Abstract
In the title compound, [Ni(C7H4N3O2)2(C12H8N2)2]·CH3OH, the NiII atom is six-coordinated in a distorted N6 octahedral geometry and is chelated by two phenanthroline ligands and two phenylcyanamide groups which occupy cis positions. The (4-nitrophenyl)cyanamide anions act as monodentate ligands. There is one classical intermolecular O—H⋯N hydrogen bond and several C—H⋯O hydrogen bonds are also observed.
Related literature
For background to phenylcyanamide ligands and their complexes, see: Crutchley (2001 ▶). For mononuclear complexes of phenylcyanamide complexes, see: Letcher et al. (1993 ▶); Kim et al. (2002 ▶); Shen et al. (1999 ▶). For polynuclear complexes of phenylcyanamide ligands, see: Ainscough et al. (1991 ▶); Chiniforoshan et al. (2009 ▶, 2010 ▶, 2012 ▶); Escuer et al. (2004 ▶). For related structures, see: Wu et al. (2004 ▶); Cheng et al. (2002 ▶); Shen et al. (1999 ▶). For the preparation of 4-nitro-phenylcyanamide used in the synthesis of the title compound, see: Crutchley & Naklicki (1989 ▶).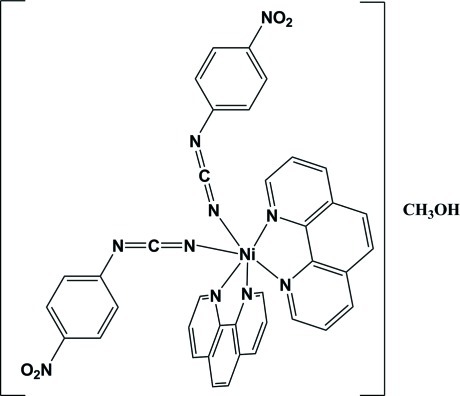
Experimental
Crystal data
[Ni(C7H4N3O2)2(C12H8N2)2]·CH4O
M r = 775.40
Triclinic,
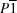
a = 10.019 (2) Å
b = 11.307 (2) Å
c = 16.403 (3) Å
α = 103.54 (3)°
β = 92.96 (3)°
γ = 99.77 (3)°
V = 1772.3 (7) Å3
Z = 2
Mo Kα radiation
μ = 0.61 mm−1
T = 298 K
0.25 × 0.20 × 0.10 mm
Data collection
Stoe IPDS II diffractometer
Absorption correction: numerical (X-RED and X-SHAPE; Stoe & Cie, 2005 ▶) T min = 0.862, T max = 0.938
19823 measured reflections
9512 independent reflections
6693 reflections with I > 2σ(I)
R int = 0.053
Refinement
R[F 2 > 2σ(F 2)] = 0.061
wR(F 2) = 0.139
S = 1.06
9512 reflections
501 parameters
1 restraint
H atoms treated by a mixture of independent and constrained refinement
Δρmax = 0.65 e Å−3
Δρmin = −0.31 e Å−3
Data collection: X-AREA (Stoe & Cie, 2005 ▶); cell refinement: X-AREA; data reduction: X-AREA; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: ORTEP-3 for Windows (Farrugia, 1997 ▶); software used to prepare material for publication: WinGX (Farrugia, 1999 ▶).
Supplementary Material
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S1600536812009890/bt5812sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536812009890/bt5812Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| O5—H5A⋯N9i | 0.91 (3) | 1.98 (4) | 2.883 (4) | 174 (5) |
| C39—H39B⋯O4ii | 0.96 | 2.55 | 3.435 (7) | 153 |
| C22—H22⋯O1iii | 0.93 | 2.57 | 3.469 (6) | 162 |
| C16—H16⋯O2iv | 0.93 | 2.45 | 3.312 (6) | 154 |
Symmetry codes: (i) 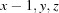 ; (ii)
; (ii) 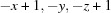 ; (iii)
; (iii) 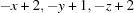 ; (iv)
; (iv) 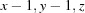 .
.
Acknowledgments
The authors acknowledge financial support from Isfahan University of Technology.
supplementary crystallographic information
Comment
Phenylcyanmide ligands (pcyd) can act as monodentate (Letcher et al., 1993; Kim et al., 2002; Shen et al., 1999) and also as bridging ligands (Crutchley, 2001). In the bridging mode, the cyanamido group (NCN) is coordinated in the end-to-end mode, forming polynuclear complexes (Chiniforoshan et al. 2009, 2010, 2012; Escuer et al., 2004; Ainscough et al., 1991).
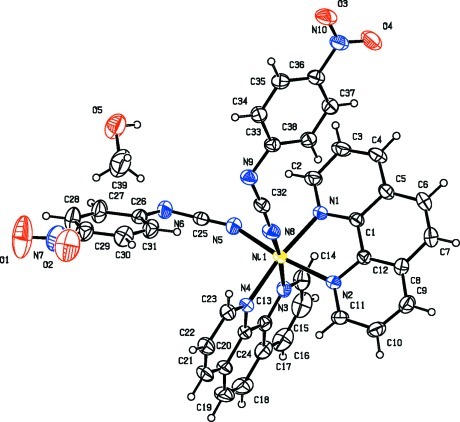
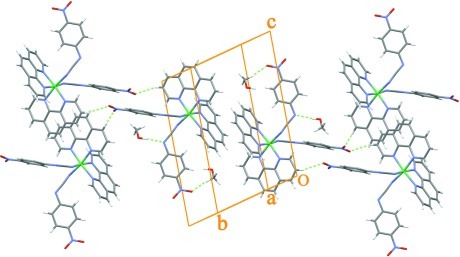
Following our work with this family of ligands, we report here the synthesis and crystal structure of mononuclear [Ni(Phen)2(4-NO2-pcyd)2].CH3OH compound of (4-nitrophenyl)cyanamide ligand, Crutchley & Naklicki (1989). The asymmetric unit of the title compound is shown in Fig. 1. In the structure of the title compound, nickel(II) atom has a distorted octahedral geometry (Fig. 1). The coordination environment consist of four nitrogen atoms from two 1,10-phenanthroline ligand and two anionic 4-NO2-phenylcyanamide ligands which occupy cis position. Bond lengths and angles are in the normal ranges reported for similar structures (Wu et al., 2004; Cheng et al., 2002; Shen et al., 1999). Crutchley (2001) has shown that the angle of a metal atom with the axial CN moiety ranges from 180° to 120°. These angles for the title compound are equal to 153.2 (2) and 150.6 (3)° for Ni(1)—N(5)—C(25) and Ni(1)—N(8)—C(32), respectively. There are several intermolecular O—H···N and C—H···O hydrogen bonds which play important role in the stabilization of crystal structure (Table 1 & Fig. 2).
Experimental
A solution of Ni(OAc)2.4H2O (0.24 gr, 0.1 mmol) in 25 ml of methanol was slowly added to methanolic solution (in 35 ml) of 4-nitrophenylcynamide (Crutchley & Naklicki, 1989) (0.32 gr, 0.2 mmol) and 1,10-phenanthroline (0.39 gr, 0.2 mmol). The mixture was stirred at ambient temperature and the yellow solid filtered after 5 h. The yellow crystals suitable for X-ray structure determination were obtained by dissolving this solid in DMF then diffused by methanol after 3 weeks.
Refinement
The hydrogen atom attached to oxygen atom of the methanol was found in difference Fourier map and refined isotropically with distance restraint of O—H = 0.91 (3)Å. All H atoms bonded to C were positioned geometrically and refined as riding atoms with C—H = 0.93 Å and Uiso(H) = 1.2 Ueq(C) for aromatic C, C—H = 0.96 Å and Uiso(H) = 1.5 Ueq(C) for methyl groups.
Figures
Fig. 1.
The asymmetric unit of the title compound with displacement ellipsoids drawn at 30% probability level.
Fig. 2.
The packing diagram of [Ni(Phen)2(4-NO2-pcyd)2].CH3OH. The intermolecular O—H···N and C—H···O hydrogen bonds are shown as green dashed lines.
Crystal data
| [Ni(C7H4N3O2)2(C12H8N2)2]·CH4O | Z = 2 |
| Mr = 775.40 | F(000) = 800 |
| Triclinic, P1 | Dx = 1.453 Mg m−3 |
| Hall symbol: -P 1 | Mo Kα radiation, λ = 0.71073 Å |
| a = 10.019 (2) Å | Cell parameters from 9512 reflections |
| b = 11.307 (2) Å | θ = 2.1–29.2° |
| c = 16.403 (3) Å | µ = 0.61 mm−1 |
| α = 103.54 (3)° | T = 298 K |
| β = 92.96 (3)° | Plate, yellow |
| γ = 99.77 (3)° | 0.25 × 0.2 × 0.1 mm |
| V = 1772.3 (7) Å3 |
Data collection
| Stoe IPDS II diffractometer | 9512 independent reflections |
| Radiation source: fine-focus sealed tube | 6693 reflections with I > 2σ(I) |
| Graphite monochromator | Rint = 0.053 |
| Detector resolution: 0.15 mm pixels mm-1 | θmax = 29.2°, θmin = 2.1° |
| rotation method scans | h = −12→13 |
| Absorption correction: numerical (X-RED and X-SHAPE; Stoe & Cie, 2005) | k = −15→15 |
| Tmin = 0.862, Tmax = 0.938 | l = −22→20 |
| 19823 measured reflections |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.061 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.139 | H atoms treated by a mixture of independent and constrained refinement |
| S = 1.06 | w = 1/[σ2(Fo2) + (0.0512P)2 + 0.8996P] where P = (Fo2 + 2Fc2)/3 |
| 9512 reflections | (Δ/σ)max = 0.001 |
| 501 parameters | Δρmax = 0.65 e Å−3 |
| 1 restraint | Δρmin = −0.31 e Å−3 |
Special details
| Experimental. shape of crystal determined optically |
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| C39 | 0.1500 (7) | 0.3400 (5) | 0.7660 (5) | 0.146 (3) | |
| H39A | 0.2378 | 0.3493 | 0.7450 | 0.219* | |
| H39B | 0.1205 | 0.2554 | 0.7678 | 0.219* | |
| H39C | 0.1557 | 0.3928 | 0.8217 | 0.219* | |
| O1 | 0.8551 (5) | 0.7156 (4) | 1.0013 (4) | 0.197 (3) | |
| O2 | 1.0049 (4) | 0.6144 (4) | 0.9548 (3) | 0.1357 (15) | |
| Ni1 | 0.62348 (4) | −0.11112 (3) | 0.714358 (19) | 0.04018 (11) | |
| N2 | 0.7128 (2) | −0.2733 (2) | 0.68911 (13) | 0.0435 (5) | |
| N3 | 0.4682 (2) | −0.2034 (2) | 0.77044 (14) | 0.0484 (5) | |
| N1 | 0.5513 (2) | −0.1913 (2) | 0.58779 (13) | 0.0449 (5) | |
| N4 | 0.7020 (2) | −0.0547 (2) | 0.83983 (13) | 0.0445 (5) | |
| N8 | 0.7899 (3) | −0.0105 (3) | 0.67656 (16) | 0.0583 (6) | |
| N5 | 0.5203 (3) | 0.0329 (2) | 0.72202 (17) | 0.0590 (6) | |
| C12 | 0.6933 (3) | −0.3347 (2) | 0.60642 (15) | 0.0418 (5) | |
| C5 | 0.5907 (3) | −0.3441 (3) | 0.46523 (17) | 0.0538 (7) | |
| C24 | 0.6222 (3) | −0.1045 (3) | 0.89187 (16) | 0.0469 (6) | |
| C13 | 0.4963 (3) | −0.1838 (3) | 0.85447 (17) | 0.0490 (6) | |
| C11 | 0.7872 (3) | −0.3163 (3) | 0.74050 (18) | 0.0532 (7) | |
| H11 | 0.8019 | −0.2746 | 0.7972 | 0.064* | |
| C20 | 0.6575 (4) | −0.0808 (3) | 0.97889 (18) | 0.0628 (9) | |
| C6 | 0.6549 (4) | −0.4473 (3) | 0.4333 (2) | 0.0713 (10) | |
| H6 | 0.6451 | −0.4833 | 0.3757 | 0.086* | |
| C1 | 0.6097 (3) | −0.2884 (2) | 0.55207 (15) | 0.0438 (6) | |
| N9 | 0.8915 (3) | 0.1479 (2) | 0.60677 (15) | 0.0534 (6) | |
| C38 | 0.8241 (3) | 0.0062 (3) | 0.46698 (18) | 0.0505 (6) | |
| H38 | 0.7834 | −0.0565 | 0.4906 | 0.061* | |
| C22 | 0.8588 (4) | 0.0513 (3) | 0.9591 (2) | 0.0735 (10) | |
| H22 | 0.9395 | 0.1066 | 0.9805 | 0.088* | |
| C33 | 0.8870 (3) | 0.1206 (3) | 0.51983 (17) | 0.0460 (6) | |
| C8 | 0.7502 (3) | −0.4389 (3) | 0.57381 (19) | 0.0540 (7) | |
| C14 | 0.3505 (3) | −0.2741 (3) | 0.7350 (2) | 0.0651 (9) | |
| H14 | 0.3292 | −0.2865 | 0.6774 | 0.078* | |
| C32 | 0.8361 (3) | 0.0614 (3) | 0.64100 (17) | 0.0486 (6) | |
| C36 | 0.8833 (3) | 0.0780 (3) | 0.34610 (17) | 0.0502 (6) | |
| C35 | 0.9464 (3) | 0.1919 (3) | 0.39645 (19) | 0.0571 (7) | |
| H35 | 0.9871 | 0.2539 | 0.3722 | 0.069* | |
| C34 | 0.9486 (3) | 0.2129 (3) | 0.48241 (19) | 0.0546 (7) | |
| H34 | 0.9915 | 0.2893 | 0.5163 | 0.065* | |
| C37 | 0.8216 (3) | −0.0150 (3) | 0.38064 (18) | 0.0532 (7) | |
| H37 | 0.7790 | −0.0911 | 0.3461 | 0.064* | |
| C23 | 0.8176 (3) | 0.0209 (3) | 0.87307 (19) | 0.0561 (7) | |
| H23 | 0.8735 | 0.0551 | 0.8377 | 0.067* | |
| C2 | 0.4720 (3) | −0.1491 (3) | 0.53848 (19) | 0.0578 (7) | |
| H2 | 0.4306 | −0.0834 | 0.5627 | 0.069* | |
| C3 | 0.4480 (4) | −0.1994 (3) | 0.4516 (2) | 0.0665 (9) | |
| H3 | 0.3917 | −0.1674 | 0.4189 | 0.080* | |
| C19 | 0.5667 (6) | −0.1375 (4) | 1.0287 (2) | 0.0860 (13) | |
| H19 | 0.5901 | −0.1234 | 1.0862 | 0.103* | |
| C9 | 0.8266 (4) | −0.4821 (3) | 0.6308 (2) | 0.0634 (8) | |
| H9 | 0.8647 | −0.5520 | 0.6121 | 0.076* | |
| C21 | 0.7808 (5) | 0.0001 (4) | 1.0112 (2) | 0.0774 (11) | |
| H21 | 0.8089 | 0.0185 | 1.0685 | 0.093* | |
| C17 | 0.4077 (4) | −0.2368 (3) | 0.9054 (2) | 0.0660 (9) | |
| C10 | 0.8445 (4) | −0.4207 (3) | 0.7137 (2) | 0.0625 (8) | |
| H10 | 0.8948 | −0.4486 | 0.7522 | 0.075* | |
| O3 | 0.9376 (3) | 0.1389 (3) | 0.22532 (15) | 0.0832 (8) | |
| N10 | 0.8849 (3) | 0.0551 (3) | 0.25539 (16) | 0.0626 (7) | |
| O4 | 0.8363 (4) | −0.0475 (3) | 0.21191 (16) | 0.1014 (10) | |
| C4 | 0.5077 (4) | −0.2955 (3) | 0.41506 (18) | 0.0659 (9) | |
| H4 | 0.4936 | −0.3287 | 0.3571 | 0.079* | |
| C7 | 0.7291 (4) | −0.4929 (3) | 0.4848 (2) | 0.0706 (9) | |
| H7 | 0.7677 | −0.5612 | 0.4622 | 0.085* | |
| C18 | 0.4481 (6) | −0.2108 (4) | 0.9942 (3) | 0.0871 (14) | |
| H18 | 0.3906 | −0.2458 | 1.0285 | 0.104* | |
| C15 | 0.2576 (4) | −0.3304 (4) | 0.7812 (4) | 0.0866 (13) | |
| H15 | 0.1766 | −0.3807 | 0.7544 | 0.104* | |
| C16 | 0.2862 (4) | −0.3115 (4) | 0.8657 (3) | 0.0862 (13) | |
| H16 | 0.2244 | −0.3485 | 0.8970 | 0.103* | |
| N6 | 0.4741 (3) | 0.2393 (2) | 0.78229 (17) | 0.0612 (7) | |
| C25 | 0.5024 (3) | 0.1317 (3) | 0.75123 (17) | 0.0502 (7) | |
| C26 | 0.5794 (3) | 0.3316 (3) | 0.82375 (18) | 0.0535 (7) | |
| C31 | 0.7151 (4) | 0.3199 (3) | 0.8274 (2) | 0.0640 (8) | |
| H31 | 0.7387 | 0.2463 | 0.7986 | 0.077* | |
| C30 | 0.8150 (4) | 0.4140 (3) | 0.8722 (3) | 0.0731 (10) | |
| H30 | 0.9053 | 0.4043 | 0.8742 | 0.088* | |
| C29 | 0.7803 (4) | 0.5236 (3) | 0.9144 (2) | 0.0706 (9) | |
| C27 | 0.5480 (4) | 0.4449 (3) | 0.8667 (3) | 0.0801 (11) | |
| H27 | 0.4581 | 0.4561 | 0.8646 | 0.096* | |
| N7 | 0.8870 (5) | 0.6246 (4) | 0.9598 (3) | 0.1013 (12) | |
| C28 | 0.6476 (5) | 0.5385 (4) | 0.9115 (3) | 0.0856 (12) | |
| H28 | 0.6253 | 0.6127 | 0.9401 | 0.103* | |
| O5 | 0.0518 (4) | 0.3745 (3) | 0.7102 (3) | 0.1121 (12) | |
| H5A | 0.001 (5) | 0.301 (4) | 0.681 (3) | 0.14 (2)* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| C39 | 0.175 (6) | 0.078 (3) | 0.169 (6) | 0.031 (4) | −0.033 (5) | 0.007 (4) |
| O1 | 0.132 (4) | 0.093 (3) | 0.301 (7) | 0.003 (3) | −0.012 (4) | −0.062 (4) |
| O2 | 0.088 (2) | 0.105 (3) | 0.196 (4) | −0.015 (2) | 0.031 (3) | 0.020 (3) |
| Ni1 | 0.0489 (2) | 0.04276 (18) | 0.02925 (15) | 0.01033 (14) | 0.00388 (12) | 0.00828 (12) |
| N2 | 0.0498 (13) | 0.0472 (12) | 0.0352 (10) | 0.0105 (10) | 0.0052 (9) | 0.0125 (9) |
| N3 | 0.0533 (14) | 0.0422 (12) | 0.0478 (12) | 0.0082 (10) | 0.0090 (10) | 0.0071 (10) |
| N1 | 0.0528 (13) | 0.0485 (12) | 0.0353 (10) | 0.0150 (10) | 0.0015 (9) | 0.0106 (9) |
| N4 | 0.0549 (13) | 0.0440 (12) | 0.0337 (10) | 0.0103 (10) | 0.0015 (9) | 0.0075 (9) |
| N8 | 0.0629 (16) | 0.0673 (16) | 0.0467 (13) | 0.0077 (13) | 0.0109 (11) | 0.0199 (12) |
| N5 | 0.0680 (17) | 0.0530 (15) | 0.0569 (15) | 0.0198 (13) | 0.0024 (12) | 0.0099 (12) |
| C12 | 0.0457 (14) | 0.0413 (13) | 0.0382 (12) | 0.0080 (11) | 0.0069 (10) | 0.0091 (10) |
| C5 | 0.0667 (19) | 0.0549 (17) | 0.0357 (13) | 0.0074 (14) | 0.0038 (12) | 0.0061 (12) |
| C24 | 0.0654 (18) | 0.0447 (14) | 0.0349 (12) | 0.0209 (13) | 0.0090 (12) | 0.0097 (10) |
| C13 | 0.0628 (18) | 0.0446 (14) | 0.0460 (14) | 0.0204 (13) | 0.0207 (13) | 0.0131 (11) |
| C11 | 0.0613 (18) | 0.0604 (18) | 0.0442 (14) | 0.0168 (14) | 0.0043 (13) | 0.0219 (13) |
| C20 | 0.097 (3) | 0.0649 (19) | 0.0360 (14) | 0.0398 (19) | 0.0114 (15) | 0.0131 (13) |
| C6 | 0.100 (3) | 0.066 (2) | 0.0414 (15) | 0.0221 (19) | 0.0067 (16) | −0.0039 (14) |
| C1 | 0.0502 (15) | 0.0450 (14) | 0.0347 (12) | 0.0050 (11) | 0.0051 (10) | 0.0092 (10) |
| N9 | 0.0590 (15) | 0.0565 (14) | 0.0452 (12) | 0.0045 (12) | 0.0082 (11) | 0.0176 (11) |
| C38 | 0.0542 (16) | 0.0490 (15) | 0.0496 (15) | 0.0060 (13) | 0.0079 (12) | 0.0168 (12) |
| C22 | 0.089 (3) | 0.066 (2) | 0.0548 (19) | 0.0179 (19) | −0.0261 (18) | −0.0005 (16) |
| C33 | 0.0447 (14) | 0.0527 (15) | 0.0444 (13) | 0.0121 (12) | 0.0077 (11) | 0.0163 (12) |
| C8 | 0.0627 (18) | 0.0445 (15) | 0.0544 (16) | 0.0136 (13) | 0.0079 (14) | 0.0086 (12) |
| C14 | 0.0533 (18) | 0.0570 (19) | 0.078 (2) | 0.0074 (15) | 0.0052 (16) | 0.0051 (16) |
| C32 | 0.0472 (15) | 0.0584 (17) | 0.0404 (13) | 0.0120 (13) | 0.0062 (11) | 0.0110 (12) |
| C36 | 0.0489 (16) | 0.0638 (18) | 0.0424 (14) | 0.0161 (13) | 0.0069 (12) | 0.0175 (13) |
| C35 | 0.0649 (19) | 0.0590 (18) | 0.0526 (16) | 0.0087 (15) | 0.0142 (14) | 0.0240 (14) |
| C34 | 0.0625 (18) | 0.0491 (16) | 0.0509 (15) | 0.0034 (14) | 0.0116 (13) | 0.0135 (13) |
| C37 | 0.0540 (17) | 0.0546 (17) | 0.0494 (15) | 0.0075 (13) | 0.0041 (13) | 0.0115 (13) |
| C23 | 0.0627 (18) | 0.0497 (16) | 0.0507 (16) | 0.0066 (14) | −0.0062 (13) | 0.0072 (13) |
| C2 | 0.0635 (19) | 0.069 (2) | 0.0455 (15) | 0.0245 (15) | −0.0018 (13) | 0.0161 (14) |
| C3 | 0.075 (2) | 0.083 (2) | 0.0442 (16) | 0.0182 (18) | −0.0085 (15) | 0.0212 (16) |
| C19 | 0.132 (4) | 0.105 (3) | 0.0445 (18) | 0.059 (3) | 0.033 (2) | 0.033 (2) |
| C9 | 0.074 (2) | 0.0528 (18) | 0.071 (2) | 0.0258 (16) | 0.0141 (17) | 0.0182 (15) |
| C21 | 0.114 (3) | 0.082 (2) | 0.0356 (15) | 0.042 (2) | −0.0144 (18) | 0.0012 (16) |
| C17 | 0.077 (2) | 0.0609 (19) | 0.077 (2) | 0.0283 (18) | 0.0401 (18) | 0.0321 (17) |
| C10 | 0.070 (2) | 0.064 (2) | 0.0648 (19) | 0.0242 (16) | 0.0056 (16) | 0.0302 (16) |
| O3 | 0.110 (2) | 0.0929 (19) | 0.0529 (13) | 0.0118 (16) | 0.0165 (13) | 0.0331 (13) |
| N10 | 0.0711 (18) | 0.0763 (19) | 0.0446 (13) | 0.0176 (15) | 0.0061 (12) | 0.0204 (13) |
| O4 | 0.153 (3) | 0.088 (2) | 0.0470 (13) | −0.0056 (19) | 0.0008 (16) | 0.0078 (13) |
| C4 | 0.084 (2) | 0.077 (2) | 0.0333 (13) | 0.0120 (19) | −0.0025 (14) | 0.0111 (14) |
| C7 | 0.095 (3) | 0.0550 (19) | 0.0598 (19) | 0.0275 (18) | 0.0138 (18) | −0.0008 (15) |
| C18 | 0.123 (4) | 0.099 (3) | 0.073 (2) | 0.057 (3) | 0.061 (3) | 0.052 (2) |
| C15 | 0.053 (2) | 0.069 (2) | 0.133 (4) | 0.0011 (17) | 0.020 (2) | 0.020 (2) |
| C16 | 0.076 (3) | 0.074 (2) | 0.124 (4) | 0.019 (2) | 0.053 (3) | 0.043 (3) |
| N6 | 0.0699 (17) | 0.0549 (15) | 0.0593 (15) | 0.0258 (13) | 0.0045 (13) | 0.0055 (12) |
| C25 | 0.0619 (18) | 0.0566 (17) | 0.0366 (13) | 0.0188 (14) | 0.0046 (12) | 0.0151 (12) |
| C26 | 0.071 (2) | 0.0543 (17) | 0.0446 (14) | 0.0262 (15) | 0.0147 (13) | 0.0174 (12) |
| C31 | 0.074 (2) | 0.0528 (18) | 0.071 (2) | 0.0186 (16) | 0.0279 (17) | 0.0172 (15) |
| C30 | 0.065 (2) | 0.071 (2) | 0.088 (3) | 0.0138 (18) | 0.0259 (19) | 0.024 (2) |
| C29 | 0.079 (2) | 0.0532 (19) | 0.080 (2) | 0.0059 (17) | 0.0168 (19) | 0.0199 (17) |
| C27 | 0.077 (2) | 0.063 (2) | 0.097 (3) | 0.0332 (19) | 0.002 (2) | −0.0019 (19) |
| N7 | 0.096 (3) | 0.066 (2) | 0.135 (4) | 0.005 (2) | 0.016 (3) | 0.018 (2) |
| C28 | 0.091 (3) | 0.057 (2) | 0.104 (3) | 0.029 (2) | 0.012 (2) | −0.001 (2) |
| O5 | 0.112 (3) | 0.070 (2) | 0.144 (3) | 0.0153 (18) | −0.024 (2) | 0.016 (2) |
Geometric parameters (Å, º)
| C39—O5 | 1.471 (7) | C8—C7 | 1.434 (4) |
| C39—H39A | 0.9600 | C14—C15 | 1.394 (6) |
| C39—H39B | 0.9600 | C14—H14 | 0.9300 |
| C39—H39C | 0.9600 | C36—C37 | 1.380 (4) |
| O1—N7 | 1.195 (6) | C36—C35 | 1.383 (4) |
| O2—N7 | 1.212 (5) | C36—N10 | 1.451 (4) |
| Ni1—N5 | 2.056 (3) | C35—C34 | 1.372 (4) |
| Ni1—N8 | 2.062 (3) | C35—H35 | 0.9300 |
| Ni1—N4 | 2.076 (2) | C34—H34 | 0.9300 |
| Ni1—N1 | 2.097 (2) | C37—H37 | 0.9300 |
| Ni1—N3 | 2.098 (2) | C23—H23 | 0.9300 |
| Ni1—N2 | 2.139 (2) | C2—C3 | 1.397 (4) |
| N2—C11 | 1.323 (3) | C2—H2 | 0.9300 |
| N2—C12 | 1.358 (3) | C3—C4 | 1.360 (5) |
| N3—C14 | 1.326 (4) | C3—H3 | 0.9300 |
| N3—C13 | 1.351 (4) | C19—C18 | 1.340 (6) |
| N1—C2 | 1.325 (4) | C19—H19 | 0.9300 |
| N1—C1 | 1.358 (3) | C9—C10 | 1.362 (5) |
| N4—C23 | 1.322 (4) | C9—H9 | 0.9300 |
| N4—C24 | 1.352 (4) | C21—H21 | 0.9300 |
| N8—C32 | 1.157 (4) | C17—C16 | 1.390 (6) |
| N5—C25 | 1.156 (4) | C17—C18 | 1.438 (6) |
| C12—C8 | 1.402 (4) | C10—H10 | 0.9300 |
| C12—C1 | 1.435 (4) | O3—N10 | 1.224 (4) |
| C5—C4 | 1.402 (5) | N10—O4 | 1.215 (4) |
| C5—C1 | 1.405 (4) | C4—H4 | 0.9300 |
| C5—C6 | 1.433 (5) | C7—H7 | 0.9300 |
| C24—C20 | 1.404 (4) | C18—H18 | 0.9300 |
| C24—C13 | 1.432 (4) | C15—C16 | 1.360 (7) |
| C13—C17 | 1.407 (4) | C15—H15 | 0.9300 |
| C11—C10 | 1.390 (4) | C16—H16 | 0.9300 |
| C11—H11 | 0.9300 | N6—C25 | 1.289 (4) |
| C20—C21 | 1.403 (6) | N6—C26 | 1.373 (4) |
| C20—C19 | 1.425 (6) | C26—C31 | 1.388 (5) |
| C6—C7 | 1.342 (5) | C26—C27 | 1.406 (4) |
| C6—H6 | 0.9300 | C31—C30 | 1.368 (5) |
| N9—C32 | 1.297 (4) | C31—H31 | 0.9300 |
| N9—C33 | 1.383 (3) | C30—C29 | 1.380 (5) |
| C38—C37 | 1.378 (4) | C30—H30 | 0.9300 |
| C38—C33 | 1.402 (4) | C29—C28 | 1.368 (6) |
| C38—H38 | 0.9300 | C29—N7 | 1.451 (5) |
| C22—C21 | 1.350 (6) | C27—C28 | 1.363 (6) |
| C22—C23 | 1.395 (4) | C27—H27 | 0.9300 |
| C22—H22 | 0.9300 | C28—H28 | 0.9300 |
| C33—C34 | 1.402 (4) | O5—H5A | 0.91 (3) |
| C8—C9 | 1.401 (5) | ||
| O5—C39—H39A | 109.5 | C37—C36—N10 | 119.4 (3) |
| O5—C39—H39B | 109.5 | C35—C36—N10 | 119.3 (3) |
| H39A—C39—H39B | 109.5 | C34—C35—C36 | 119.5 (3) |
| O5—C39—H39C | 109.5 | C34—C35—H35 | 120.2 |
| H39A—C39—H39C | 109.5 | C36—C35—H35 | 120.2 |
| H39B—C39—H39C | 109.5 | C35—C34—C33 | 120.9 (3) |
| N5—Ni1—N8 | 90.67 (12) | C35—C34—H34 | 119.6 |
| N5—Ni1—N4 | 94.32 (10) | C33—C34—H34 | 119.6 |
| N8—Ni1—N4 | 92.39 (10) | C38—C37—C36 | 119.1 (3) |
| N5—Ni1—N1 | 93.16 (10) | C38—C37—H37 | 120.4 |
| N8—Ni1—N1 | 89.69 (10) | C36—C37—H37 | 120.4 |
| N4—Ni1—N1 | 172.22 (9) | N4—C23—C22 | 122.5 (3) |
| N5—Ni1—N3 | 89.86 (11) | N4—C23—H23 | 118.7 |
| N8—Ni1—N3 | 171.74 (10) | C22—C23—H23 | 118.7 |
| N4—Ni1—N3 | 79.35 (10) | N1—C2—C3 | 122.6 (3) |
| N1—Ni1—N3 | 98.51 (10) | N1—C2—H2 | 118.7 |
| N5—Ni1—N2 | 171.53 (9) | C3—C2—H2 | 118.7 |
| N8—Ni1—N2 | 91.08 (10) | C4—C3—C2 | 119.5 (3) |
| N4—Ni1—N2 | 93.89 (9) | C4—C3—H3 | 120.3 |
| N1—Ni1—N2 | 78.56 (9) | C2—C3—H3 | 120.3 |
| N3—Ni1—N2 | 89.59 (9) | C18—C19—C20 | 121.2 (3) |
| C11—N2—C12 | 117.8 (2) | C18—C19—H19 | 119.4 |
| C11—N2—Ni1 | 129.65 (19) | C20—C19—H19 | 119.4 |
| C12—N2—Ni1 | 112.50 (17) | C10—C9—C8 | 119.4 (3) |
| C14—N3—C13 | 118.2 (3) | C10—C9—H9 | 120.3 |
| C14—N3—Ni1 | 129.1 (2) | C8—C9—H9 | 120.3 |
| C13—N3—Ni1 | 112.70 (19) | C22—C21—C20 | 119.9 (3) |
| C2—N1—C1 | 118.3 (2) | C22—C21—H21 | 120.1 |
| C2—N1—Ni1 | 127.8 (2) | C20—C21—H21 | 120.1 |
| C1—N1—Ni1 | 113.33 (17) | C16—C17—C13 | 117.1 (4) |
| C23—N4—C24 | 118.1 (2) | C16—C17—C18 | 124.5 (4) |
| C23—N4—Ni1 | 128.3 (2) | C13—C17—C18 | 118.4 (4) |
| C24—N4—Ni1 | 113.61 (18) | C9—C10—C11 | 119.6 (3) |
| C32—N8—Ni1 | 150.6 (3) | C9—C10—H10 | 120.2 |
| C25—N5—Ni1 | 153.2 (2) | C11—C10—H10 | 120.2 |
| N2—C12—C8 | 122.8 (3) | O4—N10—O3 | 122.2 (3) |
| N2—C12—C1 | 116.9 (2) | O4—N10—C36 | 118.9 (3) |
| C8—C12—C1 | 120.2 (2) | O3—N10—C36 | 118.9 (3) |
| C4—C5—C1 | 117.4 (3) | C3—C4—C5 | 119.6 (3) |
| C4—C5—C6 | 124.0 (3) | C3—C4—H4 | 120.2 |
| C1—C5—C6 | 118.6 (3) | C5—C4—H4 | 120.2 |
| N4—C24—C20 | 123.0 (3) | C6—C7—C8 | 121.6 (3) |
| N4—C24—C13 | 116.9 (2) | C6—C7—H7 | 119.2 |
| C20—C24—C13 | 120.1 (3) | C8—C7—H7 | 119.2 |
| N3—C13—C17 | 122.9 (3) | C19—C18—C17 | 121.6 (3) |
| N3—C13—C24 | 117.4 (2) | C19—C18—H18 | 119.2 |
| C17—C13—C24 | 119.7 (3) | C17—C18—H18 | 119.2 |
| N2—C11—C10 | 123.1 (3) | C16—C15—C14 | 119.6 (4) |
| N2—C11—H11 | 118.5 | C16—C15—H15 | 120.2 |
| C10—C11—H11 | 118.5 | C14—C15—H15 | 120.2 |
| C21—C20—C24 | 116.8 (3) | C15—C16—C17 | 119.9 (4) |
| C21—C20—C19 | 124.3 (3) | C15—C16—H16 | 120.0 |
| C24—C20—C19 | 118.9 (4) | C17—C16—H16 | 120.0 |
| C7—C6—C5 | 121.4 (3) | C25—N6—C26 | 117.3 (3) |
| C7—C6—H6 | 119.3 | N5—C25—N6 | 176.2 (4) |
| C5—C6—H6 | 119.3 | N6—C26—C31 | 124.4 (3) |
| N1—C1—C5 | 122.5 (3) | N6—C26—C27 | 118.0 (3) |
| N1—C1—C12 | 117.7 (2) | C31—C26—C27 | 117.6 (3) |
| C5—C1—C12 | 119.8 (3) | C30—C31—C26 | 121.5 (3) |
| C32—N9—C33 | 117.8 (2) | C30—C31—H31 | 119.2 |
| C37—C38—C33 | 121.1 (3) | C26—C31—H31 | 119.2 |
| C37—C38—H38 | 119.4 | C31—C30—C29 | 119.3 (4) |
| C33—C38—H38 | 119.4 | C31—C30—H30 | 120.3 |
| C21—C22—C23 | 119.7 (3) | C29—C30—H30 | 120.3 |
| C21—C22—H22 | 120.2 | C28—C29—C30 | 120.6 (4) |
| C23—C22—H22 | 120.2 | C28—C29—N7 | 120.2 (4) |
| N9—C33—C38 | 123.8 (3) | C30—C29—N7 | 119.2 (4) |
| N9—C33—C34 | 118.1 (3) | C28—C27—C26 | 120.7 (4) |
| C38—C33—C34 | 118.1 (3) | C28—C27—H27 | 119.6 |
| C9—C8—C12 | 117.4 (3) | C26—C27—H27 | 119.6 |
| C9—C8—C7 | 124.3 (3) | O1—N7—O2 | 122.1 (5) |
| C12—C8—C7 | 118.3 (3) | O1—N7—C29 | 118.6 (5) |
| N3—C14—C15 | 122.3 (4) | O2—N7—C29 | 119.4 (4) |
| N3—C14—H14 | 118.9 | C27—C28—C29 | 120.2 (3) |
| C15—C14—H14 | 118.9 | C27—C28—H28 | 119.9 |
| N8—C32—N9 | 175.6 (3) | C29—C28—H28 | 119.9 |
| C37—C36—C35 | 121.2 (3) | C39—O5—H5A | 104 (4) |
| N8—Ni1—N2—C11 | 94.1 (3) | N2—C12—C1—C5 | 177.1 (3) |
| N4—Ni1—N2—C11 | 1.6 (3) | C8—C12—C1—C5 | −3.2 (4) |
| N1—Ni1—N2—C11 | −176.5 (3) | C32—N9—C33—C38 | 1.2 (4) |
| N3—Ni1—N2—C11 | −77.7 (3) | C32—N9—C33—C34 | −178.9 (3) |
| N8—Ni1—N2—C12 | −82.19 (19) | C37—C38—C33—N9 | 179.3 (3) |
| N4—Ni1—N2—C12 | −174.66 (18) | C37—C38—C33—C34 | −0.6 (4) |
| N1—Ni1—N2—C12 | 7.27 (18) | N2—C12—C8—C9 | 2.0 (4) |
| N3—Ni1—N2—C12 | 106.04 (19) | C1—C12—C8—C9 | −177.7 (3) |
| N5—Ni1—N3—C14 | 83.3 (3) | N2—C12—C8—C7 | −176.8 (3) |
| N4—Ni1—N3—C14 | 177.7 (3) | C1—C12—C8—C7 | 3.5 (4) |
| N1—Ni1—N3—C14 | −9.9 (3) | C13—N3—C14—C15 | −1.6 (5) |
| N2—Ni1—N3—C14 | −88.2 (3) | Ni1—N3—C14—C15 | 179.4 (3) |
| N5—Ni1—N3—C13 | −95.8 (2) | C37—C36—C35—C34 | 0.3 (5) |
| N4—Ni1—N3—C13 | −1.39 (19) | N10—C36—C35—C34 | −178.1 (3) |
| N1—Ni1—N3—C13 | 171.02 (19) | C36—C35—C34—C33 | −0.4 (5) |
| N2—Ni1—N3—C13 | 92.6 (2) | N9—C33—C34—C35 | −179.4 (3) |
| N5—Ni1—N1—C2 | 2.2 (3) | C38—C33—C34—C35 | 0.5 (5) |
| N8—Ni1—N1—C2 | −88.5 (3) | C33—C38—C37—C36 | 0.6 (5) |
| N3—Ni1—N1—C2 | 92.5 (3) | C35—C36—C37—C38 | −0.4 (5) |
| N2—Ni1—N1—C2 | −179.6 (3) | N10—C36—C37—C38 | 178.0 (3) |
| N5—Ni1—N1—C1 | 172.90 (19) | C24—N4—C23—C22 | −0.4 (4) |
| N8—Ni1—N1—C1 | 82.2 (2) | Ni1—N4—C23—C22 | −179.9 (2) |
| N3—Ni1—N1—C1 | −96.77 (19) | C21—C22—C23—N4 | 1.7 (5) |
| N2—Ni1—N1—C1 | −8.92 (18) | C1—N1—C2—C3 | −1.0 (5) |
| N5—Ni1—N4—C23 | −89.7 (3) | Ni1—N1—C2—C3 | 169.4 (3) |
| N8—Ni1—N4—C23 | 1.1 (3) | N1—C2—C3—C4 | 0.0 (5) |
| N3—Ni1—N4—C23 | −178.8 (3) | C21—C20—C19—C18 | 177.8 (4) |
| N2—Ni1—N4—C23 | 92.4 (3) | C24—C20—C19—C18 | −1.1 (6) |
| N5—Ni1—N4—C24 | 90.8 (2) | C12—C8—C9—C10 | −1.3 (5) |
| N8—Ni1—N4—C24 | −178.4 (2) | C7—C8—C9—C10 | 177.4 (3) |
| N3—Ni1—N4—C24 | 1.73 (18) | C23—C22—C21—C20 | −1.6 (6) |
| N2—Ni1—N4—C24 | −87.13 (19) | C24—C20—C21—C22 | 0.2 (5) |
| N5—Ni1—N8—C32 | −33.3 (5) | C19—C20—C21—C22 | −178.6 (4) |
| N4—Ni1—N8—C32 | −127.6 (5) | N3—C13—C17—C16 | −0.5 (5) |
| N1—Ni1—N8—C32 | 59.9 (5) | C24—C13—C17—C16 | 179.1 (3) |
| N2—Ni1—N8—C32 | 138.4 (5) | N3—C13—C17—C18 | 179.4 (3) |
| N8—Ni1—N5—C25 | −67.7 (6) | C24—C13—C17—C18 | −1.0 (4) |
| N4—Ni1—N5—C25 | 24.7 (6) | C8—C9—C10—C11 | −0.2 (5) |
| N1—Ni1—N5—C25 | −157.5 (6) | N2—C11—C10—C9 | 1.2 (5) |
| N3—Ni1—N5—C25 | 104.0 (6) | C37—C36—N10—O4 | −2.7 (5) |
| C11—N2—C12—C8 | −1.1 (4) | C35—C36—N10—O4 | 175.7 (3) |
| Ni1—N2—C12—C8 | 175.6 (2) | C37—C36—N10—O3 | 178.6 (3) |
| C11—N2—C12—C1 | 178.6 (2) | C35—C36—N10—O3 | −2.9 (5) |
| Ni1—N2—C12—C1 | −4.7 (3) | C2—C3—C4—C5 | 1.0 (5) |
| C23—N4—C24—C20 | −1.0 (4) | C1—C5—C4—C3 | −1.0 (5) |
| Ni1—N4—C24—C20 | 178.6 (2) | C6—C5—C4—C3 | 178.2 (3) |
| C23—N4—C24—C13 | 178.6 (3) | C5—C6—C7—C8 | −1.5 (6) |
| Ni1—N4—C24—C13 | −1.8 (3) | C9—C8—C7—C6 | −179.9 (4) |
| C14—N3—C13—C17 | 1.2 (4) | C12—C8—C7—C6 | −1.2 (5) |
| Ni1—N3—C13—C17 | −179.5 (2) | C20—C19—C18—C17 | 0.7 (6) |
| C14—N3—C13—C24 | −178.3 (3) | C16—C17—C18—C19 | −179.8 (4) |
| Ni1—N3—C13—C24 | 0.9 (3) | C13—C17—C18—C19 | 0.4 (6) |
| N4—C24—C13—N3 | 0.6 (4) | N3—C14—C15—C16 | 1.1 (6) |
| C20—C24—C13—N3 | −179.8 (3) | C14—C15—C16—C17 | −0.3 (6) |
| N4—C24—C13—C17 | −179.0 (3) | C13—C17—C16—C15 | 0.0 (6) |
| C20—C24—C13—C17 | 0.6 (4) | C18—C17—C16—C15 | −179.9 (4) |
| C12—N2—C11—C10 | −0.5 (4) | C26—N6—C25—N5 | 177 (100) |
| Ni1—N2—C11—C10 | −176.6 (2) | C25—N6—C26—C31 | 6.4 (5) |
| N4—C24—C20—C21 | 1.1 (4) | C25—N6—C26—C27 | −172.4 (3) |
| C13—C24—C20—C21 | −178.5 (3) | N6—C26—C31—C30 | −177.7 (3) |
| N4—C24—C20—C19 | 180.0 (3) | C27—C26—C31—C30 | 1.1 (5) |
| C13—C24—C20—C19 | 0.4 (4) | C26—C31—C30—C29 | −0.4 (5) |
| C4—C5—C6—C7 | −177.4 (4) | C31—C30—C29—C28 | −0.2 (6) |
| C1—C5—C6—C7 | 1.8 (5) | C31—C30—C29—N7 | −178.1 (4) |
| C2—N1—C1—C5 | 0.9 (4) | N6—C26—C27—C28 | 177.7 (4) |
| Ni1—N1—C1—C5 | −170.8 (2) | C31—C26—C27—C28 | −1.2 (6) |
| C2—N1—C1—C12 | −178.9 (3) | C28—C29—N7—O1 | 7.7 (8) |
| Ni1—N1—C1—C12 | 9.5 (3) | C30—C29—N7—O1 | −174.4 (6) |
| C4—C5—C1—N1 | 0.1 (4) | C28—C29—N7—O2 | −172.6 (5) |
| C6—C5—C1—N1 | −179.2 (3) | C30—C29—N7—O2 | 5.3 (7) |
| C4—C5—C1—C12 | 179.8 (3) | C26—C27—C28—C29 | 0.6 (7) |
| C6—C5—C1—C12 | 0.6 (4) | C30—C29—C28—C27 | 0.1 (7) |
| N2—C12—C1—N1 | −3.2 (4) | N7—C29—C28—C27 | 178.0 (4) |
| C8—C12—C1—N1 | 176.5 (3) |
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| O5—H5A···N9i | 0.91 (3) | 1.98 (4) | 2.883 (4) | 174 (5) |
| C39—H39B···O4ii | 0.96 | 2.55 | 3.435 (7) | 153 |
| C22—H22···O1iii | 0.93 | 2.57 | 3.469 (6) | 162 |
| C16—H16···O2iv | 0.93 | 2.45 | 3.312 (6) | 154 |
Symmetry codes: (i) x−1, y, z; (ii) −x+1, −y, −z+1; (iii) −x+2, −y+1, −z+2; (iv) x−1, y−1, z.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: BT5812).
References
- Ainscough, E. W., Baker, E. N., Brader, M. L. & Brodie, A. M. (1991). J. Chem. Soc. Dalton Trans. pp. 1243–1249.
- Cheng, Y.-Q., Liu, A.-L., Hu, M.-L. & Ng, S. W. (2002). Acta Cryst. E58, m545–m547.
- Chiniforoshan, H., Jalilpour, S., Shirinfar, B. & Khavasi, H. R. (2009). Acta Cryst. E65, m386. [DOI] [PMC free article] [PubMed]
- Chiniforoshan, H., Jazestani, M. & Notash, B. (2012). Acta Cryst. E68, m232. [DOI] [PMC free article] [PubMed]
- Chiniforoshan, H., Shirinfar, B., Jalilpour, S. & Khavasi, H. R. (2010). Acta Cryst. E66, m331. [DOI] [PMC free article] [PubMed]
- Crutchley, R. J. (2001). Coord. Chem. Rev. 219, 125–155.
- Crutchley, R. J. & Naklicki, M. L. (1989). Inorg. Chem. 28, 1955–1958.
- Escuer, A., Mautner, F. A., Sanz, N. & Vicente, R. (2004). Polyhedron, 23, 1409–1417.
- Farrugia, L. J. (1997). J. Appl. Cryst. 30, 565.
- Farrugia, L. J. (1999). J. Appl. Cryst. 32, 837–838.
- Kim, Y.-J., Joo, Y.-S., Han, J.-T. & Han, W. S. (2002). J. Chem. Soc. Dalton Trans. pp. 3611–3618.
- Letcher, R. J., Zhang, W., Bensimon, C. & Crutchley, R. J. (1993). Inorg. Chim. Acta, 210, 183–191.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Shen, X., Shan, J., Sun, H.-B. & Kang, B.-S. (1999). J. Chin. Chem. Soc. 46, 179–183.
- Stoe & Cie (2005). X-AREA, X-RED and X-SHAPE Stoe & Cie, Darmstadt, Germany.
- Wu, A.-Q., Zheng, F.-K., Guo, G.-C. & Huang, J.-S. (2004). Acta Cryst. E60, m373–m375.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S1600536812009890/bt5812sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536812009890/bt5812Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report