Abstract
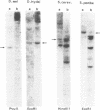
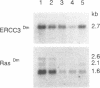
Previously the human nucleotide excision repair gene ERCC3 was shown to be responsible for a rare combination of the autosomal recessive DNA repair disorders xeroderma pigmentosum (complementation group B) and Cockayne's syndrome (complementation group C). The human and mouse ERCC3 proteins contain several sequence motifs suggesting that it is a nucleic acid or chromatin binding helicase. To study the significance of these domains and the overall evolutionary conservation of the gene, the homolog from Drosophila melanogaster was isolated by low stringency hybridizations using two flanking probes of the human ERCC3 cDNA. The flanking probe strategy selects for long stretches of nucleotide sequence homology, and avoids isolation of small regions with fortuitous homology. In situ hybridization localized the gene onto chromosome III 67E3/4, a region devoid of known D.melanogaster mutagen sensitive mutants. Northern blot analysis showed that the gene is continuously expressed in all stages of fly development. A slight increase (2-3 times) of ERCC3Dm transcript was observed in the later stages. Two almost full length cDNAs were isolated, which have different 5' untranslated regions (UTR). The SD4 cDNA harbours only one long open reading frame (ORF) coding for ERCC3Dm. Another clone (SD2), however, has the potential to encode two proteins: a 170 amino acids polypeptide starting at the optimal first ATG has no detectable homology with any other proteins currently in the data bases, and another ORF beginning at the suboptimal second startcodon which is identical to that of SD4. Comparison of the encoded ERCC3Dm protein with the homologous proteins of mouse and man shows a strong amino acid conservation (71% identity), especially in the postulated DNA binding region and seven 'helicase' domains. The ERCC3Dm sequence is fully consistent with the presumed functions and the high conservation of these regions strengthens their functional significance. Microinjection and DNA transfection of ERCC3Dm into human xeroderma pigmentosum (c.g. B) fibroblasts and group 3 rodent mutants did not yield detectable correction. One of the possibilities to explain these negative findings is that the D.melanogaster protein may be unable to function in a mammalian repair context.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bankmann M., Prakash L., Prakash S. Yeast RAD14 and human xeroderma pigmentosum group A DNA-repair genes encode homologous proteins. Nature. 1992 Feb 6;355(6360):555–558. doi: 10.1038/355555a0. [DOI] [PubMed] [Google Scholar]
- Birnstiel M. L., Busslinger M., Strub K. Transcription termination and 3' processing: the end is in site! Cell. 1985 Jun;41(2):349–359. doi: 10.1016/s0092-8674(85)80007-6. [DOI] [PubMed] [Google Scholar]
- Brennan R. G., Matthews B. W. The helix-turn-helix DNA binding motif. J Biol Chem. 1989 Feb 5;264(4):1903–1906. [PubMed] [Google Scholar]
- Broughton B. C., Barbet N., Murray J., Watts F. Z., Koken M. H., Lehmann A. R., Carr A. M. Assignment of ten DNA repair genes from Schizosaccharomyces pombe to chromosomal NotI restriction fragments. Mol Gen Genet. 1991 Sep;228(3):470–472. doi: 10.1007/BF00260641. [DOI] [PubMed] [Google Scholar]
- Cavener D. R. Comparison of the consensus sequence flanking translational start sites in Drosophila and vertebrates. Nucleic Acids Res. 1987 Feb 25;15(4):1353–1361. doi: 10.1093/nar/15.4.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbalenya A. E., Koonin E. V., Donchenko A. P., Blinov V. M. Two related superfamilies of putative helicases involved in replication, recombination, repair and expression of DNA and RNA genomes. Nucleic Acids Res. 1989 Jun 26;17(12):4713–4730. doi: 10.1093/nar/17.12.4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulyas K. D., Donahue T. F. SSL2, a suppressor of a stem-loop mutation in the HIS4 leader encodes the yeast homolog of human ERCC-3. Cell. 1992 Jun 12;69(6):1031–1042. doi: 10.1016/0092-8674(92)90621-i. [DOI] [PubMed] [Google Scholar]
- Itoh N., Slemmon J. R., Hawke D. H., Williamson R., Morita E., Itakura K., Roberts E., Shively J. E., Crawford G. D., Salvaterra P. M. Cloning of Drosophila choline acetyltransferase cDNA. Proc Natl Acad Sci U S A. 1986 Jun;83(11):4081–4085. doi: 10.1073/pnas.83.11.4081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koken M. H., Reynolds P., Jaspers-Dekker I., Prakash L., Prakash S., Bootsma D., Hoeijmakers J. H. Structural and functional conservation of two human homologs of the yeast DNA repair gene RAD6. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):8865–8869. doi: 10.1073/pnas.88.20.8865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koken M., Reynolds P., Bootsma D., Hoeijmakers J., Prakash S., Prakash L. Dhr6, a Drosophila homolog of the yeast DNA-repair gene RAD6. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3832–3836. doi: 10.1073/pnas.88.9.3832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann A. R., Hoeijmakers J. H., van Zeeland A. A., Backendorf C. M., Bridges B. A., Collins A., Fuchs R. P., Margison G. P., Montesano R., Moustacchi E. Workshop on DNA repair. Mutat Res. 1992 Jan;273(1):1–28. doi: 10.1016/0921-8777(92)90046-6. [DOI] [PubMed] [Google Scholar]
- Mount S. M. A catalogue of splice junction sequences. Nucleic Acids Res. 1982 Jan 22;10(2):459–472. doi: 10.1093/nar/10.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozer B., Marlor R., Parkhurst S., Corces V. Characterization and developmental expression of a Drosophila ras oncogene. Mol Cell Biol. 1985 Apr;5(4):885–889. doi: 10.1128/mcb.5.4.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudgett J. S., MacInnes M. A. Isolation of the functional human excision repair gene ERCC5 by intercosmid recombination. Genomics. 1990 Dec;8(4):623–633. doi: 10.1016/0888-7543(90)90248-s. [DOI] [PubMed] [Google Scholar]
- Nance M. A., Berry S. A. Cockayne syndrome: review of 140 cases. Am J Med Genet. 1992 Jan 1;42(1):68–84. doi: 10.1002/ajmg.1320420115. [DOI] [PubMed] [Google Scholar]
- Pastink A., Vreeken C., Vogel E. W. The nature of N-ethyl-N-nitrosourea-induced mutations at the white locus of Drosophila melanogaster. Mutat Res. 1988 May;199(1):47–53. doi: 10.1016/0027-5107(88)90229-1. [DOI] [PubMed] [Google Scholar]
- Robbins J., Dilworth S. M., Laskey R. A., Dingwall C. Two interdependent basic domains in nucleoplasmin nuclear targeting sequence: identification of a class of bipartite nuclear targeting sequence. Cell. 1991 Feb 8;64(3):615–623. doi: 10.1016/0092-8674(91)90245-t. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searles L. L., Ruth R. S., Pret A. M., Fridell R. A., Ali A. J. Structure and transcription of the Drosophila melanogaster vermilion gene and several mutant alleles. Mol Cell Biol. 1990 Apr;10(4):1423–1431. doi: 10.1128/mcb.10.4.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimamoto T., Kohno K., Tanaka K., Okada Y. Molecular cloning of human XPAC gene homologs from chicken, Xenopus laevis and Drosophila melanogaster. Biochem Biophys Res Commun. 1991 Dec 31;181(3):1231–1237. doi: 10.1016/0006-291x(91)92070-z. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Miura N., Satokata I., Miyamoto I., Yoshida M. C., Satoh Y., Kondo S., Yasui A., Okayama H., Okada Y. Analysis of a human DNA excision repair gene involved in group A xeroderma pigmentosum and containing a zinc-finger domain. Nature. 1990 Nov 1;348(6296):73–76. doi: 10.1038/348073a0. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Satokata I., Ogita Z., Uchida T., Okada Y. Molecular cloning of a mouse DNA repair gene that complements the defect of group-A xeroderma pigmentosum. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5512–5516. doi: 10.1073/pnas.86.14.5512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troelstra C., Odijk H., de Wit J., Westerveld A., Thompson L. H., Bootsma D., Hoeijmakers J. H. Molecular cloning of the human DNA excision repair gene ERCC-6. Mol Cell Biol. 1990 Nov;10(11):5806–5813. doi: 10.1128/mcb.10.11.5806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Houten B. Nucleotide excision repair in Escherichia coli. Microbiol Rev. 1990 Mar;54(1):18–51. doi: 10.1128/mr.54.1.18-51.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeulen W., Osseweijer P., de Jonge A. J., Hoeijmakers J. H. Transient correction of excision repair defects in fibroblasts of 9 xeroderma pigmentosum complementation groups by microinjection of crude human cell extracts. Mutat Res. 1986 May;165(3):199–206. doi: 10.1016/0167-8817(86)90055-6. [DOI] [PubMed] [Google Scholar]
- Weber C. A., Salazar E. P., Stewart S. A., Thompson L. H. ERCC2: cDNA cloning and molecular characterization of a human nucleotide excision repair gene with high homology to yeast RAD3. EMBO J. 1990 May;9(5):1437–1447. doi: 10.1002/j.1460-2075.1990.tb08260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeda G., Ma L., van Ham R. C., Bootsma D., van der Eb A. J., Hoeijmakers J. H. Characterization of the mouse homolog of the XPBC/ERCC-3 gene implicated in xeroderma pigmentosum and Cockayne's syndrome. Carcinogenesis. 1991 Dec;12(12):2361–2368. doi: 10.1093/carcin/12.12.2361. [DOI] [PubMed] [Google Scholar]
- Weeda G., van Ham R. C., Masurel R., Westerveld A., Odijk H., de Wit J., Bootsma D., van der Eb A. J., Hoeijmakers J. H. Molecular cloning and biological characterization of the human excision repair gene ERCC-3. Mol Cell Biol. 1990 Jun;10(6):2570–2581. doi: 10.1128/mcb.10.6.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeda G., van Ham R. C., Vermeulen W., Bootsma D., van der Eb A. J., Hoeijmakers J. H. A presumed DNA helicase encoded by ERCC-3 is involved in the human repair disorders xeroderma pigmentosum and Cockayne's syndrome. Cell. 1990 Aug 24;62(4):777–791. doi: 10.1016/0092-8674(90)90122-u. [DOI] [PubMed] [Google Scholar]
- Westerveld A., Hoeijmakers J. H., van Duin M., de Wit J., Odijk H., Pastink A., Wood R. D., Bootsma D. Molecular cloning of a human DNA repair gene. Nature. 1984 Aug 2;310(5976):425–429. doi: 10.1038/310425a0. [DOI] [PubMed] [Google Scholar]
- Wickens M. How the messenger got its tail: addition of poly(A) in the nucleus. Trends Biochem Sci. 1990 Jul;15(7):277–281. doi: 10.1016/0968-0004(90)90054-f. [DOI] [PubMed] [Google Scholar]
- de Jonge A. J., Vermeulen W., Klein B., Hoeijmakers J. H. Microinjection of human cell extracts corrects xeroderma pigmentosum defect. EMBO J. 1983;2(5):637–641. doi: 10.1002/j.1460-2075.1983.tb01477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Duin M., Janssen J. H., de Wit J., Hoeijmakers J. H., Thompson L. H., Bootsma D., Westerveld A. Transfection of the cloned human excision repair gene ERCC-1 to UV-sensitive CHO mutants only corrects the repair defect in complementation group-2 mutants. Mutat Res. 1988 Mar;193(2):123–130. doi: 10.1016/0167-8817(88)90042-9. [DOI] [PubMed] [Google Scholar]
- van Duin M., de Wit J., Odijk H., Westerveld A., Yasui A., Koken M. H., Hoeijmakers J. H., Bootsma D. Molecular characterization of the human excision repair gene ERCC-1: cDNA cloning and amino acid homology with the yeast DNA repair gene RAD10. Cell. 1986 Mar 28;44(6):913–923. doi: 10.1016/0092-8674(86)90014-0. [DOI] [PubMed] [Google Scholar]
- van Duin M., van den Tol J., Warmerdam P., Odijk H., Meijer D., Westerveld A., Bootsma D., Hoeijmakers J. H. Evolution and mutagenesis of the mammalian excision repair gene ERCC-1. Nucleic Acids Res. 1988 Jun 24;16(12):5305–5322. doi: 10.1093/nar/16.12.5305. [DOI] [PMC free article] [PubMed] [Google Scholar]