Abstract
Background
Hemoglobin tetramers are the major oxygen-carrying molecules within the blood. We hypothesized that a lower hemoglobin level and its reduced oxygen-carrying capacity would associate with larger infarction in acute ischemic stroke patients.
Methods
We studied 135 consecutive patients with acute ischemic stroke and perfusion brain MRI. We explored the association of admission hemoglobin with initial infarct volumes on acute images and the volume of infarct expansion on follow-up images. Multivariable linear regression was performed to analyze the independent effect of hemoglobin on imaging outcomes.
Results
Bivariate analyses showed a significant inverse correlation between hemoglobin and initial volume in diffusion-weighted imaging (r = −0.20, p = 0.02) and absolute infarct growth (r = −0.20, p = 0.02). Multivariable linear regression modeling revealed that hemoglobin remained independently predictive of larger infarct volumes acutely (p < 0.005) and with greater infarct expansion (p < 0.01) after adjusting for known covariates.
Conclusions
Hemoglobin level at the time of acute ischemic stroke associates with larger infarcts and increased infarct growth. Clarification of the mechanism of this effect may yield novel insights for therapy.
Key Words: Acute ischemia, Hemoglobin, Ischemia, Stroke volume
Introduction
Ischemic stroke is the third leading cause of death in the US, and the leading cause of disability. Reperfusion represents the mainstay of therapy, however only a small fraction of patients qualify for these treatments. A better understanding of the elements that contribute to the growth of stroke in humans may provide insights into novel treatments. Studies that have explored the influences on stroke outcomes have identified a small number of modifiable risk factors (i.e. blood glucose [1, 2] and blood pressure [3]), but interventions unfortunately have not improved neurological outcomes [3, 4]. Thus, there is an urgent need to better understand and identify additional contributors to infarct growth, and to determine whether they can be targeted therapeutically.
Since cerebral infarction results from an imbalance between energy supply and demand, exploring factors that alter this balance may yield such insights. The fact that reperfusion approaches have shown consistent, albeit variable benefit emphasizes the proximate role that energy supply plays in affecting stroke outcomes. With this in mind, we hypothesized that the oxygen-carrying capacity of the blood may influence the outcome of ischemic brain tissue. Utilizing a cohort of acute stroke patients with perfusion brain MRI scans, we sought to determine whether reduced levels of hemoglobin were associated with larger infarcts. We also evaluated whether these parameters were associated with infarct expansion.
Methods
Study Population
The study population consisted of consecutive patients with acute ischemic stroke presenting to the emergency department of a single academic center who had diffusion-weighted images (DWI) and perfusion-weighted MRI within 12 h of symptom onset and a follow-up image after 5–100 days (median 12 days, interquartile range 6–55 days). The study was a retrospective analysis of patients recruited as part of a prospective study (MRI Diffusion/Perfusion Mismatch in Human Acute Stroke) from 1996 to 2009. Part of the study population has been investigated in previous publications [5, 6]. Patients who had received any thrombolytic or investigational treatments or patients in whom the final infarct volume could not be assessed due to motion artifact, hemorrhagic conversion, massive cerebral edema or hemicraniectomy were excluded.
Variables that could potentially influence acute infarct volume were recorded in each patient. These included age, gender, the causative classification system (CCS) etiologic stroke subtypes [7], admission NIH stroke scale (NIHSS) score, admission blood glucose and time to MRI. Although there were few subjects with small-vessel stroke, exclusion of these subjects did not alter the results. Hemoglobin values were retrospectively obtained from the medical records, with the first value on presentation being recorded. For follow-up imaging analysis, we included the time to follow-up and the volume of perfusion-diffusion mismatch along with the other previously stated variables. The study was conducted at a single academic center and the study protocol was approved by the local institutional review board.
Image Acquisition
MRI was performed on 1.5- or 3-tesla whole-body scanners (GE Signa; GE Medical Systems, or Siemens Sonata; Siemens Medical Solutions). DWI were obtained using echo planar imaging with b values (diffusion weighting) of 0 and 1,000 s/mm2. DWI data sets were corrected for motion and eddy current distortion [8]. Trace DWI maps were calculated from the geometric mean of the high b value images. Perfusion-weighted images were acquired using dynamic susceptibility contrast gradient echo or spin echo planar imaging. Images were acquired during the first pass of a bolus of 0.1 (gradient echo) or 0.2 mmol/kg of body weight (spin echo) of the contrast agent gadopentetate dimeglumine (Magnevist; Berlex Laboratories) injected with an MRI-compatible power injector (MEDRAD). Mean transit time (MTT) maps were calculated using methods described previously [9] where the arterial input function was selected manually or automatically with manual review. Follow-up imaging studies consisted of either fast spin echo T2-weighted, fluid-attenuated inverted recovery (n = 104) or non-contrast CT images (n = 31).
Image Analysis
The lesion on DWI, the final T2-weighted, fluid-attenuated inverted recovery or non-contrast CT images, and MTT maps were visually identified and outlined using image display and analysis programs (ALICE, Hayden Image Processing Solutions; MRIcro, University of Arizona, and Display, McConnell Brain Imaging Center, Montreal Neurological Institute). All lesion volumes were manually outlined and (for the MTT maps) compared to the contralateral hemisphere to confirm the region of abnormality. Lesion outlines were independently reviewed for accuracy by a trained neuroradiologist, and volumes were calculated from the outlines. We previously found that the interexaminer reliability of this technique was high (intraclass correlation coefficient = 0.99) [5].
Statistics
Bivariate analyses were used to assess the relationship between initial DWI volume and the following clinical covariates: CCS Trial of Org 10172 in Acute Stroke Treatment (TOAST) etiologic stroke subtype [7], age, gender, hemoglobin, time to acute DWI, time to follow-up image, NIHSS score and admission blood glucose. Age and gender were specifically included in the analysis because of their potential confounding association with hemoglobin levels. Other variables were chosen because of their previously reported association with these imaging outcomes. Bivariate relationships were tested using Pearson's correlation, or Wilcoxon's rank sum and Kruskal-Wallis tests, as appropriate. We also explored whether hemoglobin demonstrated a U-shaped relationship with imaging outcomes by performing a bivariate quadratic regression analysis.
Standard least-square linear regression models were developed to test for independence of hemoglobin as a predictor of initial DWI volume and volume of infarct growth. All other clinical variables with an a priori hypothesized effect on hemoglobin or the imaging outcome, or with bivariate p ≤ 0.2 were entered into the multivariate models. Because DWI lesion volume did not conform to a normal distribution, the data were log transformed before analysis [10]. In the analysis of infarct growth (final stroke volume – acute stroke volume), the time to follow-up scan and the size of the perfusion-diffusion mismatch deficit (MTT-DWI) were included since these variables may influence infarct size and growth. After the final models were developed, the interaction between hemoglobin and age, and hemoglobin and gender were specifically examined by introducing these dual interaction terms. All numeric variables were expressed as means ± SD or medians (interquartile ranges, IQR). A level of p < 0.05 was considered statistically significant.
Results
A total of 3,471 consecutive patients with ischemic stroke were admitted during the study period. Of them, 594 patients met the inclusion criteria and 335 of the 594 were eligible but excluded. Reasons for exclusion were refused consent (88), death and/or comfort measure only (CMO) (47), no acute lesion on MRI (44), inability to attend follow-up (27), new claustrophobia or anxiety (26), MRI technique failure (26), loss to follow-up (19), hemicraniectomy (18), lack of a follow-up image due to protocol restrictions (17), inability to obtain consent (11), severe cerebral edema (6), subacute lesion on MRI (3), and acute hemorrhagic infarction (3). Thus, the total study population consisted of 259 patients. Of these, 153 patients were prospectively collected, and 106 patients were retrospectively collected applying identical criteria.
Within the resulting patient population, the study group was defined a priori as the natural history cohort: therefore, 72 were excluded due to intravenous tissue plasminogen activator administration, 38 due to investigational drug studies [hyperoxia (22), induced hypertension (10), and desmoteplase (6)], 6 due to intra-arterial tissue plasminogen activator, 4 due to emergency carotid bypass surgery, 3 due to intra-arterial mechanical thrombolysis, and 1 due to an investigational interventional NeuroFlo device. Further analyses included the subsequent 135 patients. Table 1 summarizes the baseline characteristics, and clinical and imaging features of this cohort. Cerebral infarcts were located within the middle cerebral artery territory in 96 patients, anterior cerebral artery territory in 4 patients, posterior cerebral artery territory in 11 patients, internal carotid artery territory in 5 patients, and basilar artery and/or cerebellar artery territories in 19 patients. Ischemic lesion volumes on acute and follow-up images and volume of growth are also listed in table 1.
Table 1.
Baseline characteristics and clinical/imaging features
| Age, years (mean ± SD) | 65 ± 15 |
| Female patients, n | 45 (33%) |
| Hemoglobin, g/dl (mean ± SD) | |
| Female | 12.9 ± 1.6 |
| Male | 14.5 ± 1.6 |
| Median admission blood glucose, mg/dl (IQR) | 117 (104–139) |
| Median admission NIHSS (IQR) | 4 (2–9) |
| CCS-TOAST stroke subtype, n of patients | |
| Large artery atherosclerosis | 37 |
| Cardioaortic embolism | 58 |
| Small artery occlusion | 3 |
| Other causes | 16 |
| Undetermined causes | 21 |
| Time to acute MRI, h (mean ± SD) | 6±3 |
| Time to admission hemoglobin, h (mean ± SD) | 5±3 |
| Median DWI lesion volume, ml (IQR) | 7.3 (1.5–30.0) |
| Median acute MTT lesion volume, ml (IQR) | 55.9 (6.5–146.6) |
| Median follow-up infarct volume, ml (IQR) | 11.9 (2.4–51.1) |
| Median time to follow-up scan, days (IQR) | 12 (6–55) |
| Median volume of infarct growth, ml (IQR) | 2.3 (-0.6 to 18.2) |
IQR = Interquartile range. Other causes include cervical artery dissection, hypercoagulability syndrome, iatrogenic causes and cocaine.
Hemoglobin level demonstrated a statistically significant inverse correlation with acute DWI volume (r = −0.20, p = 0.02) and volume of infarct growth (r = −0.20, p = 0.02). Admission blood glucose was associated with infarct growth (r = 0.18, p = 0.04), but not with initial acute DWI volume (p > 0.20), which is consistent with prior reports [1, 2, 5]. CCS-TOAST stroke subtypes demonstrated a statistically significant association with initial infarct volume [11] (p = 0.04), but not with infarct expansion (p = 0.11). NIHSS scores were available for a subset of patients (n = 85), and analysis of these patients showed correlation with acute DWI volume (r = 0.50, p < 0.001) and infarct growth (r = 0.35, p < 0.001). Time to follow-up scan was also correlated with the volume of infarct growth (r = −0.27, p < 0.01). Other comparisons, including age, gender, and time-to-acute MRI, did not demonstrate significant correlations with the above imaging outcomes.
We also evaluated the correlation between hemoglobin and the imaging outcomes, stratified by sex, since women have lower hemoglobin levels than men. We found the inverse correlation was consistent for men (n = 90; r = −0.27, p = 0.01 for acute DWI volume, and r = −0.22, p = 0.04 for infarct growth) and also present for women (n = 45; r = −0.26, p = 0.08 for acute DWI volume, and r = −0.42, p = 0.004 for infarct growth). Because hemoglobin may be a marker for chronic disease, we evaluated whether there was an association between hemoglobin level and Framingham stroke risk score [12]. In patients in whom a risk score could be calculated (n = 94), there was no significant correlation with hemoglobin (r = −0.03, p = 0.77). We also evaluated the association between hemoglobin level and individual stroke risk factors including type II diabetes (p = 0.46), coronary heart disease (p = 0.25), atrial fibrillation (p = 0.32), hypertension (p = 0.03), hyperlipidemia (p = 0.56), smoking (p = 0.67) and CCS (p = 0.08). Hypertensive subjects had higher hemoglobin levels (14.3 vs. 13.7 g/dl), but hypertension was not statistically significantly associated with either acute DWI volume or infarct growth. Together, these data argue that hemoglobin is not a marker for chronic disease based on other known risk factors for stroke.
We next constructed a multivariable regression model, which demonstrated that hemoglobin was an independent predictor for acute DWI volume (p < 0.005). Table 2 lists the covariables included in the model, including age, gender, admission glucose, time to acute MRI and CCS stroke subtype. Because the effect of hemoglobin may be influenced by either age or sex, we explored the interaction of gender·hemoglobin and age·hemoglobin post hoc in the model. However, neither interaction term had a statistically significant effect on the model (both p > 0.20). We also included admission NIHSS score into the model post hoc because this value was not available for all patients (n = 85). In this model with fewer total subjects, hemoglobin remained an independent predictor of acute DWI volume (p = 0.03, table 2).
Table 2.
Multivariate relationships with acute infarct volume as the dependent variable
| Acute infarct volume | Without NIHSS (n = 135) |
With NIHSS (n = 85) |
||
|---|---|---|---|---|
| coefficient | p | coefficient | p | |
| Age | −0.003 | 0.53 | −0.007 | 0.13 |
| Female sex | −0.22 | <0.01 | −0.23 | <0.02 |
| Admission glucose | 0.003 | 0.02 | 0.002 | 0.16 |
| Admission hemoglobin | −0.14 | <0.005 | −0.11 | 0.03 |
| Stroke subtype | 0.04 | 0.03 | ||
| Large artery | 0.08 | 0.58 | 0.09 | 0.55 |
| Cardioembolic | 0.28 | 0.04 | 0.44 | <0.01 |
| Small artery | −1.03 | <0.01 | −0.81 | 0.01 |
| Other | 0.26 | 0.17 | 0.10 | 0.65 |
| Undetermined | 0.41 | 0.02 | 0.19 | 0.34 |
| Time to acute MRI | 0.02 | 0.34 | −0.004 | 0.86 |
| NIHSS | − | − | 0.07 | <0.001 |
p values of significant differences are italicized.
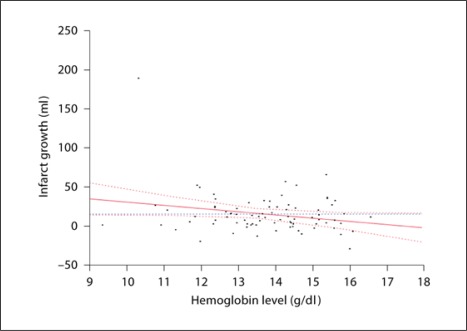
We also explored the independent effect of hemoglobin on infarct growth (table 3). We evaluated absolute infarct growth, rather than its percentage, which has been shown to be associated with neurological outcome [13]. We included the volume of the perfusion-diffusion mismatch, the time to the follow-up scans, and all other variables used previously. Hemoglobin was independently associated with infarct growth (p = 0.02). Additional variables that exhibited independent effects were gender (p = 0.01), stroke subtype (p = 0.03) and NIHSS score (p < 0.01). Based on this model, for each drop in hemoglobin of 1 g/dl, there is a 5.5 ± 2.4 cm3 increase in the volume of infarct growth (fig. 1).
Table 3.
Multivariate relationships with volume of stroke growth as the dependent variable
| Infarct growth | Coefficient | p |
|---|---|---|
| Age | −0.10 | 0.64 |
| Gender | −9.92 | 0.01 |
| Admission glucose | 0.002 | 0.97 |
| Admission hemoglobin | −4.95 | 0.02 |
| Stroke subtype | 0.03 | |
| Large artery | 0.46 | 0.94 |
| Cardioembolic | 1.53 | 0.80 |
| Small artery | −9.80 | 0.47 |
| Other | 25.88 | <0.01 |
| Undetermined | −18.07 | 0.03 |
| Time to follow up scan | −0.12 | 0.23 |
| NIHSS | 1.59 | <0.01 |
| Perfusion-diffusion mismatch volume | 0.05 | 0.21 |
p values of significant differences are italicized.
Fig. 1.
Hemoglobin level versus infarct growth.
Because elevated hematocrit has been reported to associate with infarct expansion [14], we explored whether a U-shaped polynomial association existed with hemoglobin levels. We found that in bivariate analysis, quadratic regression provided a statistically significantly improved fit compared to linear regression (p < 0.01). However, when we analyzed this relationship in multivariable regression, incorporating hemoglobin with a quadratic term did not meet statistical criteria for a better fit than linear regression (p = 0.06), perhaps due to the few number of patients at the edges of the hemoglobin range.
Finally, we performed a subset analysis only in patients who had oligemic brain tissue ‘at risk’. Although perfusion MRI is imprecise in differentiating between benign oligemia and true penumbra, we reasoned that selecting all patients with a perfusion-diffusion mismatch would provide a population of patients with greater risk for infarct growth; in 110 patients, perfusion volume was ≥120% of diffusion volume, a previously defined selection criterion [10]. Hemoglobin had a statistically significant inverse correlation with infarct growth (r = −0.20, p = 0.04), consistent with the entire cohort. In a multivariable analysis (table 4), hemoglobin remained independently significant in this subgroup (p = 0.01), as did gender (p = 0.02), CCS stroke subtype (p = 0.03) and NIHSS (p = 0.02). Conversely, in patients who did not have a mismatch on perfusion MRI (n = 25), hemoglobin did not correlate with the volume of infarct expansion.
Table 4.
Multivariate relationships with volume of stroke growth as the dependent variable (only in those patients with perfusion-diffusion mismatch >120%)
| Infarct growth in patients with mismatch | Coefficient | p |
|---|---|---|
| Age | −0.07 | 0.76 |
| Gender | −11.26 | 0.02 |
| Admission glucose | 0.003 | 0.97 |
| Admission hemoglobin | −6.71 | 0.01 |
| Stroke subtype | 0.03 | |
| Large artery | −0.17 | 0.99 |
| Cardioembolic | −2.68 | 0.76 |
| Small artery | −7.35 | 0.78 |
| Other | 31.77 | 0.01 |
| Undetermined | −21.57 | 0.06 |
| Time to follow up scan | −0.13 | 0.31 |
| NIHSS | 1.51 | 0.02 |
| Perfusion-diffusion mismatch volume | 0.05 | 0.32 |
p values of significant differences are italicized.
Discussion
Using a systematic and carefully characterized cohort of acute ischemic stroke patients, we present data that lower hemoglobin values are associated with larger acute infarcts and an increased degree of infarct growth. This effect is independent of recognized variables that contribute to infarct size, including age, sex, admission blood glucose, NIHSS, CCS-TOAST stroke subtype and MRI perfusion parameters.
The mechanism by which lower hemoglobin associates with larger infarcts is unknown. However, one possible explanation may be through its influence on energy supply. Hemoglobin carries 98% of the total blood oxygen, yet within one standard deviation of the normal range that level may vary by as much as 20% [15]. If a 20% reduced oxygen-carrying capacity occurs in the context of normally functioning cerebrovasculature, autoregulatory mechanisms typically compensate by augmenting cerebral blood flow [16]. However, in the context of ischemia, autoregulatory compensation is significantly impaired in the ischemic brain [17], with limited ability of the brain to increase the extraction of available oxygen [18]. The absolute oxygen-carrying capacity of the blood may therefore influence tissue fate under these circumstances and increase the conversion into infarct.
Available evidence in animal models of ischemic stroke suggests that lower hemoglobin reduces the threshold for ischemia [19] and results in larger infarct volumes [20, 21]. In human patients undergoing cardiopulmonary bypass, low hematocrit is associated with increased risk of clinically detected perioperative stroke [22]. This is supported by mathematical modeling which demonstrates that lower hemoglobin may adversely affect the energy balance within the penumbra [17]. Such modeling suggests a gradual and approximately linear change in oxygen metabolism at hemoglobin values >10 g/dl, with a more abrupt decline at levels below that. Our study population contained values primarily between 12 and 16 g/dl, and our data are consistent with a model whereby modest decreases in hemoglobin predict larger stroke volumes.
Prior animal [16] and some human data [14] have suggested that a high hematocrit (and therefore hemoglobin) level may also result in larger infarcts, perhaps through its influence on increasing blood viscosity. We investigated whether our data supported a deleterious effect of high hemoglobin by exploring a quadratic relationship between hemoglobin and infarct growth. While we found a U-shaped relationship in bivariate analysis, we were only able to demonstrate a trend for one in multivariable analysis. While this supports the notion that highly elevated and low hemoglobin are both associated with greater infarct expansion, further analysis in a larger cohort of patients is needed for confirmation.
There are several limitations to our analysis. First, our study was restricted only to patients with an initial brain MRI. Although rapid brain MRI scans are part of the standard of care in our stroke center, restricting our analysis to these patients could systematically bias against patients with delayed presentation and/or those patients with a contraindication to MRI. Moreoever, the median volume of strokes in our subjects was smaller than that in other populations of stroke patients [23] and this may restrict the generalizability of our results. It is possible that the higher acuity of patients with larger strokes made enrollment into our study a lower priority, where other clinical concerns may have superseded a rapid MRI. Finally, the time to the follow-up scan varied as a consequence of the retrospective nature of the study. However, when we adjusted for this variable, the association of hemoglobin with the imaging outcomes remained significant.
Our study has several strengths. It involved a relatively large sample size of systematically collected data on stroke patients, and stroke volumes were assessed in a blinded fashion. Moreover, there was temporal proximity of the initial acute MRI to the admission hemoglobin measurement. Finally, there was rigorous adjustment for potential confounders in our multivariable analyses.
Hemoglobin is the key oxygen-carrying molecule in the body, and may therefore play a role in determining tissue fate within the penumbra. Our data demonstrate that lower hemoglobin levels are associated with larger strokes and infarct growth. Future studies focused on investigating the association of hemoglobin with clinical outcome, and the mechanism of the association would ultimately guide potential treatment options.
Disclosure Statement
The authors report no conflicts of interest.
Acknowledgment
This study was supported by the American Academy of Neurology Foundation Clinical Training Research Fellowship (W.T.K.), NIH grants R01-NS059710, U01 NS069208–01 (H.A.), R01-NS051412, P50-NS051343 (A.B.S.) and R01-NS038477 (A.G.S.).
References
- 1.Baird TA, Parsons MW, Phanh T, Butcher KS, Desmond PM, Tress BM, Colman PG, Chambers BR, Davis SM. Persistent poststroke hyperglycemia is independently associated with infarct expansion and worse clinical outcome. Stroke. 2003;34:2208–2214. doi: 10.1161/01.STR.0000085087.41330.FF. [DOI] [PubMed] [Google Scholar]
- 2.Parsons MW, Barber PA, Desmond PM, Baird TA, Darby DG, Byrnes G, Tress BM, Davis SM. Acute hyperglycemia adversely affects stroke outcome: a magnetic resonance imaging and spectroscopy study. Ann Neurol. 2002;52:20–28. doi: 10.1002/ana.10241. [DOI] [PubMed] [Google Scholar]
- 3.Geeganage C, Bath PM. Interventions for deliberately altering blood pressure in acute stroke. Cochrane Database Syst Rev. 2008:CD000039. doi: 10.1002/14651858.CD000039.pub2. [DOI] [PubMed] [Google Scholar]
- 4.Gray CS, Hildreth AJ, Sandercock PA, O'Connell JE, Johnston DE, Cartlidge NE, Bamford JM, James OF, Alberti KG. Glucose-potassium-insulin infusions in the management of post-stroke hyperglycaemia: the UK Glucose Insulin in Stroke Trial (GIST-UK) Lancet Neurol. 2007;6:397–406. doi: 10.1016/S1474-4422(07)70080-7. [DOI] [PubMed] [Google Scholar]
- 5.Ay H, Arsava EM, Rosand J, Furie KL, Singhal AB, Schaefer PW, Wu O, Gonzalez RG, Koroshetz WJ, Sorensen AG. Severity of leukoaraiosis and susceptibility to infarct growth in acute stroke. Stroke. 2008;39:1409–1413. doi: 10.1161/STROKEAHA.107.501932. [DOI] [PubMed] [Google Scholar]
- 6.Wu O, Koroshetz WJ, Ostergaard L, Buonanno FS, Copen WA, Gonzalez RG, Rordorf G, Rosen BR, Schwamm LH, Weisskoff RM, Sorensen AG. Predicting tissue outcome in acute human cerebral ischemia using combined diffusion- and perfusion-weighted MR imaging. Stroke. 2001;32:933–942. doi: 10.1161/01.str.32.4.933. [DOI] [PubMed] [Google Scholar]
- 7.Ay H, Benner T, Arsava EM, Furie KL, Singhal AB, Jensen MB, Ayata C, Towfighi A, Smith EE, Chong JY, Koroshetz WJ, Sorensen AG. A computerized algorithm for etiologic classification of ischemic stroke: the Causative Classification of Stroke System. Stroke. 2007;38:2979–2984. doi: 10.1161/STROKEAHA.107.490896. [DOI] [PubMed] [Google Scholar]
- 8.Sorensen AG, Copen WA, Ostergaard L, Buonanno FS, Gonzalez RG, Rordorf G, Rosen BR, Schwamm LH, Weisskoff RM, Koroshetz WJ. Hyperacute stroke: simultaneous measurement of relative cerebral blood volume, relative cerebral blood flow, and mean tissue transit time. Radiology. 1999;210:519–527. doi: 10.1148/radiology.210.2.r99fe06519. [DOI] [PubMed] [Google Scholar]
- 9.Ostergaard L, Sorensen AG, Kwong KK, Weisskoff RM, Gyldensted C, Rosen BR. High resolution measurement of cerebral blood flow using intravascular tracer bolus passages. Part II: experimental comparison and preliminary results. Magn Reson Med. 1996;36:726–736. doi: 10.1002/mrm.1910360511. [DOI] [PubMed] [Google Scholar]
- 10.Ay H, Koroshetz WJ, Vangel M, Benner T, Melinosky C, Zhu M, Menezes N, Lopez CJ, Sorensen AG. Conversion of ischemic brain tissue into infarction increases with age. Stroke. 2005;36:2632–2636. doi: 10.1161/01.STR.0000189991.23918.01. [DOI] [PubMed] [Google Scholar]
- 11.Adams HP, Jr, Davis PH, Leira EC, Chang KC, Bendixen BH, Clarke WR, Woolson RF, Hansen MD. Baseline NIH Stroke Scale score strongly predicts outcome after stroke: a report of the Trial of Org 10172 in Acute Stroke Treatment (TOAST) Neurology. 1999;53:126–131. doi: 10.1212/wnl.53.1.126. [DOI] [PubMed] [Google Scholar]
- 12.D'Agostino RB, Wolf PA, Belanger AJ, Kannel WB. Stroke risk profile: adjustment for antihypertensive medication. The Framingham Study. Stroke. 1994;25:40–43. doi: 10.1161/01.str.25.1.40. [DOI] [PubMed] [Google Scholar]
- 13.Barrett KM, Ding YH, Wagner DP, Kallmes DF, Johnston KC. Change in diffusion-weighted imaging infarct volume predicts neurologic outcome at 90 days: results of the Acute Stroke Accurate Prediction (ASAP) trial serial imaging substudy. Stroke. 2009;40:2422–2427. doi: 10.1161/STROKEAHA.109.548933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allport LE, Parsons MW, Butcher KS, MacGregor L, Desmond PM, Tress BM, Davis SM. Elevated hematocrit is associated with reduced reperfusion and tissue survival in acute stroke. Neurology. 2005;65:1382–1387. doi: 10.1212/01.wnl.0000183057.96792.a8. [DOI] [PubMed] [Google Scholar]
- 15.Myers AM, Saunders CR, Chalmers DG. The haemoglobin level of fit elderly people. Lancet. 1968;ii:261–263. doi: 10.1016/s0140-6736(68)92358-1. [DOI] [PubMed] [Google Scholar]
- 16.Todd MM, Wu B, Maktabi M, Hindman BJ, Warner DS. Cerebral blood flow and oxygen delivery during hypoxemia and hemodilution: role of arterial oxygen content. Am J Physiol. 1994;267:H2025–H2031. doi: 10.1152/ajpheart.1994.267.5.H2025. [DOI] [PubMed] [Google Scholar]
- 17.Dexter F, Hindman BJ. Effect of haemoglobin concentration on brain oxygenation in focal stroke: a mathematical modelling study. Br J Anaesth. 1997;79:346–351. doi: 10.1093/bja/79.3.346. [DOI] [PubMed] [Google Scholar]
- 18.Heiss WD. Ischemic penumbra: evidence from functional imaging in man. J Cereb Blood Flow Metab. 2000;20:1276–1293. doi: 10.1097/00004647-200009000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Astrup J, Siesjo BK, Symon L. Thresholds in cerebral ischemia – the ischemic penumbra. Stroke. 1981;12:723–725. doi: 10.1161/01.str.12.6.723. [DOI] [PubMed] [Google Scholar]
- 20.Lee SH, Heros RC, Mullan JC, Korosue K. Optimum degree of hemodilution for brain protection in a canine model of focal cerebral ischemia. J Neurosurg. 1994;80:469–475. doi: 10.3171/jns.1994.80.3.0469. [DOI] [PubMed] [Google Scholar]
- 21.Reasoner DK, Ryu KH, Hindman BJ, Cutkomp J, Smith T. Marked hemodilution increases neurologic injury after focal cerebral ischemia in rabbits. Anesth Analg. 1996;82:61–67. doi: 10.1097/00000539-199601000-00011. [DOI] [PubMed] [Google Scholar]
- 22.Karkouti K, Djaiani G, Borger MA, Beattie WS, Fedorko L, Wijeysundera D, Ivanov J, Karski J. Low hematocrit during cardiopulmonary bypass is associated with increased risk of perioperative stroke in cardiac surgery. Ann Thorac Surg. 2005;80:1381–1387. doi: 10.1016/j.athoracsur.2005.03.137. [DOI] [PubMed] [Google Scholar]
- 23.Davis SM, Donnan GA, Parsons MW, Levi C, Butcher KS, Peeters A, Barber PA, Bladin C, De Silva DA, Byrnes G, Chalk JB, Fink JN, Kimber TE, Schultz D, Hand PJ, Frayne J, Hankey G, Muir K, Gerraty R, Tress BM, Desmond PM. Effects of alteplase beyond 3 h after stroke in the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET): a placebo-controlled randomised trial. Lancet Neurol. 2008;7:299–309. doi: 10.1016/S1474-4422(08)70044-9. [DOI] [PubMed] [Google Scholar]