Abstract
Ultraviolet light radiation (UVR) has profound effects upon human skin. Yet, the exact targets for UVR are unclear. Inasmuch as UVR is a known pro-oxidative stressor, one potential target for UVR could be oxidatively modified glycerophosphocholines (GPC). Importantly, recent studies demonstrate that these oxidized GPCs (ox-GPC) are potent agonists for the platelet-activating factor receptor and peroxisome proliferator-activated receptor gamma. This review discusses these new biologically active lipids and their down-stream receptor targets that provide a unique system of biosensors for detecting and responding to UVR photo-oxidation.
Keywords: Oxidation, ultraviolet rays, peroxisome proliferator-activated receptor gamma, platelet activating factor receptor, glycerophosphoscholines
INTRODUCTION
UV radiation has profound effects upon human skin. Both UVB (290–320 nm) and UVA (320–400 nm) are important environmental carcinogens, and skin is the major target (reviewed in 1, 2). In addition to its ability to damage DNA, UVA and UVB can modify epithelial gene expression. The known UV response consists of the rapid activation of numerous genes with reparative, protective or apoptotic effects, as well as genes encoding cytokines and other signaling molecules with potent immunomodulatory effects 3, 4. The exact mechanisms by which UVA and UVB exert these effects are not entirely clear. Potential targets for UVR include DNA and cis-urocanic acid 5. The ability of both UVA and UVB to act as a pro-oxidative stressor could also provide additional targets for UV-mediated effects. Amongst the cellular molecules that could be modified by oxidative stress to form biologically active agents are lipids, especially polyunsaturated fatty acids incorporated into phospholipids 6. The important role that oxidized phospholipids play in pathologic processes is possibly best understood in relation to cardiovascular disease, in which oxidized low density lipoproteins (LDL) are thought to be key mediators of atherogenesis (reviewed in 7, 8).
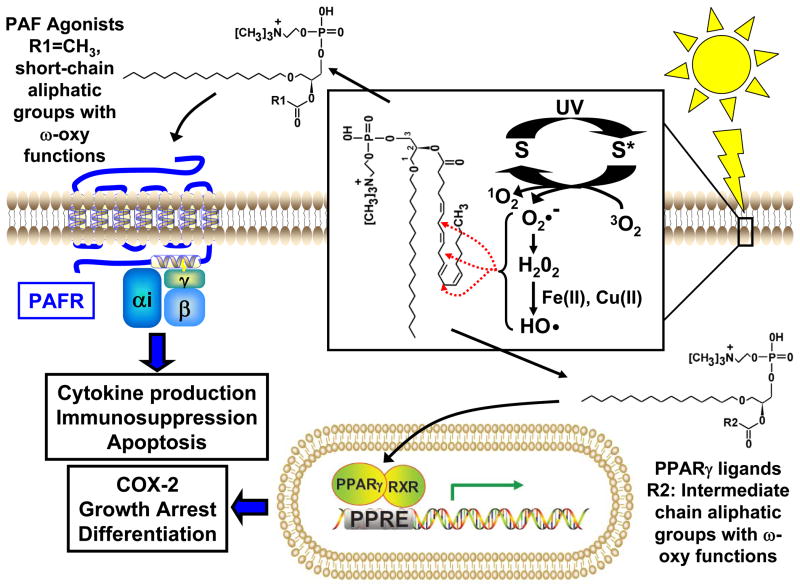
Glycerophosphocholine (GPC) is an important structural lipid in all cellular membranes. 1-alkyl GPC with a polyunsaturated fatty (such as arachidonate) esterified to the sn-2 position is found in reasonable abundance in epithelial cells 9. These molecules are precursors of bioactive lipid platelet-activating factor (1-alkyl-2-acetyl GPC), which is formed upon the actions of enzymes phospholipase A2 and PAF acetyltransferase. Esterified polyunsaturated fatty acids, especially arachidonate, are susceptible to oxidation due to the presence of bisallylic hydrogens which serve as hydrogen donors for radical attack. Oxidation of esterified fatty acyl residues introduces oxy functions, rearranges bonds and fragments carbon-carbon bonds by β-scission 10 that generate a myriad of reaction products. Among these are a series of phospholipids with oxidatively fragmented sn-2 acyl residues that terminate with either an ω-oxy function or a methyl group. If the oxidatively modified sn-2 acyl residue is a 1-alkyl GPC, then the resultant products can act as potent PAF receptor or peroxisome proliferator-activated receptor gamma (PPAR-γ) agonists. Recent studies have demonstrated that UVB can generate both PAF and PPARγ agonists via its ability to act as a pro-oxidative stressor 11, 12. The significance of these novel biologically-active GPCs will be discussed in this review (see Figure 1).
Figure 1. Proposed scheme for UVB-induced PAF-R and PPARγ ligand production from oxidized glycerophosphocholines (ox-GPCs).
Ox-GPCs serve as secondary products of photoxidation initiated by the ability of UV light to shift endogenous chromophores or photosensitizers (S) to the photoexcited state (S*). In the presence of molecular oxygen (O2), relaxation to the ground state can result in the formation of reactive oxygen species (ROS). This includes superoxide (O2•−), which rapidly undergoes dismutation to form hydrogen peroxide (H2O2). In the presence of transition metals (Fe(II), Cu(II)), H2O2 is converted to highly reactive hydroxyl radicals (•OH). These ROS then act to initiate oxidative extraction of protons from polyunsaturated fatty acids in glycerophosphocholines, like 1-hexadecyl-2-arachidonoyl-glycerophosphocholine (HAPC) (as shown in the box). Bisallylic hydrogens (red dotted arrows) serve as favored sites of proton extraction and eventually introduces oxy functions, rearranges bonds and fragments carbon-carbon bonds by β-scission that generate a myriad of reaction products. OxGPCs with PAFR activity likely have short-chain aliphatic groups ending with a methyl group or an ω-oxy function esterified at the sn-2 position (R1)(PAF, R1=CH3). OxGPCs with PPARγ activity likely have intermediate chain aliphatic groups (< 9 carbons) ending with an ω-oxy function esterified at the sn-2 position (R2). Once formed, PAF ligands activate the PAF-R, a Giα coupled G-protein coupled receptor. PAF-R activation is likely important in mediating UVB-induced cytokine production, cytotoxicity, and photoimmunosuppression. UVB-induced PPARγ ligands act to regulate gene expression, targeting genes containing a peroxisome proliferator response element (PPRE). PPARγ activation likely plays a role in UVB-mediated regulation of keratinocyte growth, differentiation, and cyclooxygenase 2 (COX-2) expression.
Platelet-activating Factor and Keratinocyte Function
PAF is an inflammatory phospholipid mediator that exerts its effects through a single, specific G-protein coupled receptor, the PAF receptor (reviewed in 13). The PAF receptor is expressed by cells of the innate immune system, but also by keratinocytes of the skin 14. PAF is the most potent phospholipid agonist yet identified where high affinity recognition depends on its sn-1 ether bond and a short sn-2 acetyl residue. PAF activates some cells at sub-picomolar levels and as would be anticipated, its synthesis is closely controlled.
PAF is synthesized in response to diverse stimuli including cytokines, endotoxin, and Ca++ ionophores. Of note, direct damage to keratinocytes by either heat or cold stimuli result in significant PAF production 15. The synthetic pathway consists of two enzymes; a phospholipase A2 that generates the lysolipid backbone by releasing the sn-2 fatty acyl residue from alkyl phosphatidylcholine and PAF acetyltransferase activity that transfers an acetyl residue from acetyl-CoA to this newly generated lysolipid. Both activities are tightly regulated, with increased intracellular Ca++ being the premier agent for their activation.
In addition to enzymatically catalyzed PAF production, PAF receptor ligands are also generated non-enzymatically, and thus not subject to cellular control. The alkyl phospholipid pool is enriched at the sn-2 position with arachidonoyl residues, a source of lyso-PAF and arachidonate for prostanoid and leukotriene synthesis. Importantly, these polyunsaturated arachidonoyl residues are highly susceptible to oxidation. Among these are a series of phospholipids with oxidatively fragmented sn-2 acyl residues that terminate with either an ω-oxy function or a methyl group. The latter series is one carbon atom shorter than the ω-oxy series, as expected from the β-scission reaction. Among the phospholipid reaction products are those that are ligands for the PAF-receptor. The most potent of the non-enzymatically generated PAF analogs are native PAF, as well as the butanoyl (C4-PAF) and butenoyl (C4:1-PAF) species that are one tenth as potent as PAF 16, 17. In addition, alkyl GPCs found in oxidized LDL with small chain sn-2 groups ending in an ω-oxy function, such as a 4-carbon ω-carboxylic acid (succinoyl-PAF) and 5 carbon ω-aldehydes (5-oxovaleroyl-PAF), have also been shown to serve as PAF-R ligands 17–20.
Keratinocytes synthesize PAF in response to numerous stimuli, including cellular damage, ionophores, and cytokines including IL-8 15, 17, 21. Keratinocytes also express PAF-receptors and activation of the epidermal PAF-receptor results in the expression of numerous cytokines including IL-1, IL-6, IL-8, IL-10, TNF-α, prostaglandins, and PAF itself 22–24. Moreover, the epidermal PAF-R can transactivate the epidermal growth factor receptor which can induce a mild proliferative response 25. It should be noted that the phenotype of a transgenic mouse which overexpressed the PAF-R (by using an actin promoter) included a hyperproliferative dermatitis 26.
PAF System and UVR
UVB generates PAF-receptor ligands 11, 24, and stimulation of the PAF-receptor of epithelial cells mimics many aspects of UVB effects on human keratinocytes including tumor necrosis factor-α, interleukin-8, and cyclooxygenase-2 (COX-2) production. Expression of the PAF-receptor in the PAF-receptor-negative epithelial cell line KB results in an augmentation of UVB-mediated cytokine production and cytotoxicity 11, 27. UVB is immunosuppressive and facilitates progression of non-melanoma skin cancer, and it has been proposed 24 that PAF and/or PAF-like lipids mediate this systemic immune suppression via PAF-receptor-mediated IL-10 production. Moreover, topical psoralen + UVA-mediated keratinocyte cytotoxicity has been reported to be decreased in PAF-receptor deficient mice in comparison to wild-type counterparts 28.
The mechanisms utilized by the PAF system in mediating UVB irradiation-induced systemic immunosuppression have been explored in recent studies 29. The importance of the PAF-R in photoimmunosuppression is demonstrated by the ability of CPAF (injected ip into C57BL6 mice) to mimic the ability of UVB to inhibit delayed type hypersensitivity reactions 30. In contrast, UVB irradiation (or ip injection of CPAF) of PAF-R-deficient C57BL6 mice does not result in an inhibition of delayed type contact hypersensitivity reactions. However, it was as yet unclear whether immune cells or keratinocytes mediated the PAF-R-dependent effects on photoimmunosuppression. Bone marrow transplantation studies to produce mice chimeric with respect to the PAF-R demonstrated that the PAF-R cells responsible for UVB-mediated immunosuppression are bone-marrow-derived 30. The model that is emerging is that UVB irradiation results in keratinocyte-mediated production of PAF-R agonists that then act upon immunocytes to inhibit delayed type hypersensitivity responses through an IL-10-dependent process. These studies indicate that PAF-R activation could be an important mediator of UV-mediated systemic immunosuppression, with regulated PAF ligand production occurring enzymatically in response to cellular damage and non-regulated PAF ligand occurring as a byproduct of oxidized GPC.
Of the known UV-mediated effects in keratinocytes, the PAF system has also been implicated in the early gene expression induced by UVB. Recently our group has examined the role of the epidermal PAF-R in UVB-mediated early gene expression. Affymatrix gene arrays of the PAF-R-negative epithelial cell line KB transduced with the PAF-R (KBP) versus control KB (KBM) cells 1 h following treatment with the PAF-R agonist carbamoyl-PAF resulted in statistically significant differences in the expression of many genes; 187 genes were up-regulated and 59 genes were down-regulated at least 2-fold 31. Genes altered by PAF-R activation included genes involved in adherens junctions, apoptosis, calcium signaling, cell cycle regulation, cytokines, extracellular matrix receptor interactions, JAK-STAT and MAPK signaling pathways. In particular, CPAF treatment induced mRNA encoding cytokines TNF-α (8.6 fold), IL-6 (11.3 fold), IL-8 (55.4 fold), and IL-11 (12.5 fold). In addition, activation of the PAF-R strongly induced mRNA encoding chemokines CCL20 (173.9 fold), CCL2 (24.8 fold), CXCL1 (30.7 fold), CXCL2 (24.8 fold), and CXCL3 (36.6 fold). CPAF treatment of KBP cells also induced anti-apoptotic genes including BIRC2 and BIRC3, TRAF-1 and MCL1. Consistent with the lack of functional PAF-Rs, treatment of control KB cells with CPAF did not result in significant changes in mRNA levels for any genes. UVB irradiation of PAF-R-positive KBP and control KBM cells revealed that 11 genes were selectively upregulated in KBP over KBM, including cytokines CCL20 and TNF-α. UVB irradiation of the skin of wild-type versus PAF-R-deficient mice on a C57BL6 background resulted in significantly decreased levels of CCL20 and TNF-α mRNA and protein levels in the PAF-R-deficient mice 31. These studies indicate involvement of the PAF system in UVB-mediated early gene expression, especially inflammatory cytokines.
Peroxisome Proliferator-Activated Receptors (PPAR)
PPARs are transcription factors belonging to the nuclear hormone receptor (NHR) family (reviewed in 32, 33). PPARs were first identified as NHRs which are activated by diverse lipid species and hypolipaemic xenobiotic agents. The nomenclature for this new class of NHRs was based on the fact that many of these agents were known to induce proliferation of hepatic peroxisomes 34. Three different PPARs have been identified (α β/δ, and γ) that differ in ligand specificity, tissue expression, and transcriptional targets. In addition, four distinct PPARγ transcripts (γ1-γ4) are produced by the use of alternative mRNA splicing leading to two different protein products: PPARγ1, PPARγ3, & PPARγ4 all encode a single protein product 35. While PPARγ1 is ubiquitously expressed, PPARγ2 and PPARγ3 are expressed in primarily in adipose tissue 35. Tissue-specific expression of PPARγ4 is unknown 35. Importantly, PPARα, PPARβ/δ and PPARγ are all expressed in keratinocytes and in human and rodent epidermis 32, 33, 36.
The ability of PPARs to regulate gene transcription is complex, utilizing ligand-dependent transactivation or ligand-independent target gene repression (reviewed in 37). In classic ligand-dependent transactivation, ligand binding induces a conformational change in protein structure that stabilizes heterodimerization with the 9-cis retinoic acid receptor (RXR). This in turn triggers loss of co-repressor binding, and facilitates recruitment of co-activators. The active PPAR:RXR complex mediates target gene transcription by binding to specific hexameric DNA repeat sequences termed peroxisome proliferator response elements (PPRE). PPARs can also influence the expression of genes lacking a PPRE. In this case, PPARs act through ligand-dependent transrepression to inhibit the functions of other transcription factors, most notably nuclear factor –kB (NF-kB) and activator protein-1 (AP-1) 37. This occurs through multiple mechanisms, including direct interaction of PPAR with the transcription factor, competition for co-activator recruitment, and regulation of mitogen-activated protein kinase (MAPK) family member activity 37.
PPARγ and keratinocyte function
PPARγ is an essential nuclear factor probably best understood in relationship to its role in controlling the expression of a number of regulatory genes involved in lipid metabolism and insulin sensitization (reviewed in 38). More recently, evidence suggests that PPARγ plays an important role in regulating epithelial proliferation, atherogenesis, carcinogenesis, immune cell function, and inflammation 8, 38–41. Within the epidermis, much less is known about the function, mechanisms of activation and target genes for PPARs (reviewed in 32, 36). However, it is clear that keratinocytes and epithelial cell lines express all three PPAR subtypes, and that PPARγ likely plays an important role in regulating keratinocyte proliferation and differentiation 32, 36, 42, 43. In addition, recent evidence indicates that PPARγ agonists may act as potentential therapeutic agents in inflammatory skin diseases like psoriasis 44–46. However, it is not entirely clear that keratinocyte PPARγ is the target of this anti-psoriatic activity. PPARγ has been shown to have potent effects on immune cell function (reviewed in 39); thus, it is also possible that dendritic cell or inflammatory cell PPARγ may also serve as targets in regulating the effects of PPARγ agonist therapy in inflammatory hyperplasia.
Evidence that Oxidized GPC are native PPARγ ligands
PPARγ was initially isolated in mammalian cells as an “orphan” receptor, so named because there were as yet no known endogenous ligands 47. It was later determined that thiazolidinedione-type anti-diabetic agents act as potent PPARγ ligands 48. This was soon followed by the discovery of the first endogenously produced PPARγ ligand, the prostaglandin J2 metabolite, 15-deoxy-Δ12,14-PGJ2 (15d-PGJ2) 49, 50. However, the small amounts of this product measured in biological systems, the relatively low affinity of 15d-PGJ2 (EC50 = 1 μM) 49, and recent reports that 15d-PGJ2 regulates a number of biochemical pathways independent of PPARγ have brought the potential role of this compound as a true endogenous ligand into question 51–55. This was followed by studies demonstrating that lipoxygenase products of arachidonic acid and linoleic acid found in oxidized LDL, 9- and 13-Hydroxyoctadienoic acids (HODE) and 15-hydroxytetraenoic acid (15-HETE), also exhibited the capacity to activate PPARγ 51, 56. However, these compounds have other effects, including regulating the activity of PPARγ through MAPK-mediated phosphorylation 57. In addition, high (micromolar) concentration of these lipids are needed to exert PPARγ effects; thus, this has brought their biological relevance into question 57.
Recent studies examining the ability of oxidized LDL to activate PPARγ in atherogenesis have resulted in the discovery of the first high-affinity endogenous ligands for PPARγ. This was first demonstrated by Davies et al 58, who demonstrated that non-enzymatically oxidized alkyl phospholipids present in oxidized low density lipoproteins (LDL), are high affinity ligands for PPARγ. One of these species was identified as 1-hexadecyl-2-azelaoyl phosphatidylcholine (azPC), which had a Kd for binding to the PPARγ receptor of approx. 40 nM 58.
Given that oxidized GPCs with PPARγ activity are present in oxidized LDL, our group sought to determine whether UVB-induced photo-oxidation could result in PPARγ ligand production, in addition to the PAF-R ligand activity already described above. Our studies demonstrate that UVB irradiation of epithelial cells and purified 1-hexadecyl-2-arachidonate-GPC (HAPC) also results in PPARγ activity 12. It should be noted that high-performance liquid chromatography separation of lipids derived from UVB irradiation of epithelial cells or purified HAPC revealed numerous fractions with significant PPARγ activity 12. Mass spectrometric analysis of these lipids found small amounts of azPC. It is important to note that the ability of UVR to induce not only PPARγ ligand activity, but also the PAF-R ligand activity described above, was blocked by anti-oxidants and by UV irradiation in an argon environment; thus, this demonstrates that these UVR-mediated effects were dependent on photo-oxidation 11, 12. Subsequently, we demonstrated that treatment of epithelial cells with other oxidative stressors result in the production of PPARγ ligand activity 59. It should also be noted that though these findings indicate that direct oxidation by UVB results in the production of numerous oxidized GPC with PPARγ activity, only one of these species has been identified (AzPC); the structural characterization of the numerous remaining oxidized GPC which constitute this activity have yet to be accomplished.
Finally, the theme that endogenous PPARγ ligands are produced as a consequence of oxidative stress is further supported by studies of nitric oxide dependent oxidative inflammatory reactions. Recent studies indicates that nitroalkene derivatives of unsaturated fatty acids, particularly linoleic acid (nitrolinoleic acid (NLA)) and oleic acid (nitrated oleic acid (OA-NO2)) are highly potent PPARγ agonists, with both inducing a significant increase in PPARγ transcriptional activity at concentrations as low as 100 nM, well below their physiologic concentration ranges 60, 61. A lesser degree of PPARα and PPARβ/δ activation was also noted, but at higher concentrations (> 1 μM) 61. In addition, as with many of the other proposed PPARγ ligands, NLA has recently been shown to have PPARγ-independent activity 62. It is unclear whether these nitroalkene PPARγ agonists are relevant to photobiology. However, UV irradiation is known to induce NO production through both enzymatic and non-enzymatic mechanisms 63, 64.
PPARγ and UVR
Given the importance of UVR in photocarcinogenesis and in the treatment of inflammatory hyperplastic disorders, as well as the anti-psoriatic activity of PPARγ agonists, our recent findings that UVR and other oxidative stressors induce PPARγ ligand activity suggest a potential role for PPARγ in photobiology12, 59. Thus, since both keratinocytes and immune cells express PPARγ protein, and UVR induces PPARγ activity, the exact role of PPARγ in cutaneous photobiology is an active area of investigation.
Recent studies in both our laboratory and others have provided an additional mechanism through which UVR-mediated PPARγ activation can influence epidermal photobiology. A previous study demonstrated that COX-2 contains a PPRE in the promoter region and is a target for PPAR-mediated transactivation in epithelial cells 65 and immortalized keratinocytes 66. Importantly, COX-2 induction has been implicated in UV-mediated processes ranging from carcinogenesis to local and systemic immunosuppression 29, 30, 67–69. Recent studies by our group demonstrate that cyclooxygenase-2 (COX-2) is a target of UVR-mediated PPARγ activation. First, treatment of the human epithelial cell line KB with PPARγ agonists augmented COX-2 mRNA and protein levels in response to various stimuli including IL-1β and PAF, although PPARγ agonist alone had little effect 12. Of significance, inhibition of PPARγ by a dominant-negative PPAR construct or use of the selective and specific PPARγ antagonist GW9662 inhibited UVB-induced COX-2 expression and resultant PGE2 production in KB cells 12. Second, PPARγ protein has been shown to be expressed in primary cultures of human keratinocytes and the sebaceous cell line SZ-95, and in these cell types inhibition of PPARγ blocks COX-2 expression induced by UVB or the lipid pro-oxidative stressor tert-butyl hydroperoxide (59, and unpublished data). Thus, these studies indicate that PPARγ is likely to contribute to photobiology through a COX-2-dependent signaling pathway.
A potential role for a PPARγ-COX-2 coupled response in UVB-immunoregulation may be inferred from studies by our group 30 and by Ullrich and colleagues 24: These studies indicate that UVB-induced systemic immunosuppression is mediated by a PAF-R-COX-2 coupled signaling pathway. Insofar as PPARγ also plays a role in UVB-mediated COX-2 induction, this introduces the possibility that oxidized GPCs may act through both PAF-R and PPARγ-mediated signaling to regulate photo-immunosuppression. It should also be noted that UVB-induced immunosuppression targets T helper cells polarized to the Th1 phenotype 70, 71. Given that PPARγ specifically inhibits Th1-mediated immune responses in a mouse model of multiple sclerosis 72, this suggests a potential role for PPARγ in UVB-induced Th1 suppression. Finally, it is quite possible that UVB-induced PPARγ activation acts to suppress immune responses independent of COX-2. There is growing evidence that many of the anti-inflammatory actions of PPARγ are dependent on its ability to block expression of a number of proinflammatory cytokines through several ligand-dependent transrepression mechanisms (reviewed in 37, 39).
Epidemiological evidence has long associated high fat diets with increased risk for neoplastic development, including cutaneous malignancy 73, 74. It has since become apparent that increased cancer risk from high fat diets is due in part to higher levels of oxidized lipid species 74, 75. A potential role for PPARγ in cutaneous carcinogenesis is indicated by a recent study demonstrating that mice deficient in either epidermal PPARγ or its heterodimer partner, RXR, exhibit increased rates of cutaneous neoplasia following a chemical carcinogenesis protocol 76. This study is in agreement with an earlier study demonstrating that heterozygous germ-line loss of PPARγ also augments chemically-induced cutaneous neoplasia 77. Potential mechanisms for this anti-neoplastic activity are suggested by studies indicating that PPARγ acts to suppress inflammatory hyperplasia and induce differentiation in the epidermis 42, 43, 45. In contrast to the above studies, work by He et al indicate that topical or systemic synthetic thiazolidinedione-type PPARγ agonists (rosiglitazone and troglitazone) have no affect on either photocarcinogenesis or chemical carcinogenesis 78. However, inasmuch as PPARγ is already activated by UVR 12, 59, this likely would negate the impact of exogenous ligand on PPARγ-mediated photobiological effects.
While PPARγ exhibits anti-neoplastic activity in models of cutaneous chemical carcinogenesis, the role of PPARγ in photocarcinogenesis is at yet unknown. If future studies demonstrate that loss of PPARγ similarly augments photocarcinogenesis, then it would be difficult to attribute this action to the ability of PPARγ to induce pro-carcinogenic COX-2 signaling within the epidmermis. In this scenario, this would simply indicate that UVR-induced PPARγ signaling is likely to have importantant COX-2 independent effects on photobiology. This would not be surprising, given the complexity and diversity of PPARγ-dependent transcriptional regulation 37, 39.
Mechanisms by which UVB generates ox-GPCs
Solar UV radiation can be broken down into 3 separate wavelength spectra, each with its own biological and toxic effects 1, 2. However, only long wavelength UVA (320–400 nm) and intermediate wavelength UVB (280–320) are able to penetrate the atmosphere in biologically significant quantities. Because of its long wavelength, UVA light has the greatest penetrating power of the UV spectra, reaching into the dermis. UVB, because of its shorter wavelength, has much less penetrating power, with most UVB being absorbed by the epidermis.
The ability of UVA and UVB irradiation to induce molecular changes is dependent on the first law of photochemistry, or the Grotthuss-Draper law. Simply put, a photon of light must be absorbed by a chemical substance to initiate a photochemical reaction. Once absorbed by the skin, the primary toxicologic effects of UVA radiation are due to production of reactive oxygen species (ROS) 79. This oxidative stress can occur through multiple mechanisms (reviewed in 79). However, a key mechanism occurs through photon absorption by endogenous, non-DNA chromophores, that then act as photosensitizers (S*, figure 1) that initiate ROS production 80. Like UVA, UVB is also able to induce photo-oxidation through similar mechanisms. However, unlike UVA, UVB is also directly absorbed by DNA (λmax=260 nm), resulting in direct photoexcitation, molecular rearrangement, and genotoxicity 80. Because of their much lower absorption maxima, polyunsaturated fatty acids do not absorb either UVA or UVB, thus they do not undergo direct photochemical reactions. UVR mediated changes to phospholipids is therefore thought to occur as a result of photo-oxidation reactions 80. Recent studies by photobiologists indicate that UVB generates ROS through a series of sequential steps involving: EGFR activation, ROS generation by cytosolic NADPH oxidase, ras/ERK & p38MAPK activation, p53 activation, and finally apoptotic signaling leading to mitochondrial ROS generation 81–88. Thus, UVB-mediated ROS are likely involved in the generation of ox-GPCs.
Given the above discussion, it is somewhat surprising that our group 11, 12 and others 89 show that UVB irradiation of purified GPCs directly in a cell-free system generates biologically active ox-GPCs. Given that the ox-GPC precursor molecule 1-hexadecyl-2-arachidonoyl-GPC does not absorb UVB, how does the initial photosensitization reaction take place? As shown in figure 2, 1-hexadecyl-2-arachidonoyl-GPC left exposed to room air to allow oxidation results in the formation of products that absorb significantly in the UVB spectrum. These results are also in agreement with an earlier study in which 2-arachidonoyl-phosphatidylcholine was treated with 15-lipooxygenase, then allowed to oxidize in air for several days 19. This oxidation resulted in the presence of more polar species with peak absorption at 235 nm, similar to the peak absorption seen in our studies after air oxidation alone. These findings fit with the notion that any low level amounts of cellular ROS that act upon 1-hexadecyl-2-arachidonoyl GPC could result in the production of direct targets for UVB. Even small amounts of ox-GPC could then serve as a photosensitizer and source of the initial ROS that could then propogate through non-oxidized GPCs in a free-radical reaction chain reaction. This also introduces the possibility that ox-GPCs also may serve as targets for direct photoexcitation and non-ROS-initiated photochemical reactions. Finally, given the ability of GPCs to serve as targets of photooxidation, how does this result in both PAF-R and PPARγ agonist production? Previous studies indicate that both PPARγ and PAF-R ligands are derived from intact oxidized alkyl phosphopholipids with a sn-1 ether linkage rather than the much larger pool of phospholipids with esterified fatty acids at the sn-1 position 58, 90. However, given the structure of PAF itself (sn-2 acetyl group), along with less potent succinoyl-PAF, 5-oxovaleroyl-PAF, butanoyl-PAF(C4-PAF) and butenoyl-PAF (C4:1-PAF), available evidence indicates that PAF-R agonists consist primarily of short chain sn-2 acyl groups terminating with either an ω-oxy function or a methyl group (Figure 1) 11, 18, 19.
Figure 2. Air oxidation of 1-hexadecyl-2-arachidonoyl GPC results in products that absorb in UVB range.
The absorbance of 200 μg/ml purified 1-hexadecyl-2-arachidonoyl-GPC (Avanti Polar Lipids) in ethanol was obtained after dried lipid was left at room temperature in the presence of air for one or fourteen days, and the absorbance obtained using a spectrophotometer.
In contrast to ox-GPCs with PAF-R ligand activity, we propose that ox-GPCs with highly potent PPARγ ligand activity will exhibit intermediate chain cleavage products with ω-oxy functions (Figure 1). This idea is based on a number of observations: First, the structure of AzPC consists of a 9-carbon azelaic acid esterified at the sn-2 position 58. Second, small chain products, like PAF, exhibit limited capacity to displace binding of the PPARγ ligand, rosiglitazone 58. Most importantly, we show that treatment of either AzPC or lipid extracts from UVB-irradiated KB cells with serum PAF acetylhydrolase (PAF-AH) results in loss of PPARγ-agonistic activities (Figure 3). This is important, as serum PAF-AH exhibits high substrate specificity for phospholipids with short sn-2 acyl chains; enzymatic activity is rapidly lost with saturated acyl chain lengths greater than 2 carbons, with 10-carbon acyl groups demonstrating essentially no activity 19, 91, 92. However, as seen in figure 3 for AzPC, serum PAF-AH exhibits potent activity for oxidized phospholipids with sn-2 residues of up to 9 carbons in length that contain ω-oxy functions 19, 91–93. Thus, while PAF-AH has been proposed to limit the actions of un-regulated ox-GPC production on PAF-R activation 19, 92, it is also quite likely that it serves to limit the ability of ox-GPCs to activate PPARγ. Finally, there is evidence that short to intermediate chain ox-GPCs are found in vivo. Studies by Takumura et al demonstrate that 4–9 carbon dicarboxylic acids are found in choline phosphoglycerides isolated from bovine brain, presumably as a result of oxidative cleavage 91.
Figure 3. PPARγ agonistic activity in UVB-irradiated KB cells is abolished by PAF-AH enzyme treatment.
KB cells co-transfected with PPRE-luciferase reporter and β-gal plasmids were treated with AzPC (1.5 μM), or AzPC treated with PAF-AH or PBS control, or lipid extracts from 107 KB cells either sham- or 2000 J/m2 UVB irradiated treated with PAF-AH or PBS. Cells were harvested 24 hr after treatment for luciferase and β-gal activity assay as previously described 12
Therapeutic Potential of OX-GPCs in UVR-mediated effects
Inasmuch as UV irradiation results in the production of ox-GPCs formed by free radical-mediated oxidation with PAF-R or PPARγ activity, ongoing studies are defining the exact roles of these novel biological lipids in UV-mediated responses. The data to date suggest that the PAF system is involved in production of cytokines including TNF-α, and also mediates systemic immunosuppression. The PPARγ system appears to be important in regulating keratinocyte proliferation & differentiation. The PPARγ system is also important in UVB-mediated production of COX-2 in keratinocytes, which has been linked to immunosuppression and neoplastic development. Inasmuch as UVB-mediated PAF-R and PPARγ activation likely plays a role in UV-mediated effects, an obvious question would be whether these effects could be tempered with the use of antioxidants. Of interest, McIntyre and colleagues have demonstrated that hamsters fed chow supplemented with vitamin C did not produce ox-GPCs following challenge with the pro-oxidative stressor cigarette smoke 94. Along those lines, we have demonstrated that mice fed a diet supplemented with 10g/kg vitamin C failed to exhibit a UVB-mediated decrease in contact hypersensitivity to DNFB. However, intraperitoneal injection of CPAF resulted in a similar inhibition of contact hypersensitivity in mice fed standard versus vitamin C-enriched chow (manuscript in preparation).
Recent studies have used systemic and topical antioxidants in murine model systems to modulate UV effects 95–98. The relative favorable safety profile of antioxidants has allowed human studies (reviewed in 99–101). As our understanding of the targets for UV radiation and their pathophysiological significance increases, more effective therapies can be designed to modulate the both beneficial and detrimental consequences of this ubiquitous agent.
Future considerations
From the standpoint of future studies, there are several key questions that remain unanswered regarding the role of ox-GPCs in photobiology. First, additional studies are needed to identify the molecular structure of both PAF-R and PPARγ ligands that are produced as a consequence of UVB irradiation. Once these ligands have been identified, the specific and characteristic structural differences in the oxidized sn-2 constituents that differentiate a PAF-R ligand from a PPARγ ligand should be verified. Finally, identification of these ligands will support further studies to verify that these ligands are produced in physiologically relevant concentrations within not only cultured cell lines, but also intact epidermis. These questions are active areas of investigation within our laboratories.
Acknowledgments
The authors gratefully acknowledge funding from the Prevent Cancer Foundation (RLK), a VA Merit Award grant (JBT), and NIH grants R03AR053710 (RLK), R01HL062996 (JBT) and U19A1070448 (JBT).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hussein MR. Ultraviolet radiation and skin cancer: molecular mechanisms. J Cutaneous Pathol. 2005;32(3):191–205. doi: 10.1111/j.0303-6987.2005.00281.x. [DOI] [PubMed] [Google Scholar]
- 2.Nishigori C. Cellular aspects of photocarcinogenesis. Photochem Photobiol Sci. 2006;5(2):208–14. doi: 10.1039/b507471a. [DOI] [PubMed] [Google Scholar]
- 3.Sesto A, Navarro M, Burslem F, Jorcano JL. Analysis of the ultraviolet B response in primary human keratinocytes using oligonucleotide microarrays. Proc Natl Acad Sci, USA. 2002:052678999. doi: 10.1073/pnas.052678999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Enk CD, Jacob-Hirsch J, Gal H, et al. The UVB-induced gene expression profile of human epidermis in vivo is different from that of cultured keratinocytes. Oncogene. 2006;25(18):2601–14. doi: 10.1038/sj.onc.1209292. [DOI] [PubMed] [Google Scholar]
- 5.Ullrich SE. Sunlight and skin cancer: lessons from the immune system. Mol Carcinogen. 2007;46(8):629–33. doi: 10.1002/mc.20328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gardner HW. Oxygen radical chemistry of polyunsaturated fatty acids. Free Rad Biol Med. 1989;7(1):65–86. doi: 10.1016/0891-5849(89)90102-0. [DOI] [PubMed] [Google Scholar]
- 7.Galle J, Hansen-Hagge T, Wanner C, Seibold S. Impact of oxidized low density lipoprotein on vascular cells. Atherosclerosis. 2006;185(2):219–26. doi: 10.1016/j.atherosclerosis.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 8.Nicholson AC, Hajjar DP. CD36, oxidized LDL and PPAR gamma: pathological interactions in macrophages and atherosclerosis. Vascular Pharmacol. 2004;41(4–5):139–46. doi: 10.1016/j.vph.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 9.Travers JB, Harrison KA, Johnson CA, Clay KL, Morelli JG. Platelet-activating factor biosynthesis induced by various stimuli in human HaCaT keratinocytes. J Invest Dermatol. 1996;107(1):88–94. doi: 10.1111/1523-1747.ep12298295. [DOI] [PubMed] [Google Scholar]
- 10.Frankel EN. Chemistry of free radical and singlet oxidation of lipids. Prog Lipid Res. 1984;23(4):197–221. doi: 10.1016/0163-7827(84)90011-0. [DOI] [PubMed] [Google Scholar]
- 11.Marathe GK, Johnson C, Billings SD, et al. Ultraviolet B radiation generates platelet-activating factor-like phospholipids underlying cutaneous damage. J Biol Chem. 2005;280(42):35448–57. doi: 10.1074/jbc.M503811200. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Q, Southall MD, Mezsick SM, et al. Epidermal peroxisome proliferator-activated receptor gamma as a target for ultraviolet B radiation. J Biol Chem. 2005;280(1):73–9. doi: 10.1074/jbc.M409795200. [DOI] [PubMed] [Google Scholar]
- 13.Ishii S, Nagase T, Shimizu T. Platelet-activating factor receptor. Prostaglandins Other Lipid Mediat. 2002;68–69:599–609. doi: 10.1016/s0090-6980(02)00058-8. [DOI] [PubMed] [Google Scholar]
- 14.Travers JB, Huff JC, Rola-Pleszczynski M, Gelfand EW, Morelli JG, Murphy RC. Identification of functional platelet-activating factor receptors on human keratinocytes. J Invest Dermatol. 1995;105(6):816–23. doi: 10.1111/1523-1747.ep12326581. [DOI] [PubMed] [Google Scholar]
- 15.Alappatt C, Johnson CA, Clay KL, Travers JB. Acute keratinocyte damage stimulates platelet-activating factor production. Arch Dermatol Res. 2000;292(5):256–9. doi: 10.1007/s004030050483. [DOI] [PubMed] [Google Scholar]
- 16.Marathe GK, Davies SS, Harrison KA, et al. Inflammatory platelet-activating factor-like phospholipids in oxidized low density lipoproteins are fragmented alkyl phosphatidylcholines. J Biol Chem. 1999;274(40):28395–404. doi: 10.1074/jbc.274.40.28395. [DOI] [PubMed] [Google Scholar]
- 17.Prescott SM, Zimmerman GA, Stafforini DM, McIntyre TM. Platelet-activating factor and related lipid mediators. Ann Rev Biochem. 2000;69:419–45. doi: 10.1146/annurev.biochem.69.1.419. [DOI] [PubMed] [Google Scholar]
- 18.Goetzl EJ, Derian CK, Tauber AI, Valone FH. Novel effects of 1-O-hexadecyl-2-acyl-sn-glycero-3-phosphorycholine mediators on human leukocyte function: delineation of the specific roles of the acyl substituents. Biochem Biophys Res Commun. 1980;94(3):881–8. doi: 10.1016/0006-291x(80)91317-0. [DOI] [PubMed] [Google Scholar]
- 19.Stremler KE, Stafforini DM, Prescott SM, McIntyre TM. Human plasma platelet-activating factor acetylhydrolase. Oxidatively fragmented phospholipids as substrates. J Biol Chem. 1991;266(17):11095–103. [PubMed] [Google Scholar]
- 20.Kamido H, Eguchi H, Ikeda H, et al. Core aldehydes of alkyl glycerophosphocholines in atheroma induce platelet aggregation and inhibit endothelium-dependent arterial relaxation. J Lipid Res. 2002;43(1):158–66. [PubMed] [Google Scholar]
- 21.Travers JB, Johnson C, Clay KL, et al. Identification of sn-2 acetyl glycerophosphocholines in human keratinocytes. J Lipid Mediat Cell Signal. 1997;16(3):139–45. doi: 10.1016/s0929-7855(97)00575-0. [DOI] [PubMed] [Google Scholar]
- 22.Pei Y, Barber LA, Murphy RC, et al. Activation of the Epidermal Platelet-Activating Factor Receptor Results in Cytokine and Cyclooxygenase-2 Biosynthesis. J Immunol. 1998;161(4):1954–61. [PubMed] [Google Scholar]
- 23.Dy LC, Pei Y, Travers JB. Augmentation of ultraviolet B radiation-induced tumor necrosis factor production by the epidermal platelet-activating factor receptor. J Biol Chem. 1999;274(38):26917–21. doi: 10.1074/jbc.274.38.26917. [DOI] [PubMed] [Google Scholar]
- 24.Walterscheid JP, Ullrich SE, Nghiem DX. Platelet-activating factor, a molecular sensor for cellular damage, activates systemic immune suppression. J Exp Med. 2002;195(2):171–9. doi: 10.1084/jem.20011450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marques SA, Dy LC, Southall MD, et al. The platelet-activating factor receptor activates the extracellular signal-regulated kinase mitogen-activated protein kinase and induces proliferation of epidermal cells through an epidermal growth factor-receptor-dependent pathway. J Pharmacol Exp Ther. 2002;300(3):1026–35. doi: 10.1124/jpet.300.3.1026. [DOI] [PubMed] [Google Scholar]
- 26.Ishii S, Nagase T, Tashiro F, et al. Bronchial hyperreactivity, increased endotoxin lethality and melanocytic tumorigenesis in transgenic mice overexpressing platelet-activating factor receptor. EMBO J. 1997;16(1):133–42. doi: 10.1093/emboj/16.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barber LA, Spandau DF, Rathman SC, et al. Expression of the platelet-activating factor receptor results in enhanced ultraviolet B radiation-induced apoptosis in a human epidermal cell line. J Biol Chem. 1998;273(30):18891–7. doi: 10.1074/jbc.273.30.18891. [DOI] [PubMed] [Google Scholar]
- 28.Wolf P, Nghiem DX, Walterscheid JP, et al. Platelet-activating factor is crucial in psoralen and ultraviolet A-induced immune suppression, inflammation, and apoptosis. Am J Pathol. 2006;169(3):795–805. doi: 10.2353/ajpath.2006.060079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shreedhar V, Giese T, Sung VW, Ullrich SE. A Cytokine Cascade Including Prostaglandin E2, IL-4, and IL-10 Is Responsible for UV-Induced Systemic Immune Suppression. J Immunol. 1998;160(8):3783–9. [PubMed] [Google Scholar]
- 30.Zhang Q, Yao Y, Konger RL, et al. UVB Radiation-Mediated Inhibition of Contact Hypersensitivity Reactions Is Dependent on the Platelet-Activating Factor System. J Invest Dermatol. 2008 doi: 10.1038/sj.jid.5701251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Travers JB, Edenberg HJ, Zhang Q, et al. Augmentation of UVB Radiation-Mediated Early Gene Expression by the Epidermal Platelet-Activating Factor Receptor. J Invest Dermatol. 2007;128(2):455–60. doi: 10.1038/sj.jid.5701083. [DOI] [PubMed] [Google Scholar]
- 32.Friedmann PS, Cooper HL, Healy E. Peroxisome proliferator-activated receptors and their relevance to dermatology. Acta Dermato-Venereologica. 2005;85(3):194–202. doi: 10.1080/00015550510030104. [DOI] [PubMed] [Google Scholar]
- 33.Michalik L, Wahli W. Peroxisome proliferator-activated receptors (PPARs) in skin health, repair and disease. Biochim Biophys Acta. 2007;1771(8):991–8. doi: 10.1016/j.bbalip.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 34.Issemann I, Green S. Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature. 1990;347(6294):645–50. doi: 10.1038/347645a0. [DOI] [PubMed] [Google Scholar]
- 35.Meirhaeghe A, Amouyel P. Impact of genetic variation of PPARγ in humans. Mol Gen Metab. 2004;83(1–2):93–102. doi: 10.1016/j.ymgme.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 36.Kuenzli S, Saurat JH. Peroxisome proliferator-activated receptors in cutaneous biology. Br J Dermatol. 2003;149(2):229–36. doi: 10.1046/j.1365-2133.2003.05532.x. [DOI] [PubMed] [Google Scholar]
- 37.Ricote M, Glass CK. PPARs and molecular mechanisms of transrepression. Biochim Biophys Acta. 2007;1771(8):926–35. doi: 10.1016/j.bbalip.2007.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heikkinen S, Auwerx J, Argmann CA. PPARgamma in human and mouse physiology. Biochim Biophys Acta. 2007;1771(8):999–1013. doi: 10.1016/j.bbalip.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Genolet R, Wahli W, Michalik L. PPARs as drug targets to modulate inflammatory responses? Cur Drug Targets - Inflamm Allergy. 2004;3(4):361–75. doi: 10.2174/1568010042634578. [DOI] [PubMed] [Google Scholar]
- 40.Szeles L, Torocsik D, Nagy L. PPARgamma in immunity and inflammation: cell types and diseases. Biochimica et Biophysica Acta. 2007;1771(8):1014–30. doi: 10.1016/j.bbalip.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 41.Krishnan A, Nair SA, Pillai MR. Biology of PPAR gamma in cancer: a critical review on existing lacunae. Cur Mol Med. 2007;7(6):532–40. doi: 10.2174/156652407781695765. [DOI] [PubMed] [Google Scholar]
- 42.Mao-Qiang M, Fowler AJ, Schmuth M, et al. Peroxisome-proliferator-activated receptor (PPAR)-gamma activation stimulates keratinocyte differentiation. J Invest Dermatol. 2004;123(2):305–12. doi: 10.1111/j.0022-202X.2004.23235.x. [DOI] [PubMed] [Google Scholar]
- 43.Demerjian M, Man MQ, Choi EH, et al. Topical treatment with thiazolidinediones, activators of peroxisome proliferator-activated receptor-gamma, normalizes epidermal homeostasis in a murine hyperproliferative disease model. Exp Dermatol. 2006;15(3):154–60. doi: 10.1111/j.1600-0625.2006.00402.x. [DOI] [PubMed] [Google Scholar]
- 44.Kuenzli S, Saurat JH. Peroxisome proliferator-activated receptors as new molecular targets in psoriasis. Cur Drug Targets - Inflamm Allergy. 2004;3(2):205–11. doi: 10.2174/1568010043343976. [DOI] [PubMed] [Google Scholar]
- 45.Ellis CN, Varani J, Fisher GJ, et al. Troglitazone Improves Psoriasis and Normalizes Models of Proliferative Skin Disease: Ligands for Peroxisome Proliferator-Activated Receptor-γ Inhibit Keratinocyte Proliferation. Arch Dermatol. 2000;136(5):609–16. doi: 10.1001/archderm.136.5.609. [DOI] [PubMed] [Google Scholar]
- 46.Varani J, Bhagavathula N, Ellis CN, Pershadsingh HA. Thiazolidinediones: potential as therapeutics for psoriasis and perhaps other hyperproliferative skin disease. Exp Opinion Investigational Drugs. 2006;15(11):1453–68. doi: 10.1517/13543784.15.11.1453. [DOI] [PubMed] [Google Scholar]
- 47.Zhu Y, Alvares K, Huang Q, Rao MS, Reddy JK. Cloning of a new member of the peroxisome proliferator-activated receptor gene family from mouse liver. J Biol Chem. 1993;268(36):26817–20. [PubMed] [Google Scholar]
- 48.Lehmann JM, Moore LB, Smith-Oliver TA, Wilkison WO, Willson TM, Kliewer SA. An Antidiabetic Thiazolidinedione Is a High Affinity Ligand for Peroxisome Proliferator-activated Receptor γ (PPARγ) J Biol Chem. 1995;270(22):12953–6. doi: 10.1074/jbc.270.22.12953. [DOI] [PubMed] [Google Scholar]
- 49.Forman BM, Tontonoz P, Chen J, Brun RP, Spiegelman BM, Evans RM. 15-Deoxy-delta 12, 14-prostaglandin J2 is a ligand for the adipocyte determination factor PPAR gamma. Cell. 1995;83(5):803–12. doi: 10.1016/0092-8674(95)90193-0. [DOI] [PubMed] [Google Scholar]
- 50.Kliewer SA, Lenhard JM, Willson TM, Patel I, Morris DC, Lehmann JM. A prostaglandin J2 metabolite binds peroxisome proliferator-activated receptor gamma and promotes adipocyte differentiation. Cell. 1995;83(5):813–9. doi: 10.1016/0092-8674(95)90194-9. [DOI] [PubMed] [Google Scholar]
- 51.Rosen ED, Spiegelman BM. PPARgamma: a Nuclear Regulator of Metabolism, Differentiation, and Cell Growth. J Biol Chem. 2001;276(41):37731–4. doi: 10.1074/jbc.R100034200. [DOI] [PubMed] [Google Scholar]
- 52.Han H, Shin SW, Seo CY, et al. 15-Deoxy-delta 12,14-prostaglandin J2 (15d-PGJ 2) sensitizes human leukemic HL-60 cells to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis through Akt downregulation. Apoptosis. 2007;12(11):2101–14. doi: 10.1007/s10495-007-0124-2. [DOI] [PubMed] [Google Scholar]
- 53.Lim HJ, Lee KS, Lee S, et al. 15d-PGJ2 stimulates HO-1 expression through p38 MAP kinase and Nrf-2 pathway in rat vascular smooth muscle cells. Toxicol Applied Pharmacol. 2007;223(1):20–7. doi: 10.1016/j.taap.2007.04.019. [DOI] [PubMed] [Google Scholar]
- 54.Xiang Z, Lin T, Reeves SA. 15d-PGJ2 induces apoptosis of mouse oligodendrocyte precursor cells. J Neuroinflammation. 2007;4:18. doi: 10.1186/1742-2094-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zingarelli B, Hake PW, Mangeshkar P, et al. Diverse cardioprotective signaling mechanisms of peroxisome proliferator-activated receptor-gamma ligands, 15-deoxy-Delta12,14-prostaglandin J2 and ciglitazone, in reperfusion injury: role of nuclear factor-kappaB, heat shock factor 1, and Akt. Shock. 2007;28(5):554–63. doi: 10.1097/shk.0b013e31804f56b9. [DOI] [PubMed] [Google Scholar]
- 56.Nagy L, Tontonoz P, Alvarez JGA, Chen H, Evans RM. Oxidized LDL Regulates Macrophage Gene Expression through Ligand Activation of PPARγ. Cell. 1998;93(2):229–40. doi: 10.1016/s0092-8674(00)81574-3. [DOI] [PubMed] [Google Scholar]
- 57.Hsi LC, Wilson LC, Eling TE. Opposing Effects of 15-Lipoxygenase-1 and -2 Metabolites on MAPK Signaling in Prostate. Alteration in Peroxisome Proliferator-Activated Receptor gamma. J Biol Chem. 2002;277(43):40549–56. doi: 10.1074/jbc.M203522200. [DOI] [PubMed] [Google Scholar]
- 58.Davies SS, Pontsler AV, Marathe GK, et al. Oxidized alkyl phospholipids are specific, high affinity peroxisome proliferator-activated receptor gamma ligands and agonists. J Biol Chem. 2001;276(19):16015–23. doi: 10.1074/jbc.M100878200. [DOI] [PubMed] [Google Scholar]
- 59.Zhang Q, Seltmann H, Zouboulis CC, Konger RL. Involvement of PPARgamma in oxidative stress-mediated prostaglandin E(2) production in SZ95 human sebaceous gland cells. J Invest Dermatol. 2006;126(1):42–8. doi: 10.1038/sj.jid.5700028. [DOI] [PubMed] [Google Scholar]
- 60.Baker PRS, Lin Y, Schopfer FJ, et al. Fatty acid transduction of nitric oxide signaling: multiple nitrated unsaturated fatty acid derivatives exist in human blood and urine and serve as endogenous peroxisome proliferator-activated receptor ligands. J Biol Chem. 2005;280(51):42464–75. doi: 10.1074/jbc.M504212200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schopfer FJ, Lin Y, Baker PRS, et al. Nitrolinoleic acid: an endogenous peroxisome proliferator-activated receptor gamma ligand. Proc Natl Acad Sci, USA. 2005;102(7):2340–5. doi: 10.1073/pnas.0408384102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wright MM, Schopfer FJ, Baker PRS, et al. Fatty acid transduction of nitric oxide signaling: nitrolinoleic acid potently activates endothelial heme oxygenase 1 expression. Proc Natl Acad Sci, USA. 2006;103(11):4299–304. doi: 10.1073/pnas.0506541103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Paunel AN, Dejam A, Thelen S, et al. Enzyme-independent nitric oxide formation during UVA challenge of human skin: characterization, molecular sources, and mechanisms. Free Rad Biol Med. 2005;38(5):606–15. doi: 10.1016/j.freeradbiomed.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 64.Weller R. Nitric oxide: a key mediator in cutaneous physiology. Clin Exp Dermatol. 2003;28(5):511–4. doi: 10.1046/j.1365-2230.2003.01365.x. [DOI] [PubMed] [Google Scholar]
- 65.Meade EA, McIntyre TM, Zimmerman GA, Prescott SM. Peroxisome Proliferators Enhance Cyclooxygenase-2 Expression in Epithelial Cells. J Biol Chem. 1999;274(12):8328–34. doi: 10.1074/jbc.274.12.8328. [DOI] [PubMed] [Google Scholar]
- 66.Chene G, Dubourdeau M, Balard P, et al. n-3 and n-6 polyunsaturated fatty acids induce the expression of COX-2 via PPARgamma activation in human keratinocyte HaCaT cells. Biochim Biophys Acta. 2007;1771(5):576–89. doi: 10.1016/j.bbalip.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 67.Kuwamoto K, Miyauchi-Hashimoto H, Tanaka K, et al. Possible involvement of enhanced prostaglandin E2 production in the photosensitivity in xeroderma pigmentosum group A model mice. J Invest Dermatol. 2000;114(2):241–6. doi: 10.1046/j.1523-1747.2000.0883x.x. [DOI] [PubMed] [Google Scholar]
- 68.Chun KS, Akunda JK, Langenbach R. Cyclooxygenase-2 inhibits UVB-induced apoptosis in mouse skin by activating the prostaglandin E2 receptors, EP2 and EP4. Cancer Res. 2007;67(5):2015–21. doi: 10.1158/0008-5472.CAN-06-3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fischer SM, Pavone A, Mikulec C, Langenbach R, Rundhaug JE. Cyclooxygenase-2 expression is critical for chronic UV-induced murine skin carcinogenesis. Mol Carcinogen. 2007;46(5):363–71. doi: 10.1002/mc.20284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Boonstra A, van Oudenaren A, Barendregt B, An L, Leenen PJM, Savelkoul HFJ. UVB irradiation modulates systemic immune responses by affecting cytokine production of antigen-presenting cells. Int Immunol. 2000;12(11):1531–8. doi: 10.1093/intimm/12.11.1531. [DOI] [PubMed] [Google Scholar]
- 71.Kremer IB, Hilkens CM, Sylva-Steenland RM, et al. Reduced IL-12 production by monocytes upon ultraviolet-B irradiation selectively limits activation of T helper-1 cells. J Immunol. 1996;157(5):1913–8. [PubMed] [Google Scholar]
- 72.Bright JJ, Natarajan C, Muthian G, Barak Y, Evans RM. Peroxisome Proliferator-Activated Receptor-γ-Deficient Heterozygous Mice Develop an Exacerbated Neural Antigen-Induced Th1 Response and Experimental Allergic Encephalomyelitis. J Immunol. 2003;171(11):5743–50. doi: 10.4049/jimmunol.171.11.5743. [DOI] [PubMed] [Google Scholar]
- 73.Black HS. Photocarcinogenesis and diet. Federation Proc. 1987;46(5):1901–5. [PubMed] [Google Scholar]
- 74.Logani MK, Davies RE. Lipid oxidation: biologic effects and antioxidants--a review. Lipids. 1980;15(6):485–95. doi: 10.1007/BF02534079. [DOI] [PubMed] [Google Scholar]
- 75.Bartsch H, Nair J. Chronic inflammation and oxidative stress in the genesis and perpetuation of cancer: role of lipid peroxidation, DNA damage, and repair. Langenbecks Arch Surg. 2006;391(5):499–510. doi: 10.1007/s00423-006-0073-1. [DOI] [PubMed] [Google Scholar]
- 76.Indra AK, Castaneda E, Antal MC, et al. Malignant transformation of DMBA/TPA-induced papillomas and nevi in the skin of mice selectively lacking retinoid-X-receptor alpha in epidermal keratinocytes. J Invest Dermatol. 2007;127(5):1250–60. doi: 10.1038/sj.jid.5700672. [DOI] [PubMed] [Google Scholar]
- 77.Nicol CJ, Yoon M, Ward JM, et al. PPARgamma influences susceptibility to DMBA-induced mammary, ovarian and skin carcinogenesis. Carcinogen. 2004;25(9):1747–55. doi: 10.1093/carcin/bgh160. [DOI] [PubMed] [Google Scholar]
- 78.He G, Muga S, Thuillier P, Lubet RA, Fischer SM. The effect of PPARgamma ligands on UV- or chemically-induced carcinogenesis in mouse skin. Mol Carcinogen. 2005;43(4):198–206. doi: 10.1002/mc.20111. [DOI] [PubMed] [Google Scholar]
- 79.Wondrak GT, Jacobson MK, Jacobson EL. Identification of Quenchers of Photoexcited States as Novel Agents for Skin Photoprotection. J Pharmacol Exp Ther. 2005;312(2):482–91. doi: 10.1124/jpet.104.075101. [DOI] [PubMed] [Google Scholar]
- 80.Wondrak GT, Jacobson MK, Jacobson EL. Endogenous UVA-photosensitizers: mediators of skin photodamage and novel targets for skin photoprotection. Photochem Photobiol Sci. 2006;5(2):215–37. doi: 10.1039/b504573h. [DOI] [PubMed] [Google Scholar]
- 81.Wan Y, Wang Z, Shao Y, Xu Y, Voorhees J, Fisher G. UV-induced expression of GADD45 is mediated by an oxidant sensitive pathway in cultured human keratinocytes and in human skin in vivo. Internatl J Mol Med. 2000;6(6):683–8. doi: 10.3892/ijmm.6.6.683. [DOI] [PubMed] [Google Scholar]
- 82.Jiang Q, Zhou C, Healey S, et al. UV radiation down-regulates Dsg-2 via Rac/NADPH oxidase-mediated generation of ROS in human lens epithelial cells. Internatl J Mol Med. 2006;18(2):381–7. [PubMed] [Google Scholar]
- 83.Wang H, Kochevar IE. Involvement of UVB-induced reactive oxygen species in TGF-beta biosynthesis and activation in keratinocytes. Free Rad Biol Med. 2005;38(7):890–7. doi: 10.1016/j.freeradbiomed.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 84.Fan C, Katsuyama M, Nishinaka T, Yabe-Nishimura C. Transactivation of the EGF receptor and a PI3 kinase-ATF-1 pathway is involved in the upregulation of NOX1, a catalytic subunit of NADPH oxidase. FEBS Letters. 2005;579(5):1301–5. doi: 10.1016/j.febslet.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 85.Rezvani HR, Dedieu S, North S, et al. Hypoxia-inducible factor-1alpha, a key factor in the keratinocyte response to UVB exposure. J Biol Chem. 2007;282(22):16413–22. doi: 10.1074/jbc.M611397200. [DOI] [PubMed] [Google Scholar]
- 86.Pani G, Bedogni B, Colavitti R, Anzevino R, Borrello S, Galeotti T. Cell compartmentalization in redox signaling. IUBMB Life. 2001;52(1–2):7–16. doi: 10.1080/15216540252774702. [DOI] [PubMed] [Google Scholar]
- 87.Zhao Y, Chaiswing L, Bakthavatchalu V, Oberley TD, St Clair DK. Ras mutation promotes p53 activation and apoptosis of skin keratinocytes. Carcinogen. 2006;27(8):1692–8. doi: 10.1093/carcin/bgl037. [DOI] [PubMed] [Google Scholar]
- 88.Van Laethem A, Nys K, Van Kelst S, et al. Apoptosis signal regulating kinase-1 connects reactive oxygen species to p38 MAPK-induced mitochondrial apoptosis in UVB-irradiated human keratinocytes. Free Rad Biol Med. 2006;41(9):1361–71. doi: 10.1016/j.freeradbiomed.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 89.Gruber F, Oskolkova O, Leitner A, et al. Photooxidation Generates Biologically Active Phospholipids That Induce Heme Oxygenase-1 in Skin Cells. J Biol Chem. 2007;282(23):16934–41. doi: 10.1074/jbc.M702523200. [DOI] [PubMed] [Google Scholar]
- 90.Pontsler AV, St Hilaire A, Marathe GK, Zimmerman GA, McIntyre TM. Cyclooxygenase-2 is induced in monocytes by peroxisome proliferator activated receptor gamma and oxidized alkyl phospholipids from oxidized low density lipoprotein. J Biol Chem. 2002;277(15):13029–36. doi: 10.1074/jbc.M109546200. [DOI] [PubMed] [Google Scholar]
- 91.Stremler KE, Stafforini DM, Prescott SM, Zimmerman GA, McIntyre TM. An oxidized derivative of phosphatidylcholine is a substrate for the platelet-activating factor acetylhydrolase from human plasma. J Biol Chem. 1989;264(10):5331–4. [PubMed] [Google Scholar]
- 92.Stafforini DM, McIntyre TM, Zimmerman GA, Prescott SM. Platelet-activating Factor Acetylhydrolases. J Biol Chem. 1997;272(29):17895–8. doi: 10.1074/jbc.272.29.17895. [DOI] [PubMed] [Google Scholar]
- 93.Stafforini DM, Sheller JR, Blackwell TS, et al. Release of free F2-isoprostanes from esterified phospholipids is catalyzed by intracellular and plasma platelet-activating factor acetylhydrolases. J Biol Chem. 2006;281(8):4616–23. doi: 10.1074/jbc.M507340200. [DOI] [PubMed] [Google Scholar]
- 94.Lehr HA, Weyrich AS, Saetzler RK, et al. Vitamin C blocks inflammatory platelet-activating factor mimetics created by cigarette smoking. J Clin Invest. 1997;99(10):2358–64. doi: 10.1172/JCI119417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Black HS, Lenger WA, Gerguis J, Thornby JI. Relation of antioxidants and level of dietary lipid to epidermal lipid peroxidation and ultraviolet carcinogenesis. Cancer Res. 1985;45(12 Pt 1):6254–9. [PubMed] [Google Scholar]
- 96.Sharma SD, Meeran SM, Katiyar SK. Dietary grape seed proanthocyanidins inhibit UVB-induced oxidative stress and activation of mitogen-activated protein kinases and nuclear factor-κB signaling in in vivo SKH-1 hairless mice. Mol Cancer Ther. 2007;6(3):995–1005. doi: 10.1158/1535-7163.MCT-06-0661. [DOI] [PubMed] [Google Scholar]
- 97.D’Agostini F, Balansky RM, Camoirano A, De Flora S. Modulation of light-induced skin tumors by N-acetylcysteine and/or ascorbic acid in hairless mice. Carcinogen. 2005;26(3):657–64. doi: 10.1093/carcin/bgi008. [DOI] [PubMed] [Google Scholar]
- 98.Staniforth V, Chiu LT, Yang NS. Caffeic acid suppresses UVB radiation-induced expression of interleukin-10 and activation of mitogen-activated protein kinases in mouse. Carcinogenesis. 2006;27(9):1803–11. doi: 10.1093/carcin/bgl006. [DOI] [PubMed] [Google Scholar]
- 99.Swindells K, Rhodes LE. Influence of oral antioxidants on ultraviolet radiation-induced skin damage in humans. Photodermatol Photoimmunol Photomed. 2004;20:297–304. doi: 10.1111/j.1600-0781.2004.00121.x. [DOI] [PubMed] [Google Scholar]
- 100.Mukhtar H. Eat Plenty of Green Leafy Vegetables for Photoprotection: Emerging Evidence. J Investig Dermatol. 2003;121(2):viii–viii. doi: 10.1046/j.1523-1747.2003.12377.x. [DOI] [PubMed] [Google Scholar]
- 101.F’Guyer S, Afaq F, Mukhtar H. Photochemoprevention of skin cancer by botanical agents. Photodermatol Photoimmunol Photomed. 2003;19(2):56–72. doi: 10.1034/j.1600-0781.2003.00019.x. [DOI] [PubMed] [Google Scholar]