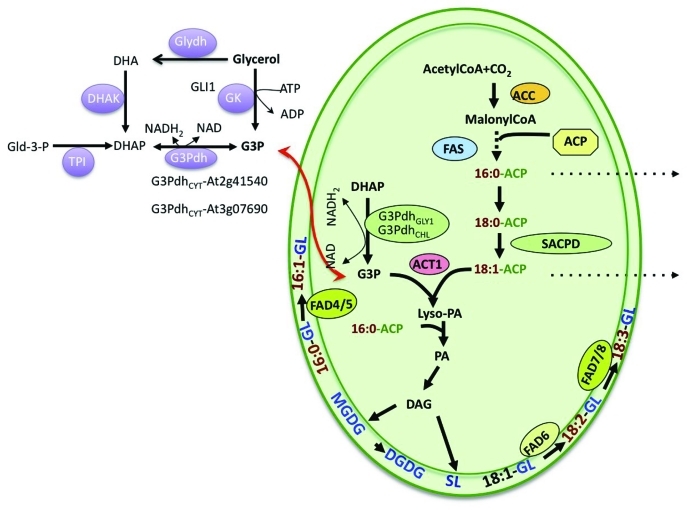
Glycerol-3-phosphate (G3P) is an obligatory component of energy-producing reactions including glycolysis and glycerolipid biosynthesis.1,2 G3P levels in the plant are regulated by enzymes directly/indirectly involved in G3P biosynthesis, as well as those involved in G3P catabolism. G3P is synthesized via the glycerol kinase (GK)-mediated phosphorylation of glycerol,3 or the G3P dehydrogenase (G3Pdh)-mediated reduction of dihydroxyacetone phosphate (DHAP)4 (Fig. 1). DHAP is derived from glycolysis via triosephosphate isomerase activity on glyceraldehyde-3-phosphate, or from the conversion of glycerol to dihydroxacetone (DHA) by glycerol dehydrogenase (Glydh) followed by phosphorylation of DHA to DHAP by DHA kinase (DHAK). G3P is catabolized either upon its conversion to glycerol by glycerol-3-phoshatase (GPP) or its utilization in glycerolipid/triacylglycerol biosynthesis. In Arabidopsis, the total G3P pool is derived from the activities of five G3Pdh isoforms and one GK isoform present in three cellular locations5-9; GK and two of the G3Pdh isoforms are present in the cytoplasm, two other G3Pdh isoforms localize to plastids, and one to the mitochondria. One of the plastid localized G3Pdh isoforms, designated GLY1, was previously shown to be required for glycerolipid biosynthesis; a mutation in GLY1 compromised lipids synthesized via the plastidal pathway of lipid biosynthesis. The fact that exogenous application of glycerol to gly1 plants normalizes plastidal lipid levels10 and that GLY1 encodes a G3Pdh4 suggests that the G3P pool generated via the GLY1 catalyzed reaction is required for the biosynthesis of plastidal lipids. Intriguingly, unlike GLY1, neither the chloroplastic, nor the two cytosolic isoforms of G3Pdh, contribute to plastidal and/or extraplastidal lipid biosynthesis.9
Figure 1.
A condensed scheme of glycerol-3-phosphate metabolism in plants. Glycerol is phosphorylated to glycerol-3-phosphate (G3P) by glycerol kinase (GK; GLI1). G3P can also be generated by G3P dehydrogenase (G3Pdh) via the reduction of dihydroxyacetone phosphate (DHAP). DHAP is derived from glycolysis via triosephosphate isomerase (TPI) activity on glyceraldehyde-3-phosphate (Gld-3-P), or from the conversion of glycerol to dihydroxacetone (DHA) by glycerol dehydrogenase (Glydh) followed by phosphorylation of DHA to DHAP by DHA kinase (DHAK). G3Pdh isoforms are present in both the cytosol and the plastids (represented by the oval). GLY1 is one of the two plastidial G3Pdh isoforms that plays an important role in plastidial glycerolipid biosynthesis. In the plastids, G3P is acylated with oleic acid (18:1) by the ACT1-encoded G3P acyltransferase. This ACT1-utilized 18:1 is derived from the stearoyl-acyl carrier protein (ACP)-desaturase (SACPD)-catalyzed desaturation of stearic acid (18:0). The 18:1-ACP generated by SACPD either enters the prokaryotic lipid biosynthetic pathway through acylation of G3P or is exported out (dotted line) of the plastids as a coenzyme A (CoA)-thioester to enter the eukaryotic lipid biosynthetic pathway. Membranous fatty acid desaturases (FAD) catalyze desaturation of FAs present on membranous glycerolipids. Other abbreviations used are: GL, glycerolipid; FAS, fatty acid synthase; ACC, acetyl-CoA carboxylase; Lyso-PA, acyl-G3P; PA, phosphatidic acid; PG, phosphatidylglycerol; MGDG, monogalactosyldiacylglycerol; DGDG, digalactosyldiacylglycerol; SL, sulfolipid; DAG, diacylglycerol.
For glycerolipid biosynthesis, G3P is first acylated with the fatty acid (FA) oleic acid (18:1), to form lyso-phosphatidic acid (lyso-PA) via the activity of the soluble G3P acyltransferase (GPAT) encoded by the ACT1 gene in Arabidopsis11 (Fig. 1). 18:1 in turn is derived from the saturated FA, stearic acid (18:0), via the activity of soluble stearoyl-acyl carrier protein desaturases (SACPD),12 which introduce a single cis double bond in 18:0. The 18:1 generated via this reaction is either exported out of the plastids or acylated at the sn-1 position of G3P. Previously, we have shown that 18:1 levels are important regulators of plant defense signaling. In Arabidopsis, 18:1 is synthesized via the SSI2/FAB2-encoded SACPD,12 which uses 18:0 as a substrate. A mutation in SSI2 results in the accumulation of 18:0 and a reduction in 18:1 levels. The mutant plants show stunting, spontaneous lesion formation, constitutive PR gene expression, and enhanced resistance to bacterial and oomycete pathogens.4,12-17 Characterization of ssi2 suppressor mutants has shown that the altered defense-related phenotypes are the result of the reduction in the levels of the unsaturated FA, 18:1, which causes induction of several resistance (R) genes.4,14,18,19 Restoration of 18:1 levels, via mutations in ACT1,14 GLY14 or ACP4,18 normalizes R gene expression in ssi2 plants. The low 18:1-mediated induction of R gene expression and the associated defense signaling can also be suppressed by simultaneous mutations in EDS1 and the genes governing salicylic acid (SA) biosynthesis (SID2, EDS5).19 Furthermore, the functional redundancy between EDS1 and SA likely masks the requirement for EDS1 by several coiled coil (CC)- nucleotide binding site (NBS)- leucine rich repeat (LRR) proteins,19 previously thought to function independent of EDS1.20 Thus, the reliance on EDS1 for signaling mediated by CC-NBS-LRR proteins becomes evident only in the absence of SA.
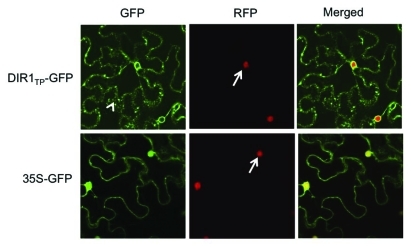
The plastidal 18:1 levels are also regulated via the chloroplastic G3P pool and vice-versa. However, 18:1 and G3P appear to function distinctly in defense signaling. For example, G3P levels are important for basal defense against the hemibiotrophic fungus, Colletotrichum higginsianum.21,22 Genetic mutations affecting G3P synthesis in Arabidopsis enhance susceptibility to C. higginsianum. Conversely, plants accumulating increased G3P show enhanced resistance. More recently, we demonstrated roles for G3P in R-mediated defense leading to systemic acquired resistance (SAR).9 R-mediated defense against the avirulent bacterial pathogen P. syringae is associated with a rapid increase in G3P levels; G3P levels peak within 6 h of inoculation with avirulent bacteria (avrRpt2), in resistant plants expressing the R gene RPS2. Strikingly, accumulation of G3P, in the infected and systemic tissues, precedes the accumulation of other metabolites known to be essential for SAR; SA,23,24 jasmonic acid (JA)25 and azelaic acid (AA)26 accumulated at least 24 h post pathogen inoculation. Furthermore, mutants defective in G3P synthesis are compromised in SAR but accumulated normal levels of SA, AA, and JA. Compromised SAR in G3P deficient mutants was restored by exogenous application of G3P, thus arguing a role for G3P in SAR. This was further supported by the fact that exogenous G3P induced SAR in the absence of the primary pathogen in both Arabidopsis and soybean.9 That fact that G3P is a conserved metabolite common to prokaryotes, plants, and humans further corroborates the conserved nature of SAR signaling. Interestingly, although exogenous G3P did not induce SA biosynthesis, SAR conferred by exogenous G3P was dependent on SA. These results suggest that the onset and/or establishment of SAR likely requires basal, but not induced levels of SA, in the distal tissues. It is possible that the relatively small increase in SA observed in the systemic tissues during SAR is an indirect response that contributes to generalized resistance, rather than SAR itself. Interestingly, both G3P conferred SAR, and the systemic movement of G3P were dependent on the lipid transfer protein, DIR1, a well-known positive regulator of SAR.27 Conversely, systemic movement of DIR1 required G3P. These findings did not correlate with the fact that G3P is cytosolic while DIR1 was a predicted apoplastic protein. To resolve this issue, we studied the localization of DIR1, and found that it is in fact a symplastic protein. The symplastic location of DIR1 was further corroborated when GFP fused to the signal peptide from DIR1 localized to the endoplasmic reticulum, rather than the typical cytoplasmic and nuclear location of GFP (Fig. 2). These results suggested that the symplastic movement of DIR1 is likely critical for SAR, and supported the facts that G3P and DIR1 are interdependent for translocation to systemic tissues. However, these findings could not explain how a lipid transfer-like protein might associate with the phosphorylated sugar G3P, to move systemically. Analysis of G3P in the leaf extracts showed that it was derivatized into an unknown compound before/during translocation. It is likely that the G3P derivative has a lipid moiety via which it associates with DIR1 for transfer. In summary, we showed that DIR1 together with a G3P-derived compound are sufficient for the induction of SAR in wild type plants. Our findings provide strong evidence in support of a direct defense-signaling role for G3P and warranty further analysis of its metabolic pathway(s) for their role(s) in various modes of plant defense.
Figure 2.
Confocal micrograph showing localization of GFP fused to DIR1 transit peptide (TP) or GFP alone in Nicotiana benthamiana plants expressing RFP-tagged nuclear histone protein H2B. Arrow indicates nucleus, arrowhead indicates endoplasmic reticulum.
Acknowledgments
This work was supported by grants from the National Science Foundation (IOS#0749731) to AK and PK and United Soybean Board (#1244) to AK.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/17901
References
- 1.Kachroo A, Kachroo P. Fatty acid derived signals. Annu Rev Phytopathol. 2009;47:153–176. doi: 10.1146/annurev-phyto-080508-081820. [DOI] [PubMed] [Google Scholar]
- 2.Ohlrogge J, Browse J. Lipid biosynthesis. Plant Cell. 1995;7:957–70. doi: 10.1105/tpc.7.7.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kang L, Li J, Zhao T, Xiao F, Tang X, Thilmony R, et al. Interplay of the Arabidopsis nonhost resistance gene NHO1 with bacterial virulence. Proc Natl Acad Sci USA. 2003;100:3919–24. doi: 10.1073/pnas.0637377100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kachroo A, Venugopal SC, Lapchyk L, Falcone D, Hildebrand D, Kachroo P. Oleic acid levels regulated by glycerolipid metabolism modulate defense gene expression in Arabidopsis. Proc Natl Acad Sci USA. 2004;101:5152–7. doi: 10.1073/pnas.0401315101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wei Y, Periappuram C, Datla R, Selvaraj G, Zou J. Molecular and biochemical characterization of a plastidic glycerol-3-phosphate dehydrogenase from Arabidopsis. Plant Physiol Biochem. 2001;39:841–8. doi: 10.1016/S0981-9428(01)01308-0. [DOI] [Google Scholar]
- 6.Shen W, Wei Y, Dauk M, Zheng Z, Zou J. Identification of a mitochondrial glycerol-3-phosphate dehydrogenase from Arabidopsis thaliana: evidence for a mitochondrial glycerol-3-phosphate shuttle in plants. FEBS Lett. 2003;536:92–6. doi: 10.1016/S0014-5793(03)00033-4. [DOI] [PubMed] [Google Scholar]
- 7.Shen W, Wei Y, Dauk M, Tan Y, Taylor DC, Selvaraj G, et al. Involvement of a glycerol-3-phosphate dehydrogenase in modulating the NADH/NAD ratio provides evidence of a mitochondrial glycerol-3-phosphate shuttle in Arabidopsis. Plant Cell. 2006;18:422–41. doi: 10.1105/tpc.105.039750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quettier A-L, Shaw E, Eastmond PJ. SUGAR-DEPENDENT6 encodes a mitochondrial flavin adenine dinucleotide-dependent glycerol-3-P dehdrogenase, which is required for glycerol catabolism and postgerminative seedling growth in Arabidopsis. Plant Physiol. 2008;148:519–28. doi: 10.1104/pp.108.123703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chanda B, Xia Y, Mandal M, Yu K, Sekine K, Gao Q-M, et al. Glycerol-3-phosphate, a critical mobile inducer of systemic immunity in plants. Nat Genet. 2011;43:421–7. doi: 10.1038/ng.798. [DOI] [PubMed] [Google Scholar]
- 10.Miquel M, Cassagne C, Browse J. A new class of Arabidopsis mutants with reduced hexadecatrienoic acid fatty acid levels. Plant Physiol. 1998;117:923–30. doi: 10.1104/pp.117.3.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kunst L, Browse J, Somerville C. Altered regulation of lipid biosynthesis in a mutant of Arabidopsis deficient in chloroplast glycerol-3-phosphate acyltransferase activity. Proc Natl Acad Sci USA. 1988;85:4143–7. doi: 10.1073/pnas.85.12.4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kachroo P, Shanklin J, Shah J, Whittle EJ, Klessig DF. A Fatty acid desaturase modulates the activation of defense signaling pathways in Plants. Proc Natl Acad Sci USA. 2001;98:9448–53. doi: 10.1073/pnas.151258398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kachroo P, Venugopal SC, Navarre DA, Lapchyk L, Kachroo A. Role of salicylic acid and fatty acid desaturation pathways in ssi2-mediated signaling. Plant Physiol. 2005;139:1717–35. doi: 10.1104/pp.105.071662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kachroo A, Lapchyk L, Fukushigae H, Hildebrand D, Klessig D, Kachroo P. Plastidial fatty acid signaling modulates salicylic acid- and jasmonic acid-mediated defense pathways in the Arabidopsis ssi2 mutant. Plant Cell. 2003;15:2952–65. doi: 10.1105/tpc.017301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kachroo A, Shanklin J, Lapchyk L, Whittle E, Hildebrand D, Kachroo P. The Arabidopsis stearoyl-acyl carrier protein-desaturase family and the contribution of leaf isoforms to oleic acid synthesis. Plant Mol Biol. 2007;63:257–71. doi: 10.1007/s11103-006-9086-y. [DOI] [PubMed] [Google Scholar]
- 16.Chandra-Shekara AC, Navarre D, Kachroo A, Kang H-G, Klessig DF, Kachroo P. Signaling requirements and role of salicylic acid in HRT-and rrt- mediated resistance against turnip crinkle virus in Arabidopsis. Plant J. 2004;40:647–59. doi: 10.1111/j.1365-313X.2004.02241.x. [DOI] [PubMed] [Google Scholar]
- 17.Kachroo P, Kachroo A, Lapchyk L, Hildebrand D, Klessig D. Restoration of defective cross talk in ssi2 mutants; Role of salicylic acid, jasmonic acid and fatty acids in SSI2-mediated signaling. Mol Plant Microbe Interact. 2003;16:1022–9. doi: 10.1094/MPMI.2003.16.11.1022. [DOI] [PubMed] [Google Scholar]
- 18.Xia Y, Gao Q-M, Yu K, Lapchyk L, Navarre D, Hildebrand D, et al. An intact cuticle in distal tissues is essential for the induction of systemic acquired resistance in plants. Cell Host Microbe. 2009;5:151–65. doi: 10.1016/j.chom.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 19.Venugopal SC, Jeong R-D, Mandal M, Zhu S, Chandra-Shekara AC, Xia Y, et al. ENHANCED DISEASE SUSCEPTIBILITY 1 and SALICYLIC ACID act redundantly to regulate resistance gene expression and low oleate-induced defense signaling. PLoS Genet. 2009;5:e1000545. doi: 10.1371/journal.pgen.1000545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aarts N, Metz M, Holub E, Staskawicz BJ, Daniels MJ, Parker J. Different requirements for EDS1 and NDR1 by disease resistance genes define at least two R gene-mediated signaling pathways in Arabidopsis. Proc Natl Acad Sci USA. 1998;95:10306–11. doi: 10.1073/pnas.95.17.10306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chanda B, Venugopal SC, Kulshrestha S, Navarre D, Downie B, Vaillancourt L, et al. Glycerol-3-phosphate levels are associated with basal resistance to the hemibiotrophic fungus Colletotrichum higginsianum in Arabidopsis. Plant Physiol. 2008;147:2017–29. doi: 10.1104/pp.108.121335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Venugopal SC, Chanda B, Vaillancourt L, Kachroo A, Kachroo P. The common metabolite glycerol-3-phosphate is a novel regulator of plant defense signaling. Plant Signal Behav. 2009;4:746–9. doi: 10.4161/psb.4.8.9111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vlot CA, Dempsey DA, Klessig DF. Salicylic acid, a multifaceted hormone to combat disease. Annu Rev Phytopathol. 2009;47:177–206. doi: 10.1146/annurev.phyto.050908.135202. [DOI] [PubMed] [Google Scholar]
- 24.Park S-W, Kaimoyo E, Kumar D, Mosher S, Klessig D. Methyl salicylate is a critical mobile signal for plant systemic acquired resistance. Science. 2007;318:113–6. doi: 10.1126/science.1147113. [DOI] [PubMed] [Google Scholar]
- 25.Truman W, Bennett MH, Kubigsteltig I, Turnbull C, Grant M. Arabidopsis systemic immunity uses conserved defense signaling pathways and is mediated by jasmonates. Proc Natl Acad Sci USA. 2007;104:1075–80. doi: 10.1073/pnas.0605423104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jung HW, Tschaplinkski TJ, Wang L, Glazebrook J, Greenberg JT. Priming in systemic plant immunity. Science. 2009;324:89–91. doi: 10.1126/science.1170025. [DOI] [PubMed] [Google Scholar]
- 27.Maldonado AM, Doerner P, Dixon RA, Lamb CJ, Cameron RK. A putative lipid transfer protein involved in systemic resistance signaling in Arabidopsis. Nature. 2002;419:399–403. doi: 10.1038/nature00962. [DOI] [PubMed] [Google Scholar]