Abstract
Background
Cell transplantation is a ‘hype and hope’ in the current scenario. It is in the early stage of development with promises to restore function in chronic diseases. Mesenchymal stem cell (MSC) transplantation in stroke patients has shown significant improvement by reducing clinical and functional deficits. They are feasible and multipotent and have homing characteristics. This study evaluates the safety, feasibility and efficacy of autologous MSC transplantation in patients with chronic stroke using clinical scores and functional imaging (blood oxygen level-dependent and diffusion tensor imaging techniques).
Methods
Twelve chronic stroke patients were recruited; inclusion criteria were stroke lasting 3 months to 1 year, motor strength of hand muscles of at least 2, and NIHSS of 4–15, and patients had to be conscious and able to comprehend. Fugl Meyer (FM), modified Barthel index (mBI), MRC, Ashworth tone grade scale scores and functional imaging scans were assessed at baseline, and after 8 and 24 weeks. Bone marrow was aspirated under aseptic conditions and expansion of MSC took 3 weeks with animal serum-free media (Stem Pro SFM). Six patients were administered a mean of 50–60 × 106 cells i.v. followed by 8 weeks of physiotherapy. Six patients served as controls. This was a non-randomized experimental controlled trial.
Results
Clinical and radiological scanning was normal for the stem cell group patients. There was no mortality or cell-related adverse reaction. The laboratory tests on days 1, 3, 5 and 7 were also normal in the MSC group till the last follow-up. The FM and mBI showed a modest increase in the stem cell group compared to controls. There was an increased number of cluster activation of Brodmann areas BA 4 and BA 6 after stem cell infusion compared to controls, indicating neural plasticity.
Conclusion
MSC therapy aiming to restore function in stroke is safe and feasible. Further randomized controlled trials are needed to evaluate its efficacy.
Key Words: fMRI, Functional imaging, Neurological rehabilitation, Neuronal plasticity, Stem cell transplantation, Stroke
Introduction
Stroke is the leading cause of morbidity worldwide with a prevalence rate of approximately 250–300/100,000 in the total population [1] in Asian countries, and 28.5 million disability adjusted life years are estimated to be lost [2] due to stroke leaving 60% of stroke survivors dependent on caregivers for their daily activities. Urged by the expectation of functional neuronal replacement, stem cell transplantation has emerged as a ‘hope’ to cure disease in the last decade. Several studies have evidenced a potential benefit of stem cell grafts in animal models and clinical trials of stroke [3]. It is also postulated that stem cells operate not only through one unidirectional mechanism (e.g. generating neurons) but rather as cellular mediators of a multitude of biological activities that could provide a favorable outcome for neurogenic diseases [4].
Mesenchymal Stem Cells
Mesenchymal stem cells (MSC) are self-renewing, multipotent cells that can differentiate into bone, cartilage, adipose tissues and neural precursors [5, 6, 7]. Intravenously administered MSC have been shown to promote engraftment of hematopoietic lineages in cancers and ameliorate graft-versus-host disease [8, 9, 10]. The only published trial with a 5-year follow-up of functional potential in stroke patients receiving autologous MSC transplantation reported significant improvement in the activities of daily living [11]. MSC possess immunomodulatory properties, limit the local inflammatory response by decreasing activation of microglia and macrophages and impair T-lymphocyte maturation [12].
Recovery as Neural Plasticity
Initial recovery following an acute insult/stroke is mediated by spontaneous internal events, and after a late phase this recovery is remodeled by both external and internal perturbations [13, 14]. It has been suggested that 3 months is the time point recognized generally to get the functional status of a stroke victim [15]. Functional neuroimaging investigates the biological mechanism of treatment effects in which blood oxygen level-dependent (BOLD) and diffusion tensor imaging (DTI) techniques are used to study the functionality and integrity of brain areas [16, 17].
Stem Cells as Regenerative Medicine
The emergence of stem cell biology has led to perennial applications in regenerative medicine for stroke aiming to restore plasticity for improving functional recovery [18]. When introduced into the lesioned central nervous system, stem cells can have a positive influence through their intrinsic neuroprotective properties, e.g. production of growth and trophic factors, stimulation of endogenous neurogenesis and modulation of neuroinflammation. They are known to influence the environment by reorganizing the brain, and promoting learning in the form of synaptogenesis and dendritic sprouting [19, 20]. Transplanted stem cells also interact with the host tissue by forming connections and by providing ‘scaffolds’ to an injured brain in order to rescue dysfunctional and dormant cells [21].
Cell therapies are setting new paradigms in regenerative medicine. This research evaluates the safety, tolerance and possible efficacy of intravenous autologous MSC transplantation in chronic stroke assessed clinically and by functional imaging (BOLD and DTI).
Methods
Patients diagnosed with stroke (index event) 3 months to 1 year ago, with MRC (Medical Research Council) grade of muscle power for the wrist and hand extensor or flexor muscles of at least 2, and NIHSS (National Institute of Health Stroke Scale) between 4 and 15 who were conscious and able to comprehend were recruited for this study. We excluded patients with bleeding disorders, chronic liver and/or renal failure, progressive neurological worsening, unilateral neglect, neoplasia and with contraindications to MRI, and immunocompromised and pregnant patients.
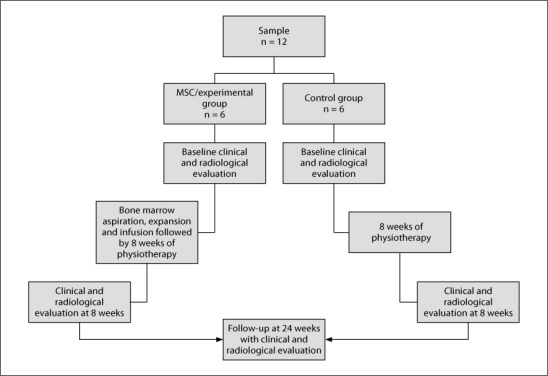
The patients were divided into two groups matched for age, disease severity, time of stroke onset and lesion size. All the patients were examined by a neurologist and a neurophysiotherapist regarding muscle power (MRC), tone (modified Ashworth) and Fugl Meyer (FM) scale for upper limbs and modified Barthel index (mBI) at baseline, and the 8- and 24-week follow-ups [22]. We used the Edinburgh handedness inventory to assess hand dominance [23]. The detailed flow chart of the study is explained in figure 1. BOLD and DTI techniques were performed at baseline, and after 8 and 24 weeks of cell therapy.
Fig. 1.
Flow chart depicting the plan of the study.
The trial protocol and informed consent form were approved by the Institute Committee for Stem Cell Research and Therapy. A neurologist helped in treatment allocation but was not part of the study. Only the functional imaging parameters were blinded in this research.
Procedures
Bone Marrow Aspiration, Expansion and Transplantation. Bone marrow was aspirated under aseptic conditions from the posterior superior iliac crest of 6 chronic stroke patients. The aspirate was diluted with phosphate-buffered saline, layered over Ficoll density medium and centrifuged at 1,800 r.p.m. for 25 min. The collected mononuclear cell layer was plated at a density of 1 × 106 cells/cm2 with Stem Pro MSC SFM basal medium (A-10334, Invitrogen) in a T-25 tissue culture flask and incubated at 37°C/5% CO2. The cells were harvested and seeded at 3,000 or 10,000 cells/cm2 in triplicate wells of 6-well plates. Throughout the duration of the culture, individual wells remained separate and were propagated individually. On reaching 70–90% confluence, all MSC cultures were harvested using TrypLE™ Express (Invitrogen), counted flow cytometrically and reseeded at the same seeding density till the final required dose of cells was obtained. Nonadherent cells were removed after 24 h and fresh media were again added for incubation [24, 25]. The media were exchanged every 3 days and at confluency, the cells were trypsinized and subcultured. All samples were tested for mycoplasma and endotoxins at every third passage using commercially available kits according to the manufacturer's instructions. MSC expansion took around 23 ± 3 days. We used an aseptic infusion technique. Cells were directly dissolved in a 250-ml saline bottle and infused intravenously over 2–3 h using a sterile 50-ml syringe. We used a 20-gauge cannula for cell injection. Patients were evaluated for safety, i.e. laboratory tests (hemoglobin, red and white blood cells, platelets, liver and kidney function tests and partial thromboplastin time) 1, 3 and 7 days and 24 weeks after transplantation. As the patients received an 8-week physiotherapy regime after cell transplantation, they were examined and evaluated daily for any adverse reaction.
Neuromotor Rehabilitation. The physiotherapy regime administered to all 12 patients was based on the motor imagery [26, 27] incorporating learning and repetition of motor learning and lasted 60–90 min 5 days/week for 8 weeks.
fMRI Acquisition. Subjects were asked to perform the motor task with the affected hand, with self-paced (minimum 0.5 Hz) fist clenching/extension of the wrist or metacarpophalangeal joint of the hand. BOLD data were acquired using the gradient echoplanar imaging sequence using a 1.5-tesla MR scanner (Avanto, Siemens, Germany). Block design with alternate baseline and activation cycles was used with a total of 90 whole-brain echoplanar imaging measurements (TR = 4,520 ms, TE = 44 ms, slices = 31 and slice thickness = 4 mm) and MPrage sequence with 176 contiguous slices of 1.0-mm thickness [28, 29].
Diffusion Tensor Images. They were acquired with single-shot echoplanar technique with b values of 0, 400 and 1,000 s/mm2 in 20 directions, 128 × 128 matrix, field of view: 230 × 230 mm, TE = 76 ms, TR = 10,726 ms, echoplanar imaging factor = 127 and a slice thickness of 2.3 mm. The termination criteria used were fractional anisotropy (FA) <0.2 with an angle change >45°. Seed points as regions of interest (ROI) were drawn in the infarcted area and corticospinal tract in the affected and unaffected hemispheres [30, 31]. The selection of ROI for FA were repeated thrice by one rater, and the average value was regarded as the unit of measurement (intrarater reliability 0.88).
Statistics
Statistical analysis was done by SPSS 11.5. We used parametric paired sample t test/Kruskal-Wallis test for intragroup and 2-sample t test/Mann-Whitney test for intergroup comparisons with p = 0.05. Repeated-measure ANOVA was used to calculate the difference at baseline, and 8 and 24 weeks.
Results
Establishment of Safety
The routine laboratory tests 1, 3 and 7 days after the stem cell transplantation were normal for all patients. Flow-cytometric analysis showed phenotype markers which justify these cells as MSC or stromal cells. The cells expressed CD90, CD73 and CD105, and were negative for HLA class II. The mean CD90, CD73 and CD105 were 61, 57.1 and 40%, respectively. The mean cell viability at transplantation was 98% (performed with trypan blue stain); the cells were sterile and endotoxin free during expansion and at the time of injection. There were no early and late adverse reactions observed in patients during and after transplantation. The patients did not report any tumorigenesis, ectopic tissue formation or any behavioral abnormality during the follow-up.
Clinical Results
In the MSC or experimental group (males:females = 2:4), all had right-hand dominance with age = 42 ± 16.4 years (mean ± SD); the mean FM score was 16.6 ± 5.27 at baseline and 29 ± 7.45 after 8 weeks. Patients showed statistically significant improvement when transplanted with stem cells (p = 0.001, t = −8.357). At the 24-week follow-up, the mean FM score was 36.6 ± 7.4, which was higher than the mean at 8 weeks in these patients exhibiting statistically significant improvement (p = 0.001, t = −8.174). Repeated-measure ANOVA was found to be statistically significant at all time measurements, i.e. at baseline and after 8 and 24 weeks. The mean mBI in the MSC group were 48.75 ± 10.56 and 68.75 ± 12.27 at baseline and 8 weeks, respectively (p = 0.0001), and increased to 78.6 ± 11.34 at 24 weeks (p < 0.05). In the control group (all males with right-hand dominance), mean age was 47.08 ± 9.9 years. The mean FM scores were 16.8 ± 6.1 and 27.1 ± 6.4 at baseline and 8 weeks, respectively. These patients also showed statistically significant improvement between baseline and 8 weeks after therapy, and between 8 and 24 weeks (p < 0.05) for both FM and mBI scores (table 1).
Table 1.
Demographic and clinical data in the MSC and the control group at baseline, and 8 and 24 weeks
| No. | Group | Age, years/sex | Months after stroke | Lesion area all MCA territory | Lesion volume ml | CD90 % | CD73 % | CD105 % | Baseline |
8 weeks |
24 weeks |
|||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FM n/66 | mBI n/100 | FM n/66 | mBI n/100 | FM n/66 | mBI n/100 | |||||||||
| 1 | E | 28/F | 11 | R frontoparietal (I) | 12 | 40 | 40 | 44 | 22 | 52 | 36 | 72 | 44 | 80 |
| 2 | E | 20/F | 12 | R frontoparietal (H) | 58 | 52 | 58 | 38 | 11 | 30 | 22 | 42 | 30 | 60 |
| 3 | E | 59/F | 7 | R frontal (I) | 34.6 | 67 | 65 | 23 | 11 | 40 | 26 | 55 | 32 | 70 |
| 4 | E | 35/F | 8 | L internal capsule (H) | 13.5 | 45 | 43 | 48 | 14 | 32 | 20 | 53 | 28 | 65 |
| 5 | E | 55/M | 9 | R frontal (I) | 14.2 | 76 | 76 | 52 | 22 | 52 | 38 | 65 | 44 | 78 |
| 6 | E | 55/M | 8 | R frontal (I) | 15.1 | 86 | 61 | 35 | 20 | 58 | 32 | 70 | 42 | 82 |
| Mean | 42 | 9.3 | 24.5 | 61 | 57.1 | 40 | 16.6 | 44 | 29 | 59.5 | 36.6 | 72.5 | ||
| 1 | C | 40/M | 10 | L frontal (I) | 55 | _ | _ | _ | 11 | 40 | 20 | 55 | 30 | 65 |
| 2 | C | 28/M | 12 | R parietal (I) | 12.2 | − | − | − | 20 | 52 | 31 | 62 | 38 | 78 |
| 3 | C | 42/M | 8 | R internal capsule (I) | 15.4 | − | − | − | 12 | 35 | 26 | 52 | 36 | 65 |
| 4 | C | 30/M | 12 | L temporoparietal (I) | 45.5 | − | − | − | 11 | 42 | 20 | 50 | 30 | 68 |
| 5 | C | 60/M | 7 | L caudate (I) | 10 | − | − | − | 24 | 55 | 36 | 65 | 33 | 73 |
| 6 | C | 50/M | R frontoparietal (H) | 11.2 | − | − | − | 23 | 50 | 30 | 68 | 38 | 72 | |
| Mean | 46.5 | 9.3 | 24.8 | - | - | - | 16.8 | 45.6 | 27.1 | 58.6 | 34.1 | 70.1 | ||
C = Control; E = MSC; R = right; L = left; I = ischemia; H = hemorrhage.
BOLD Activation Pattern
The laterality index (LI) was calculated in each subject with a threshold of 10 voxels. It was measured with the formula LI = (CL – I)/(CL + I), where CL = the number of voxels of the contralateral hemisphere to the affected hand and I = the number of voxels for the ipsilateral hemisphere to the affected hand. In the MSC group, the LI of the ipsilesional primary motor cortex (BA 4) did not show statistically significant improvement between 0 and 8 weeks (p = 0.15) and between 8 and 24 weeks (p = 0.98; table 2). The LI of the ipsilesional premotor motor cortex (BA 6) showed statistically significant improvement (p = 0.01) between 0 and 8 weeks but was not significant between 8 and 24 weeks (p > 0.05). In the control group, the LI for the primary motor and premotor cortex (BA 4 and BA 6, respectively) was not statistically significant when calculated between 0 and 8 weeks and 8 and 24 weeks.
Table 2.
BOLD brain activation pattern and voxel counts in BA 4, BA 6 and cerebellum in the MSC and the control group
| Group | Task | Baseline |
8 weeks |
24 weeks |
||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BA4 |
LI of CL BA4 | BA6 |
LI of CL BA6 | CB |
I CB activ. ratio | BA4 |
LI of CL BA4 | BA6 |
LI of CL BA6 | CB |
I CB activ. ratio | BA4 |
LI of CL BA4 | BA6 |
LI of CL BA6 | CB |
I CB activ. ratio | |||||||||||
| L | R | L | R | L | R | L | R | L | R | L | R | L | R | L | R | L | R | |||||||||||
| MSC | LFM | − | 59 | 1 | 86 | 150 | 0.27 | 176 | − | − | − | 390 | 1 | − | 250 | 1 | 176 | − | − | − | − | − | 866 | 1,254 | 0.98 | 660 | − | 1 |
| MSC | LFM | 786 | − | −1 | 1,044 | − | −1 | − | − | − | − | − | − | 670 | 230 | −0.6 | 946 | 179 | 0.84 | 220 | 220 | 0 | 620 | 3,330 | 0.66 | 7,270 | 7,270 | 0 |
| MSC | LFM | − | − | − | 172 | 256 | 0.19 | 1,012 | − | 1 | − | − | − | 13 | 734 | 0.96 | 430 | − | 1 | − | 232 | 1 | 65 | 1,544 | 0.95 | 640 | 730 | 0.46 |
| MSC | RFM | 78 | − | 1 | − | − | − | 6,490 | − | 1 | 513 | − | 1 | 513 | − | 1 | 540 | − | 1 | − | − | 543 | − | 1 | 634 | 866 | 0.57 | |
| MSC | LFM | 10 | 24 | 0.72 | 98 | 462 | 0.65 | 229 | − | 1 | 14 | 78 | 0.78 | 47 | 1,126 | 0.91 | 1,344 | − | 1 | − | 770 | 1 | − | 1,801 | 1 | − | − | − |
| MSC | LFM | − | 239 | 1 | 56 | 140 | 0.42 | 44 | 142 | 0.23 | − | 605 | 1 | − | 575 | 1 | 221 | − | 1 | − | 550 | 1 | 110 | 430 | 0.59 | 1,012 | 572 | 0.63 |
| C | RFM | − | − | − | 499 | − | 1 | − | 371 | 1 | − | − | − | 643 | − | 1 | 329 | 465 | 0.41 | − | − | 712 | − | − | 650 | 650 | − | |
| C | LFM | − | − | − | − | 586 | 1 | 1,238 | 748 | 0.62 | − | 212 | 1 | − | 824 | 1 | 1,295 | 382 | 0.77 | − | 350 | 1 | − | 724 | 1 | 138 | 461 | |
| C | LFM | − | 132 | 1 | 221 | 257 | 0.11 | 112 | 223 | 0.33 | − | 650 | 1 | 13 | 730 | 0.96 | 230 | − | 1 | − | 750 | 1 | − | 830 | 1 | 212 | − | 1 |
| C | RFM | − | 138 | 1 | 1,893 | 1,462 | 0.12 | − | − | − | − | − | − | 712 | 223 | 0.49 | 232 | 462 | 0.66 | − | − | 532 | 126 | 0.61 | 512 | 512 | 0.5 | |
| C | RFM | 812 | 421 | 0.31 | 232 | − | 1 | 289 | 631 | 0.69 | 1,627 | − | 1 | 1,462 | 1 | 1,128 | − | 1 | 1,192- | 1 | 931 | − | 1 | 720 | − | − | ||
| C | LFM | − | 2,861 | 1 | − | 2,861 | 1 | 2,861 | − | 1 | − | 3,542 | 1 | − | 4,180 | 1 | 3,881 | − | 1 | − | − | − | 2,921 | 1 | 2,691 | 2,691 | 0.5 | |
C = Control; L = left; R = right; RFM/LFM = right/left fist making; CB = cerebellum; BA 4 = primary motor cortex; BA 6 = supplementary/premotor cortex; BA 1–3 = sensory motor cortices; LI = (CL – I)/(CL + I); CL = contralateral (to the paretic hand); I = ipsilateral (to the paretic hand); CB ratio = I CB/(I CB + CL CB); cluster: 2*2*2 mm3.
The FA ratio was calculated as FA of the affected hemisphere (ah) divided by the unaffected hemisphere (uh). The FAah was decreased compared to FAuh in both groups. We also calculated the fiber density by calculating the number of fibers (FN) and the fiber length (FL) in the given ROI in the affected and the unaffected hemispheres. We calculated the FLah/FLuh ratio, and the FNah/FNuh ratio. In the MSC group, the mean FLah (mm) were 0.12 ± 0.05 mm at week 0, and 0.14 ± 0.05 and 0.15 ± 0.06 mm after 8 and 24 weeks, whereas in the control arm they were 0.09 ± 0.03, 0.10 ± 0.04 and 0.11 ± 0.04 mm, respectively. There was no significant change in FL in the MSC and control groups after 8 and 24 weeks. The mean fiber density (number) ratio (FNah/FNuh) in the MSC group was 0.28, 0.40 and 0.48 at baseline, and 8 and 24 weeks, and 0.25, 0.29 and 0.34, respectively, in the control group (nonsignificant: p > 0.05).
Comparison between MSC and Control Groups
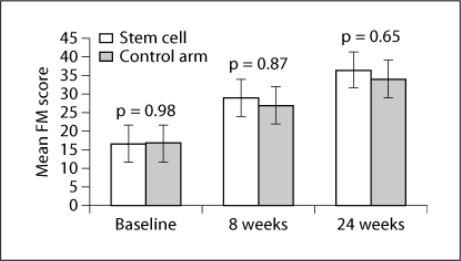
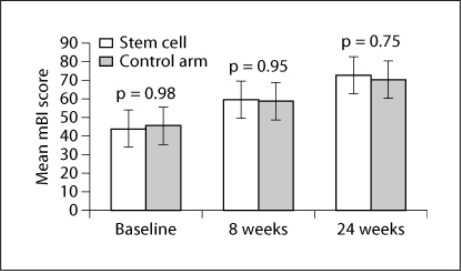
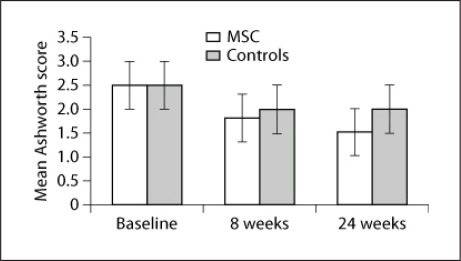
There was no significant difference in baseline clinical and radiological scores between the MSC and control groups, suggesting that the two groups were comparable to study the effectiveness of therapy after 8 and 24 weeks. There was no significant difference in FM and mBI scores after therapy (8 weeks: p = 0.87, t = 0.161 and p = 0.95, t = 0.065, respectively; fig. 2) and at follow-up (p = 0.65 and p = 0.75, respectively; fig. 3). A meager reduction in the Ashworth tone scale was observed between the two groups (fig. 4).
Fig. 2.
Mean FM scores at baseline, and at the 8- and 24-week follow-ups with p values in the MSC (stem cell) and control groups.
Fig. 3.
Mean mBI scores at baseline, and the 8- and 24-week follow-ups with p values in the MSC (stem cell) and control groups.
Fig. 4.
Mean Ashworth scores between the MSC and control groups.
The LI of BA 4 and 6 between MSC and control groups was also statistically insignificant after 8 and 24 weeks (p > 0.5). There was no statistically significant (p > 0.05) change in the FA ratio, FL ratio and FN ratio between the two groups after 8 and 24 weeks.
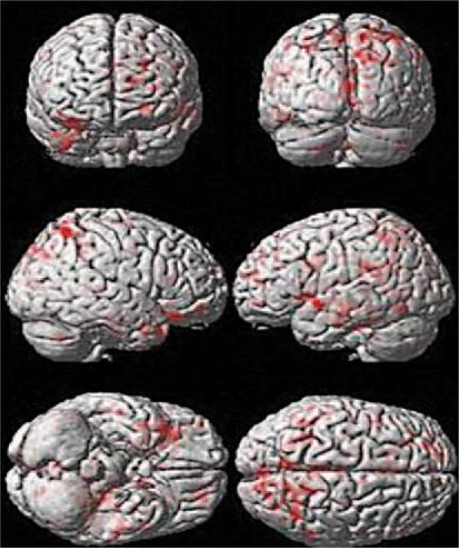
We compared the results of right-hemispheric stroke with the control arm patients after 8 weeks (table 3). It was found that BA 4 and 6, and the inferior temporal gyrus (fig. 5) of the affected hemisphere were active with a cluster count of 40 and 112 voxels, respectively. The results were similar at 24 weeks (cluster activation of 71 and 112 voxels of BA 4 and BA 6, respectively).
Table 3.
Comparison of BOLD activation between the MSC and the control group at 24 weeks in right-hemispheric stroke
| Cluster | z score | MNI coordinates (x, y, z), mm | Hemisphere | Area of activation | Brodmann area |
|---|---|---|---|---|---|
| 71 | 4.02 | 16, −50, 54 | right cerebrum | precentral sulcus | BA 4 |
| 112 | 2.95 | 18, −36,46 | right cerebrum | medial frontal gyrus | BA 6 |
| 56 | 4.05 | −54, −36,26 | right cerebrum | inferior parietal lobule | BA 40 |
| 40 | 4.56 | −48,4, −8 | left cerebrum | superior temporal gyrus | BA 22.21 |
| 92 | 3.97 | 8, −40, 28 | right cerebrum | cingulate gyrus | BA 31 |
| 95 | 3.36 | −2,18,34 | left cerebrum | cingulate gyrus | BA 32 |
| 87 | 2.99 | −14, −12,20 | left cerebrum | caudate | caudate body |
| 115 | 2.50 | −48, −14, −26 | left cerebrum | inferior temporal gyrus | BA 20, 21 |
One cluster 2*2 *2 mm3. MNI = Montreal Neurologic Institute.
Fig. 5.
Comparison of BOLD activation between the MSC and control groups in right-hemispheric stroke overlaid on rendered images.
Discussion
This is the first known trial (to our knowledge) that establishes the safety and tolerance of MSC derived using serum-free media for expansion unlike bovine serum used in the earlier study [11, 12, 24, 25]. We were able to procure 50–60 million cells at a mean of 4 passages in all the 6 patients, which made autologous MSC transplantation feasible. No immunosuppressants were required following transplantation, eliminating the risks associated with MSC therapy. The clinical, laboratory and radiological evaluations did not report any deaths, cell-related complications, stroke recurrence and structural changes in imaging up to the 6-month follow-up.
The MSC group had 5 cortical and 1 subcortical lesions, 2 patients (patients 2 and 4) were hemorrhagic, and the remainder was ischemic with a lesion volume of 12–58 ml. All patients in this group showed significant improvement after 8 and 24 weeks versus baseline. The control patients had 4 cortical and 2 subcortical lesions (volume: 10–55 ml). All patients in this group showed significant improvement after 8 and 24 weeks versus baseline. Using the 2-sample t test, we observed no statistically significant (p > 0.05) difference in FM, mBI, LI and FA ratios between both study groups after 8 and 24 weeks. However, the mean percentage change in FM scores between 8 weeks and baseline levels and between 8 and 24 weeks were 74.6 and 26.2%, respectively, in the MSC group and 61.3 and 25.8% in the control arm, respectively. The change from baseline to the 24-week follow-up was 120% in the MSC group compared to 102% in the control group, suggesting a minor trend to improvement in patients administered MSC. There was only a minor change in the power of wrist and hand muscles (subjective analysis) compared to the control group, though we observed a decrease in tone in spastic muscles, a lower pain threshold and a better functional performance in the MSC group (fig. 4).
In 1 MSC patient (No. 2) who had a large right temporoparietal lesion, we observed a shift in the LI from negative to positive (table 3). At baseline, there was an increased activation in the supplementary motor cortex (BA 6) compared with the primary execution area (BA 4) in nearly all patients (recruitment principle of plasticity) [32, 33]. After an 8-week physiotherapeutic regime, there was an increased number of voxels in BA 6 in both groups. In the MSC group, the premotor and supplementary motor areas (BA 6) had an increased number of voxels active at the 24-week follow-up suggesting the ‘focusing’ principle of neural plasticity although the LI at the 24-week follow-up was not statistically significant (p < 0.05) between both groups. We noticed a considerable increase in LI and signal intensity [34] from baseline to 8 weeks indicating that a focused exercise regime which involves vigorous training of the hand leads to increased force of activation performing better in the fMRI task compared to the performance at 24 weeks.
We correlated FM scores with FA ratios at 24 weeks and found no significant difference in improvement between the MSC and the control group, but 4 of 6 patients (patients 1, 3, 5 and 6) in the MSC group showed good recovery at follow-up with FA >0.6, whereas in the control group only 3 of 6 patients improved. This minute change in the integrity of the motor tract system might have been due to axonal remodeling imposed by the transplanted MSC [35, 36] although there was no statistical significant change in FL and FN ratios between the groups.
The dosage of stem cells prescribed in our study was in congruence with previously published trials [11, 12] and still ongoing studies [37, 38]. The earlier research groups administered 2 × 50 [11], and 200–400 [39, 40], 5–10 [41] and 34.6 × 106 cells [42]. Our findings were in agreement with those of other clinical trials as adverse reactions, mortality or any other risk factor were not noted in association with MSC administration of up to 50–60 × 106 cells in chronic stroke patients.
It has been proved that stem cells given intravenously home in the infarcted regions thus promoting functional recovery in chronic stroke rats [43]. However, cell-enhanced recovery has been reported with chronic delivery of cells even 1 month after ischemia [44, 45]. The best route of transplantation still needs to be established considering the specific cell type or the mechanism of action underlying the beneficial effect.
We did not find any significant change in the pattern of recovery of ischemic and hemorrhagic stroke. Previous clinical trials recruited both ischemic and hemorrhagic stroke patients but could not elucidate the effect of cells on the type of stroke [39, 40, 41, 42]. We tried to match the patients with respect to lesion volume but could not find any significant change in the volume and the correlation of the same with MSC therapy.
In our study, the safety and feasibility of expansion of these cells was established in 6 patients. However, our results showed no statistically significant improvement in all clinical scores and functional imaging parameters at 8 and 24 weeks. This could be attributed to a very small sample size and the heterogeneity in MSC group patients (1 patient, No. 2, had a large temporoparietal hemorrhage with a very low potential to recover). We explored infusion with stem cells and physiotherapy as a combination therapy to look into behavioral recovery after stroke. Our aim to administer stem cells intravenously was based on the hypothesis that intravenously administered stem cells will help to upregulate growth factors within the body and brain making the host environment conducive for behavioral recovery [18, 19], as it is known that enriched environment, physical activity, stress or molecules such as BDNF, VEGF and dextroamphetamines lead to reorganization of brain areas [46, 47, 48].
Due to the small sample size, non-randomized trial, dose of cells, site and mode of transplantation, cellular milieu and recovery factors after stroke, we could not reach a definite conclusion regarding the potential of MSC in chronic stroke [49, 50]. This study shows, however, that autologous MSC transplantation is safe and feasible. More research is required to evaluate the efficacy of MSC transplantation.
References
- 1.Banerjee T, Das S. Epidemiology of stroke in India. Neurol Asia. 2006;11:1–4. [Google Scholar]
- 2.Dalal PM. Burden of Stroke – an Indian Perspective. J Assoc Physicians India. 2004;52:692–696. [PubMed] [Google Scholar]
- 3.Onteniente B, Polentes J. Regenerative medicine for stroke – are we there yet? Cerebrovasc Dis. 2011;31:544–551. doi: 10.1159/000324325. [DOI] [PubMed] [Google Scholar]
- 4.Locatelli F, Bersano A, Strazzer S, Bresolin N, Corti S. Stem cell therapy in stroke: review. Cell Mol Life Sci. 2009;66:757–772. doi: 10.1007/s00018-008-8346-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9:641–650. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 6.Pittenger MF, Mackay AM, Beck SC. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 7.Bianco P, Riminucci M, Gronthos S. Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells. 2001;19:180–192. doi: 10.1634/stemcells.19-3-180. [DOI] [PubMed] [Google Scholar]
- 8.Majumdar MK, Thiede MA, Mosca JD, Moorman M, Gerson SL. Phenotypic and functional comparison of cultures of marrow-derived mesenchymal stem cells (MSCs) and stromal cells. J Cell Physiol. 1998;176:57–66. doi: 10.1002/(SICI)1097-4652(199807)176:1<57::AID-JCP7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 9.Devine SM, Cobbs C, Jennings M, Bartholomew A, Hoffman R. Mesenchymal stem cells distribute to a wide range of tissues following systemic infusion into nonhuman primates. Blood. 2003;101:2999–3001. doi: 10.1182/blood-2002-06-1830. [DOI] [PubMed] [Google Scholar]
- 10.Lazarus HM, Koc ON, Devine SM. Cotransplantation of HLA-identical sibling culture-expanded mesenchymal stem cells and hematopoietic stem cells in hematologic malignancy patients. Biol Blood Marrow Transplant. 2005;11:389–398. doi: 10.1016/j.bbmt.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 11.Bang OY, Lee JS, Lee PH, Lee G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol. 2005;57:874–881. doi: 10.1002/ana.20501. [DOI] [PubMed] [Google Scholar]
- 12.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 13.Bonita R, Beaglehole R. Recovery of motor function after stroke. Stroke. 1999;19:1497–1500. doi: 10.1161/01.str.19.12.1497. [DOI] [PubMed] [Google Scholar]
- 14.Kreisel SH, Hennerici MG, Bäzner H. Pathophysiology of stroke rehabilitation: the natural course of clinical recovery, use-dependent plasticity and rehabilitative outcome. Cerebrovasc Dis. 2007;23:243–255. doi: 10.1159/000098323. [DOI] [PubMed] [Google Scholar]
- 15.Ovbiagele B, Saver JL. Day-90 acute ischemic stroke outcomes can be derived from early functional activity level. Cerebrovasc Dis. 2010;29:50–56. doi: 10.1159/000255974. [DOI] [PubMed] [Google Scholar]
- 16.Calautti C, Baron J. Functional neuroimaging studies of motor recovery after stroke in adults: a review. Stroke. 2003;34:1553–1566. doi: 10.1161/01.STR.0000071761.36075.A6. [DOI] [PubMed] [Google Scholar]
- 17.Liang Z, Zeng J, Zhang C, Liu S, Ling X, Xu A, Ling L, Wang F, Pei Z. Longitudinal investigations on the anterograde and retrograde degeneration in the pyramidal tract following pontine infarction with diffusion tensor imaging. Cerebrovasc Dis. 2008;25:209–216. doi: 10.1159/000113858. [DOI] [PubMed] [Google Scholar]
- 18.Cramer SC. Repairing the human brain after stroke: II. Restorative therapies. Ann Neurol. 2008;63:549–560. doi: 10.1002/ana.21412. [DOI] [PubMed] [Google Scholar]
- 19.Rodríguez-González R, Hurtado O, Sobrino T, Castillo J. Neuroplasticity and cellular therapy in cerebral infarction. Cerebrovasc Dis. 2007;24(suppl 1):167–180. doi: 10.1159/000107393. [DOI] [PubMed] [Google Scholar]
- 20.Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, Chopp M. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001;32:1005–1011. doi: 10.1161/01.str.32.4.1005. [DOI] [PubMed] [Google Scholar]
- 21.Steindler DA. Neural stem cells, scaffolds and chaperones. Nature. 2002;20:1091–1093. doi: 10.1038/nbt1102-1091. [DOI] [PubMed] [Google Scholar]
- 22.Loewen SC, Anderson BA. Reliability of modified motor assessment scale and the Barthel index. Phys Ther. 1988;68:1077–1081. doi: 10.1093/ptj/68.7.1077. [DOI] [PubMed] [Google Scholar]
- 23.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 24.Chase LG, Lakshmipathy U, Rao SM, Vemuri MC. A novel serum-free medium for the expansion of human mesenchymal stem cells. Stem Cell Res Ther. 2010;1:8–13. doi: 10.1186/scrt8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lindroos B, Boucher S, Chase L, Kuokkanen H, Huhtala H, Haataja R, et al. Serum-free, xeno-free culture media maintain the proliferation rate and multipotentiality of adipose stem cells in vitro. Cytotherapy. 2009;11:958–972. doi: 10.3109/14653240903233081. [DOI] [PubMed] [Google Scholar]
- 26.Gaggioli A, Meneghini A, Morganti F, Alcaniz M, Riva G. A strategy for computer-assisted mental practice in stroke rehabilitation. NeurorehabilNeural Repair. 2006;20:503–507. doi: 10.1177/1545968306290224. [DOI] [PubMed] [Google Scholar]
- 27.Maeda F, Kleiner-Fisman G, Pascual-Leone A. Motor facilitation while observing hand actions: specificity of the effect and role of observer's orientation. J Neurophysiol. 2002;87:1329–1335. doi: 10.1152/jn.00773.2000. [DOI] [PubMed] [Google Scholar]
- 28.Steiner CM, Barber PA, Smale PR. Functional potential in chronic stroke patients depends on corticospinal tract integrity. Brain. 2007;130:170–180. doi: 10.1093/brain/awl333. [DOI] [PubMed] [Google Scholar]
- 29.Weiller C, Chollet F, Friston KJ, Wise RJ, Frackowiak RS. Functional reorganization of the brain in recovery from striatocapsular infarction in man. Ann Neurol. 1992;31:463–472. doi: 10.1002/ana.410310502. [DOI] [PubMed] [Google Scholar]
- 30.Grabel D, Ringer TM, Fitzek C, Fitzek S, Kohl M, Kaiser WA, Witte OW, Axer H. Wallerian degeneration of pyramidal tract after paramedian pons infarct. Cerebrovasc Dis. 2010;30:380–388. doi: 10.1159/000319573. [DOI] [PubMed] [Google Scholar]
- 31.Jang HS, Bai D, Son SM, Lee J, et al. Motor outcome prediction using diffusion tensor tractography in pontine infarct. Ann Neurol. 2008;64:460–465. doi: 10.1002/ana.21444. [DOI] [PubMed] [Google Scholar]
- 32.Feydy A, Carlier R, Brami AR, Bussel B, Cazalis F, Pierot L, Burnod LY, Maier MA. Longitudinal study of motor recovery after stroke: recruitment and focusing of brain activation. Stroke. 2002;33:1610–1617. doi: 10.1161/01.str.0000017100.68294.52. [DOI] [PubMed] [Google Scholar]
- 33.Ward N, Brown M, Thompson A, Frackowiak R. Neural correlates of motor recovery after stroke: a longitudinal fMRI study. Brain. 2003;126:2476–2496. doi: 10.1093/brain/awg245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pineiro R, Pendlebury S, Johansen-Berg H, Matthews PM. Altered hemodynamic responses in patients after subcortical stroke measured by functional MRI. Stroke. 2002;30:103–109. doi: 10.1161/hs0102.100482. [DOI] [PubMed] [Google Scholar]
- 35.Jiang Q, Zhang Z, Ding G, Siver B. MRI detects white matter reorganization after neural progenitor cell treatment of stroke. Neuroimage. 2006;32:1080–1089. doi: 10.1016/j.neuroimage.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 36.Komitova M, Mattsson B, Johansson BB, Eriksson PS. Enriched environment increases neural stem/progenitor cell proliferation and neurogenesis in the subventricular zone of stroke-lesioned adult rats. Stroke. 2005;36:1278–1282. doi: 10.1161/01.STR.0000166197.94147.59. [DOI] [PubMed] [Google Scholar]
- 37.www.clinicaltrials.gov/ct2/show/NCT00473057
- 38.www.clinicaltrials.gov/ct2/show/NCT00395200
- 39.Kondziolka D, Wechsler L, Goldstein S, et al. Transplantation of cultured neuronal cells for patients with stroke. Neurology. 2000;55:565–570. doi: 10.1212/wnl.55.4.565. [DOI] [PubMed] [Google Scholar]
- 40.Kondziolka D, Steinberg GK, Wechsler L, et al. Neurotransplantation for patients with subcortical motor stroke: a phase 2 randomized trial. J Neurosurg. 2005;103:38–45. doi: 10.3171/jns.2005.103.1.0038. [DOI] [PubMed] [Google Scholar]
- 41.Savitz SI, Dinsmore J, Wu J, et al. Neurotransplantation of fetal porcine cells in patients with basal ganglia infarcts: a preliminary safety and feasibility study. Cerebrovasc Dis. 2005;20:101–107. doi: 10.1159/000086518. [DOI] [PubMed] [Google Scholar]
- 42.Suárez-Monteagudo C, Hernández-Ramírez P, Alvarez-González L, et al. Autologous bone marrow stem cell neurotransplantation in stroke patients. An open study. Rest Neurol Neurosci. 2009;27:151–161. doi: 10.3233/RNN-2009-0483. [DOI] [PubMed] [Google Scholar]
- 43.Willing A, Lixian J, Milliken M, Poulus S, et al. Intravenous versus intrastriatal cord blood administration in a rodent model of stroke. J Neurosci Res. 2003;73:296–307. doi: 10.1002/jnr.10659. [DOI] [PubMed] [Google Scholar]
- 44.Shen SH, Zang Z, Lu M, et al. Therapeutic benefit of bone marrow stromal cells administered 1 month after stroke. J Cereb Blood Flow Metab. 2007;27:6–13. doi: 10.1038/sj.jcbfm.9600311. [DOI] [PubMed] [Google Scholar]
- 45.Chen J, Zhang ZG, Li Y, et al. Intravenous administration of human bone marrow stromal cells induces angiogenesis in the ischemic boundary zone after stroke in rats. Circ Res. 2003;92:692–699. doi: 10.1161/01.RES.0000063425.51108.8D. [DOI] [PubMed] [Google Scholar]
- 46.Wechsler L. Stem cell transplantation for stroke. Hong Kong Med J. 2001;7:9–13. [Google Scholar]
- 47.Jin K, Zhu Y, Sun Y. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci USA. 2002;99:11946–11950. doi: 10.1073/pnas.182296499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kreisel SH, Bäzner H, Hennerici MG. Pathophysiology of stroke rehabilitation: temporal aspects of neurofunctional recovery. Cerebrovasc Dis. 2006;21:6–17. doi: 10.1159/000089588. [DOI] [PubMed] [Google Scholar]
- 49.Wechsler L, Steindler D, Borlongan C, Chopp M, Caplan L, Hess D. Stem Cell Therapies as an Emerging Paradigm in Stroke (STEPS): bridging basic and clinical science for cellular and neurogenic factor therapy in treating stroke. Stroke. 2009;40:510–515. doi: 10.1161/STROKEAHA.108.526863. [DOI] [PubMed] [Google Scholar]
- 50.Gladstone DJ, Black SE, Hakim AH. Towards wisdom from failure: lessons from neuroprotective stroke trials and new therapeutic directions. Stroke. 2002;33:2123–2136. doi: 10.1161/01.str.0000025518.34157.51. [DOI] [PubMed] [Google Scholar]