Abstract
Nerve growth factor (NGF) is a polypeptide which, in addition to its effect on nerve cells, is believed to play a role in inflammatory responses and in tissue repair. Because fibroblasts represent the main target and effector cells in these processes, to investigate whether NGF is involved in lung and skin tissue repair, we studied the effect of NGF on fibroblast migration, proliferation, collagen metabolism, modulation into myofibroblasts, and contraction of collagen gel. Both skin and lung fibroblasts were found to produce NGF and to express tyrosine kinase receptor (trkA) under basal conditions, whereas the low-affinity p75 receptor was expressed only after prolonged NGF exposure. NGF significantly induced skin and lung fibroblast migration in an in vitro model of wounded fibroblast and skin migration in Boyden chambers. Nevertheless NGF did not influence either skin or lung fibroblast proliferation, collagen production, or metalloproteinase production or activation. In contrast, culture of both lung and skin fibroblasts with NGF modulated their phenotype into myofibroblasts. Moreover, addition of NGF to both fibroblast types embedded in collagen gel increased their contraction. Fibrotic human lung or skin tissues displayed immunoreactivity for NGF, trkA, and p75. These data show a direct pro-fibrogenic effect of NGF on skin and lung fibroblasts and therefore indicate a role for NGF in tissue repair and fibrosis.
Keywords: fibrosis, migration, trkA, p75, gel contraction
Nerve growth factor (NGF) is a polypeptide that plays an important role for cells belonging to the nervous, endocrine, and immune systems (1, 2). The biological activity of NGF is known to be mediated by the tyrosine kinase receptor (trkA) and the low-affinity glycoprotein receptor p75 present on the surface of the responsive cells (3). Various studies have shown that circulating NGF levels increase in patients affected with chronic-inflammatory disorders, including allergy and neurofibromatosis (4–6). A role for NGF in repair processes has recently been proposed (4, 5), especially since the finding that NGF healed otherwise untreatable corneal ulcers (7). NGF has also been viewed as a reparative factor for its new-innervation (8) and new-vascularization (9) properties, particularly in the nervous system. We have been studying the role of mast cells and eosinophils, key cells of allergic inflammatory reactions, in repair processes that take place after or concomitantly with the tissue damage caused by inflammation (10, 11). These two cells are known to produce and to be influenced by NGF (12–14). We have hypothesized that the enhanced levels of NGF found in the serum of allergic patients in addition to pro-inflammatory activities (4, 15) could be related to NGF activity in tissue remodeling. Therefore, to investigate the NGF role in repair we exposed skin and lung fibroblasts to NGF and evaluated whether NGF could influence some of the functional and biochemical properties of fibroblasts.
Materials and Methods
Fibroblast and Fibroblast Cultures.
Human skin biopsies were obtained from volunteers after informed consent, according to guidelines established by the Hadassah-Hebrew University, following the principles expressed in the Declaration of Helsinki. The biopsies were put as explants, and fibroblasts were obtained, grown, and subcultured as previously described (11). Human lung fibroblasts (MCR-5) were obtained from American Type Culture Collection. For the experiments, fibroblasts were used between the 3rd and 7th generation and cultured in medium (Dulbecco's modified Eagle's medium supplemented with 10% heat-inactivated fetal calf serum, 2 mM l-glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin; Biological Industries, Beit Haemek, Israel) containing, according to the different experiments, one of the following: murine NGF (0.1–1000 ng/ml); purified human transforming growth factor β1 (TGF-β1; 20 ng/ml; R & D Systems); neutralizing goat anti-NGF antibodies, raised against ultrapure murine 2.5S NGF and further purified by column chromatography (16); or mouse anti-TGF-β1 monoclonal antibodies (10 μg/ml; R & D Systems). Murine NGF was purified from mouse submaxillary gland (17). It shares a close homology with human NGF and is suitable for studies with human tissues (18). Biological activity of the protein was tested by using an in vitro neurite outgrow bioassay (1). Sterile tissue culture plasticware was obtained from Falcon and Nunc.
NGF Determination.
The NGF content in fibroblast conditioned media collected after 2, 4, and 6 days of culture was measured by a modified highly sensitive two-site immunoenzymatic assay (ELISA), using anti-NGF antibodies (clone 27/21, Chemicon) (19, 20). This assay specifically recognizes human NGF but not brain-derived neurotrophic factor, with a detection limit of 0.5 pg/ml.
Confocal Microscopical Analysis of trkA and p75 Expression.
The fibroblasts were cultured until confluence on sterilized coverslips placed into 24-well plates. After 0, 2, 4, and 6 days of culturing in the presence of NGF (5–500 ng/ml) or medium alone, coverslips were washed in phosphate-buffered saline (PBS), fixed in 2% phosphate-buffered paraformaldehyde, and processed for immunofluorescence. The following specific antisera were used: rabbit anti-human trkA antibody (2 μg/ml; Santa Cruz Biotechnology), which does not cross-react with trkB or trkC (21, 22), and mouse anti-human p75 antibody (hybridoma HB2836, American Type Culture Collection). The specific binding of the primary antibody was detected by using an anti-rabbit/mouse F(ab′)2 fragment-FITC, (20 μg/ml, Boehringer Mannheim) at 37°C for 1 h. After washing, the nuclei were counterstained with propidium iodide (Sigma) and mounted with an antifade solution (AF1; Citifluor, Cambridge, U.K.). The coverslips were analyzed by using a confocal microscope equipped with image software (Leica, U.K.). To detect nonspecific binding, control immunofluorescence was performed by replacing the primary antibody with nonspecific purified immunoglobulins (IgG).
Fibroblast Migration Assays.
Migration was assessed on fibroblasts (103 cells per well) cultured in serum-free DMEM with NGF and/or anti-NGF and/or irrelevant IgG antibodies by using tissue culture inserts in a 24-well plate (Boyden chamber) (23). Cells were left to migrate for 16 h at 37°C under a 5% CO2/95% air atmosphere. Migration was also assessed in an in vitro wound model in which a linear wound midline is produced in a confluent fibroblast monolayer and half of it is then scraped cell free (24). Immediately after wounding, NGF or anti-NGF antibodies were added and 30 min and 2, 4, 12, 48, and 72 h later, the cells migrating across the wound line were counted (inverted microscope). The migration from the wound line was estimated by counting the number of optic grids from the wounded line to the farthest-migrating fibroblasts (×5).
Fibroblast Proliferation, Collagen Production, and Metalloproteinase Activity.
Subconfluent fibroblasts (4 × 103 cells in 200 μl) or confluent fibroblasts (1 × 104 cells in 200 μl) were incubated with NGF, respectively, and their proliferation was assessed by [3H]thymidine incorporation (11) and their collagen production was assessed by [3H]proline incorporation (11). Metalloproteinase activity was detected by gelatin zymography (11).
Immunostaining for α Smooth Muscle Actin (α-SMA) and Cell Surface ELISA for α-SMA on Fibroblasts.
Fibroblasts were seeded on coverslips until confluence and then incubated either with NGF, TGF-β1, anti-NGF, or anti-TGF-β1 neutralizing antibodies. After 2, 4, and 6 days of culturing, the coverslips were washed with PBS, postfixed in buffered 2% paraformaldehyde for 30 min, pretreated with 0.3% H2O2 in aqueous solution, and incubated with 2% BSA/10% normal serum solution. The specific incubations were carried out overnight at 4°C with monoclonal mouse anti-human α-SMA antibodies (hybridoma supernatant, diluted 1/250, a kind gift from Giulio Gabbiani, Dept of Pathology, University of Geneva, Switzerland). The specific binding of the primary antibody was detected by the avidin–biotin–peroxidase method, following the procedure suggested by the kit manufacturer (Vectastain Elite ABC kit, Vector Laboratories). To detect nonspecific binding, control immunostaining was carried out in parallel, with replacement of the primary antibody by isotype-specific irrelevant antibodies. The ELISA for determination of cell surface α-SMA expression was performed on confluent cultures in 96-well plates incubated with NGF (25).
Tridimensional Collagen Gel Contraction.
The fibroblasts were added to bacteriological 35-mm dishes (105 cells per dish) containing 2% fetal calf serum medium and 100 mM NaOH immediately after the collagen type I [2 μg/ml from rat tail tendon in 18 mM acetic acid (11)] was added in medium alone or in the presence of NGF, anti-NGF antibodies, or irrelevant anti-IgG isotype antibodies. Gel diameter was evaluated blindly on days 2, 4, and 6 by placing the dishes on a graduate ruler over a black surface.
Immunohistochemical Detection of NGF, trkA, and p75 on Scar Skin and Lung Fibrotic Human Biopsies.
Consecutive 5-μm paraffin-embedded sections of normal human lung (n = 3) and normal skin (n = 3), and fibrotic lung disease (honey comb, n = 1; other fibrotic conditions, n = 2) and scar skin (n = 5) were dewaxed and dehydrated following a standard procedure. Deparaffinized and hydrated sections were treated with hyaluronidase (1 mg/ml in 100 mM sodium acetate/0.85% NaCl), or alternatively with 0.1% trypsin in Tris⋅HCl buffer, pH 7.6 (Sigma) containing CaCl2 for 10 min at room temperature. The internal peroxidase blocking as well as the blocking of nonspecific binding was carried out as reported above for immunocytochemistry of fibroblasts. The following specific antibodies were used: polyclonal goat anti-NGF antibody and monoclonal antibodies against both trkA and p75.
Statistical Analysis.
Data are expressed as median and range or as mean ± SEM of three independent experiments in which the different groups were tested in triplicates or quadruplicates. Nonparametric analysis (ANOVA, followed by Tukey–Kramer post hoc test) was used to compare the effects. In both cases, a probability of ≤0.05 was considered statistically significant. The statistical package used was StatView II for PC (Abacus Concepts, Berkeley, CA).
Results
Basal and Stimulated Production of NGF by Fibroblasts.
Fibroblasts were cultured in medium alone or medium containing various concentrations of NGF. The amount of NGF released from lung and skin fibroblasts in medium after 20 h was 0.91 ± 0.05 pg/ml (n = 3) and 3.81 ± 0.10 pg/ml (n = 3), respectively. After 6 days of incubation in the presence of 50 ng/ml NGF, the amount of NGF in the supernatant increased from 6.69 ± 0.15 pg/ml to 261.94 ± 10.57 pg/ml (P < 0.05, n = 3) for lung and from 6.93 ± 0.13 pg/ml to 291.88 ± 11.68 pg/ml (P < 0.05, n = 3) for skin fibroblasts.
Expression of trkA and p75 Receptors by Fibroblasts: Influence of NGF.
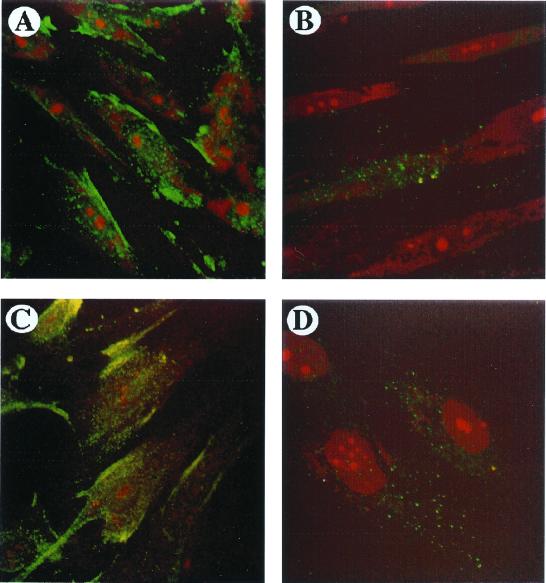
The presence of NGF-specific receptors on the cellular surface represents an index of NGF activity. We therefore assessed the presence of both trkA and p75 receptors on lung and skin fibroblasts before and after stimulation with NGF. trkA was detected on lung (Fig. 1A) and skin (Fig. 1C) fibroblasts incubated in medium alone (n = 3). After 2, 4, and 6 days of incubation with NGF (100 ng/ml) the cells continued to express trkA (data not shown). On the other hand, after 6 days of culture with 100 ng/ml NGF, p75 receptors, which were previously undetectable, were also now expressed by both the fibroblasts (Fig. 1 B and D). However, only a small portion of cultured fibroblasts—i.e., 60% for skin and 40% for lung, were positive for p75.
Figure 1.
Confocal photomicrographic analysis of trkA and p75 receptors. The basal expression of trkA by lung and skin fibroblasts (green) is shown in A and C, respectively. When cultured in the presence of 100 ng/ml NGF for 6 consecutive days, both lung and skin fibroblasts also expressed p75 (green, B and D, respectively). Red in the fibroblast nuclei is propidium iodide staining. Fibroblast monolayers incubated with nonspecific purified immunoglobulins (IgG) did not display positive staining. The experiment depicted is a representative one of three. (×40.)
NGF Stimulates Skin Fibroblast Chemotaxis.
To test whether NGF is able to influence the chemotactic activity of lung and skin fibroblasts, its effect was checked in Boyden chambers. NGF enhanced chemotaxis in skin fibroblasts starting at 300 ng/ml NGF (6.0 ± 0.1 vs. 2.5 ± 0.2 migrating cells, P < 0.05, n = 3), still increasing at 600 ng/ml (13.0 ± 0.7 vs. 2.5 ± 0.2 migrating cells, P < 0.05) and reaching a plateau at 800 ng/ml (17 ± 2.6 vs. 2.5 ± 0.2 migrating cells, P < 0.05), as compared with control. The addition of neutralizing anti-NGF together with NGF totally inhibited its effect. No chemotactic effect was observed in lung fibroblasts at all NGF concentrations tested.
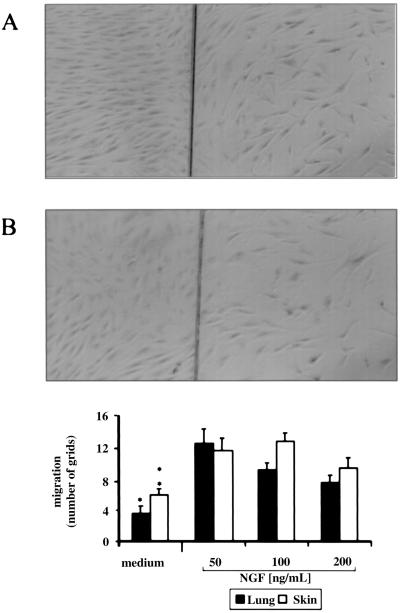
Effect of NGF in an in Vitro Wound Model.
To further explore the activity of NGF on fibroblast migration, NGF was added to skin and lung fibroblast wounded monolayers. In lung wounded fibroblasts, the addition of 50 ng/ml NGF induced the highest distance of cellular migration beyond the wound line, as compared with the controls (12.50 ± 1.80 vs. 3.5 ± 0.90 optic grids, P < 0.05, n = 3, Fig. 2). This effect was also associated with the maximal number of cells migrating in the wounded area (27.17 ± 2.51 vs. 6.83 ± 2.10 cells, P < 0.05). A similar effect was observed when NGF was added to wounded skin fibroblasts (Fig. 2). In fact, the addition of 100 ng/ml NGF significantly enhanced the migration beyond the wounded line (12.78 ± 1.01 vs. 5.89 ± 0.80 grids, P < 0.05) and increased the number of cells present in the wounded area (30.43 ± 1.39 vs. 14.83 ± 2.70 cells, P < 0.05). Addition of 200 ng/ml NGF to both lung and skin fibroblasts decreased their migration. Lung and skin fibroblasts beyond the wound line are shown in Fig. 2 A and B, respectively.
Figure 2.
Lung and skin fibroblast migration across a wound line as a function of NGF concentration. The effect of 50 ng/ml NGF on wounded lung and skin fibroblasts after 1 day is shown in A and B, respectively. (×5.) The experiment depicted is a representative one of three. (Bottom) Quantitative evaluation of fibroblast migration beyond the wound line (*, P < 0.05 for lung fibroblasts and **, P < 0.05 for skin fibroblasts, both at 50 and 100 ng/ml NGF). Experiments (n = 3) were performed in triplicates; error bars indicate SEM.
NGF Does Not Influence Fibroblast Proliferation, Collagen Production, or Metalloproteinase Activity.
NGF was added to skin and lung fibroblasts, and proliferation, collagen deposition, and metalloproteinase activity were investigated after 2, 4, and 6 days. Under our culture conditions, NGF did not influence these skin or lung fibroblast properties.
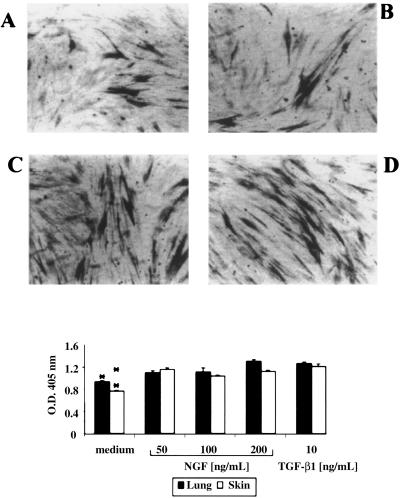
α-SMA Expression and Morphology of Fibroblasts Cultured with NGF.
Immunocytochemical analysis carried out on fibroblasts grown in monolayers and incubated with NGF for 2, 4, and 6 days revealed the expression of the contractile protein α-SMA. After 6 days of incubation with NGF both lung (Fig. 3A) and skin (Fig. 3B) fibroblasts were positive for α-SMA. Fibroblasts incubated with TGF-β1, as expected, displayed a strong immunoreactivity for this contractile protein (Fig. 3 C and D). Interestingly, skin and, to a lesser extent, lung fibroblasts preincubated with either NGF or TGF-β1 appeared more elongated, as previously reported for fibroblasts incubated with TGF-β1 (26). The semiquantitative evaluation of α-SMA expression (Fig. 3) revealed that lung and skin fibroblasts responded to different NGF concentrations by expressing significantly higher amounts of α-SMA, as compared with fibroblasts cultured in medium alone (P < 0.05 for lung fibroblasts at 50 and 200 ng/ml NGF; P < 0.05 for skin fibroblasts at 50, 100, and 200 ng/ml). In experiments in which neutralizing anti-NGF and anti-TGF-β1 antibodies were added together with NGF and TGF-β1, respectively, a significant reduction of this expression was observed, indicating the specific effect of the exogenous proteins (data not shown).
Figure 3.
Effect of NGF on α-SMA expression in lung and skin fibroblasts. Fibroblasts were cultured for 6 days with NGF or TGF-β1, and α-SMA expression by fibroblasts was evaluated by a cell surface ELISA. A and B show the effect of 100 ng/ml NGF and C and D, that of 10 ng/ml TGF-β1 on lung and skin fibroblasts, respectively. The experiment depicted is a representative one of three. (Bottom) Quantitative evaluation of α-SMA expression. α-SMA expression was found increased in both lung and skin fibroblasts (*, P < 0.05 for lung fibroblasts at 50 and 200 ng/ml NGF; **, P < 0.05 for skin fibroblasts at 50, 100, and 200 ng/ml NGF, and both for TGF-β1). Experiments (n = 3) were performed in triplicates; error bars indicate SEM.
Effect of NGF on Fibroblast-Mediated Collagen Lattice Contraction.
Collagen is the major protein of the extracellular matrix and its contraction is a physiological step that occurs during repair (11). The addition of exogenous NGF to skin and lung fibroblasts embedded in collagen gels resulted in increased contraction of the matrix. The highest contraction of the matrix was found in collagen-embedded skin fibroblasts at 100 ng/ml NGF, with contraction already apparent on day 2 (2 d: 19.5 ± 0.7 mm vs. 24 ± 0.8 mm, P < 0.05; 4 d: 13.5 ± 0.7 mm vs. 15.5 ± 0.7 mm, P < 0.05; 6 d: 11 ± 0.5 mm vs. 12.5 ± 0.7 mm, fibroblasts + NGF vs. fibroblasts alone P < 0.05, n = 3). Likewise, lung fibroblasts significantly contracted their matrix at 50 ng/ml NGF (2 d: 22.16 ± 1.7 mm vs. 24.16 ± 2.7 mm, P < 0.05; 4 d: 14.7 ± 1.5 mm vs. 19.36 ± 1.36 mm, P < 0.05; 6 d: 10.9 ± 0.8 mm vs. 15.8 ± 1.6 mm, fibroblasts + NGF vs. fibroblasts alone P < 0.05, n = 3). On the other hand, the presence of the highest NGF concentration (500 ng/ml) significantly retarded the gel contraction in skin fibroblasts (2 d: 26.25 ± 3 mm vs. 24 ± 0.8 mm, P < 0.05; 4 d: 22.5 ± 4 mm vs. 15.5 ± 0.7 mm, P < 0.05; 6 d: 18.4 ± 4 mm vs. 12.5 ± 0.7 mm, fibroblasts + NGF vs. fibroblasts alone P < 0.05, n = 3). Also for lung fibroblasts we observed a reduction in the contraction at 500 ng/ml NGF (2 d: 26.25 ± 3 mm vs. 24 ± 0.8 mm, P < 0.05; 4 d: 22.5 ± 4 mm vs. 15.5 ± 0.7 mm, P < 0.05; 6 d: 18.4 ± 4 mm vs. 12.5 ± 0.7 mm, P > 0.05).
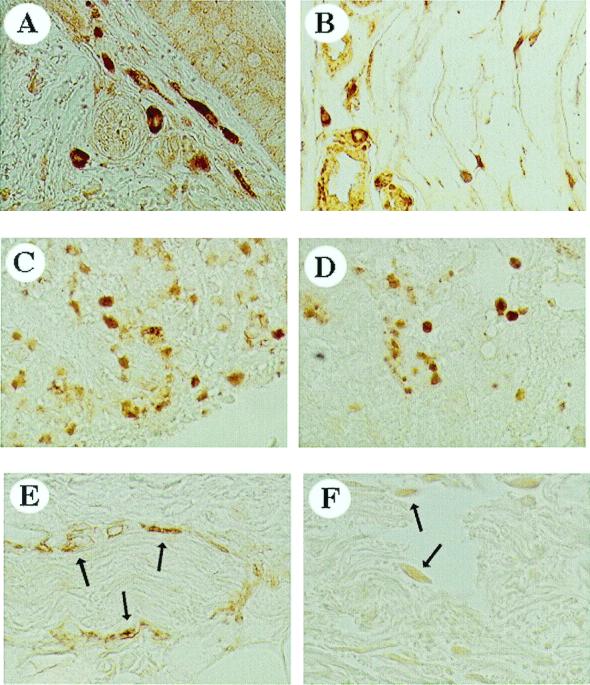
NGF, trkA, and p75 Expression in Normal and Fibrotic Tissue from Lung and Skin Biopsies.
To strengthen the association between NGF and repair, we investigated the presence of NGF and NGF receptors in healthy human tissues and fibrotic tissues from lung and skin biopsies. Increased NGF immunoreactivity was found in biopsies from scar skin and tissue with fibrotic interstitial lung disease (Fig. 4 A and B, respectively) as compared with normal ones. In both tissues, NGF-positive cells displayed different shapes. In normal tissues no positivity was detected, apart from NGF staining in the walls of arteries and veins (data not shown). trkA-positive cells were also present in both skin and lung sections, predominantly in round cells (Fig. 4 C and D). In these tissues slight immunoreactivity was also detected for p75 (Fig. 4 E and F).
Figure 4.
Immunohistochemical analysis of NGF, trkA, and p75 in skin scar tissue (A, C, and E) and fibrotic interstitial lung disease (B, D, and F) human biopsies. NGF reactivity occurs in skin tissue (A) and lung tissue (B). The heterogeneous staining indicates that NGF reactivity is localized in structurally different cells. trkA- (C and D) and p75- (E and F, indicated with arrows) positive cells are found in skin and lung tissues. (×40.)
Discussion
In the present study we have shown that NGF has direct profibrogenic effects on human lung and skin fibroblasts. In addition, we have demonstrated that these fibroblasts produce NGF and can display both trkA and p75 receptors.
NGF is present in several inflammatory conditions often associated with tissue repair and fibrosis. Therefore its participation has been recently postulated in tissue repair (4, 5). This hypothesis is in line with previous studies showing that NGF is synthesized in cutaneous wound tissues and that its high levels may contribute to the efficient wound healing in the neonate (27). The observation that NGF contributes to healing after traumatic muscle injury (28, 29), during diabetic conditions (30), and in corneal ulcers (7) is also consistent with this hypothesis.
We therefore first investigated whether fibroblasts themselves, the effector and target cells of repair, could produce NGF. We found that under basal conditions both lung and skin fibroblasts produced low levels of this factor and when they were exposed to NGF the production was significantly enhanced. According to previous data, human foreskin fibroblasts have been shown to produce NGF (31). We also found that fibroblast NGF production was enhanced after exposure to NGF (32), suggesting that endogenous NGF can be up-regulated by NGF itself. Interestingly, TGF-β1 also induced a slight but statistically significant increase in NGF from both lung and skin fibroblasts (36.70 ± 4.30 pg/ml vs. 6.69 ± 0.15 pg/ml and 22.90 ± 5.00 pg/ml vs. 6.93 ± 0.13 pg/ml, P < 0.05, n = 3, unpublished data) implying that a synergism between NGF and TGF-β1 might occur.
Since a prerequisite for the biological activity of NGF is the presence of receptors on the cellular surface (3), we investigated the expression of trkA and p75 on lung and skin fibroblasts. Both fibroblasts constitutively expressed trkA, whereas the low-affinity p75 receptor was expressed only after NGF long-term exposure. The presence of both the NGF receptors on the fibroblasts suggests the existence of paracrine/autocrine loops of the factor on these cells. trkA and p75 can regulate survival and cell death, respectively (33). In our studies we could not detect any effect of NGF on fibroblast proliferation. The observation that the p75 receptor appears after long-term culture of the fibroblasts with NGF would suggest that apoptosis could be triggered by this factor during the latest stages of repair. This mechanism could control tissue load in terminating the inflammatory process and thus in contributing to physiological tissue repair resolution. Interestingly, we also did not detect any effect of NGF on collagen production or on modulation of metalloproteinases. In contrast, it was found that at low concentrations, NGF significantly enhanced fibroblast migration, the first step in wound healing (34). To date only endothelin, insulin-like growth factor I (IGF-I) and platelet-derived growth factor (PDGF) as well as TGF-β (35–37) are known to affect this important step in repair. Interestingly, skin fibroblast migration was increased by the presence of NGF both in Boyden chambers and in an in vitro wounded monolayer system. On the other hand, lung fibroblasts did not display migration activity in the presence of NGF in Boyden chambers but they did migrate in the in vitro wound. These different effects might indicate that wounded but not intact fibroblasts might release factors that would enhance the promigratory capacity of NGF.
The last stage of proper wound repair is the contraction of the wound carried out by myofibroblasts—i.e., specialized fibroblasts that display the contractile protein α-SMA (26). NGF stimulated both skin and lung fibroblast contraction of collagen gels at low concentrations, whereas at higher concentrations the effect was inhibited. In addition we found that NGF was able to induce the expression of α-SMA, in both lung and skin fibroblasts, indicating their phenotype change into myofibroblasts. Interestingly, it has been previously reported that in vivo after wounding, most of the NGF synthesis appears to be due to the myofibroblasts (29). It is worth noting that until now only TGF-β, IGF-I, PDGF, and angiotensin II were known to be able to induce α-SMA expression (35–39). NGF had not previously been found to induce α-SMA expression.
The possibility that NGF might contribute to the repair process by inducing the expression of other growth factors cannot be ruled out (40). Indeed, it has been suggested, at least in PC12, that NGF might play either an overlapping or a cooperative role with TGF-β1, by regulating TGF-β1 gene expression, at both transcriptional and posttranscriptional level (41). The facts that tumor necrosis factor-α is involved in connective tissue metabolism (42) and in NGF production at the site of lesion (27), and connective tissue growth factor produced by fibroblasts seems to be TGF-β1 mediated (43), also suggest overlapping and/or cooperative effects of these factors on wound repair or fibrotic disorders.
In summary, we have shown that NGF influences lung and skin fibroblast migration and α-SMA expression/gel collagen contraction, indicating an important role for this factor at the beginning and at the end stages of wound repair. Therefore, NGF can now be viewed as a factor in the proper resolution of tissue repair.
Acknowledgments
We are grateful to Prof. Rita Levi-Montalcini for stimulating discussions and suggestions about our research. This work was supported by a grant from the Aimwell Charitable Trust (U.K.) to F.L.-S. F.L.-S. and R.R. are affiliated with the David R. Bloom Center for Pharmacy at The Hebrew University of Jerusalem. A.M. is the recipient of a Consiglio Nazionale delle Ricerche (Italy) fellowship for research abroad.
Abbreviations
- NGF
nerve growth factor
- TGF-β1
transforming growth factor β1
- α-SMA
α smooth muscle actin
References
- 1.Levi-Montalcini R. Science. 1987;237:1154–1162. doi: 10.1126/science.3306916. [DOI] [PubMed] [Google Scholar]
- 2.Aloe L, Bracci-Laudiero L, Bonini S, Manni L. Allergy. 1997;52:883–994. doi: 10.1111/j.1398-9995.1997.tb01247.x. [DOI] [PubMed] [Google Scholar]
- 3.Chao M V, Hempstead B L. Trends Neurosci. 1995;18:321–326. [PubMed] [Google Scholar]
- 4.Bonini S, Lambiase A, Bonini S, Angelucci F, Magrini F, Manni L, Aloe L. Proc Natl Acad Sci USA. 1996;93:10955–10960. doi: 10.1073/pnas.93.20.10955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonini S, Lambiase A, Bonini S, Levi-Schaffer F, Aloe L. Int Arch Allergy Immunol. 1999;118:159–162. doi: 10.1159/000024055. [DOI] [PubMed] [Google Scholar]
- 6.Ebadi M, Bashir R M, Heidrick M L, Hamada F M, Refaey H E, Hamed A, Helal G, Baxi M D, Cerutis D R, Lassi N K. Neurochem Int. 1997;30:347–374. doi: 10.1016/s0197-0186(96)00071-x. [DOI] [PubMed] [Google Scholar]
- 7.Lambiase A, Rama P, Bonini S, Caprioglio G, Aloe L. N Engl J Med. 1998;338:1174–1180. doi: 10.1056/NEJM199804233381702. [DOI] [PubMed] [Google Scholar]
- 8.Tuveri M, Generini S, Matucci-Cerinic M, Aloe L. Lancet. 2000;356:1739–1740. doi: 10.1016/S0140-6736(00)03212-8. [DOI] [PubMed] [Google Scholar]
- 9.Santos P M, Winterowd J G, Allen G G, Bothwell M A, Rubel E W. Otolaryngol Head Neck Surg. 1991;105:12–25. doi: 10.1177/019459989110500103. [DOI] [PubMed] [Google Scholar]
- 10.Levi-Schaffer F, Rubinchik E. J Invest Dermatol. 1995;104:999–1003. doi: 10.1111/1523-1747.ep12606237. [DOI] [PubMed] [Google Scholar]
- 11.Levi-Schaffer F, Garbuzenko E, Rubin A, Reich R, Pickholz D, Gillery P, Emonard H, Nagler A, Maquart X. Proc Natl Acad Sci USA. 1999;96:9660–9665. doi: 10.1073/pnas.96.17.9660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leon A, Buriani A, Dal Toso R, Fabris M, Romanello S, Aloe L, Levi-Montalcini R. Proc Natl Acad Sci USA. 1994;91:3739–3743. doi: 10.1073/pnas.91.9.3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aloe L, Levi-Montalcini R. Brain Res. 1977;133:358–366. doi: 10.1016/0006-8993(77)90772-7. [DOI] [PubMed] [Google Scholar]
- 14.Solomon A, Aloe L, Pe'er J, Frucht-Pery J, Bonini S, Bonini S, Levi-Schaffer F. J Allergy Clin Immunol. 1998;102:454–460. doi: 10.1016/s0091-6749(98)70135-6. [DOI] [PubMed] [Google Scholar]
- 15.Sanico A M, Stanisz A M, Gleeson T D, Bora S, Proud D, Bienenstock J, Koliatsos V E, Togias A. Am J Respir Crit Care Med. 2000;161:1631–1635. doi: 10.1164/ajrccm.161.5.9908028. [DOI] [PubMed] [Google Scholar]
- 16.Vigneti E, Bracci-Laudiero L, Aloe L. Year Immunol. 1993;7:146–149. [PubMed] [Google Scholar]
- 17.Bocchini V, Angeletti P U. Proc Natl Acad Sci USA. 1969;64:787–794. doi: 10.1073/pnas.64.2.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ullrich A, Gray A, Berman C, Coussens L, Dull T J. Cold Spring Harbor Symp Quant Biol. 1983;48:435–441. doi: 10.1101/sqb.1983.048.01.048. [DOI] [PubMed] [Google Scholar]
- 19.Weskamp G, Otten U. J Neurochem. 1987;48:1779–1786. doi: 10.1111/j.1471-4159.1987.tb05736.x. [DOI] [PubMed] [Google Scholar]
- 20.Bracci-Laudiero L, Aloe L, Levi-Montalcini R, Buttinelli C, Schilter D, Gillessen S, Scully J L, Otten U. Neurosci Lett. 1992;147:9–12. doi: 10.1016/0304-3940(92)90762-v. [DOI] [PubMed] [Google Scholar]
- 21.Martin-Zanca D, Oskam R, Mitra G, Copeland T, Barbacid M. Mol Cell Biol. 1989;9:24–33. doi: 10.1128/mcb.9.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dissen G A, Hill D F, Costa M E, Dees W L, Lara H E, Ojeda S R. Endocrinology. 1996;137:198–209. doi: 10.1210/endo.137.1.8536613. [DOI] [PubMed] [Google Scholar]
- 23.Albini A, Iwamoto Y, Kleinman H K, Martin G R, Aaronson S A, Kozlowski J M, McEwan R N. Cancer Res. 1987;47:3239–3245. [PubMed] [Google Scholar]
- 24.Levi-Schaffer F, Kupietzky A. Exp Cell Res. 1990;188:42–49. doi: 10.1016/0014-4827(90)90275-f. [DOI] [PubMed] [Google Scholar]
- 25.Piela Smith T H, Broketa G, Hand A, Korn J H. J Immunol. 1992;148:1375–1381. [PubMed] [Google Scholar]
- 26.Desmouliere A, Geinoz A, Gabbiani F, Gabbiani G. J Cell Biol. 1993;122:103–111. doi: 10.1083/jcb.122.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hattori A, Hayashi K, Kohno FEBS Lett. 1996;379:157–160. doi: 10.1016/0014-5793(95)01502-7. [DOI] [PubMed] [Google Scholar]
- 28.Poduslo J F, Curran G L, Gill J S. J Neurochem. 1998;71:1651–1660. doi: 10.1046/j.1471-4159.1998.71041651.x. [DOI] [PubMed] [Google Scholar]
- 29.Hasan W, Zhang R, Warn J D, Smith P G. Cell Tissue Res. 2000;300:97–109. doi: 10.1007/s004410000175. [DOI] [PubMed] [Google Scholar]
- 30.Kasemkijwattana C, Menetrey J, Somogyi G, Moreland M S, Fu F H, Buranapanitkit B, Watkins S C, Huard J. Cell Transplant. 1998;7:585–598. doi: 10.1177/096368979800700609. [DOI] [PubMed] [Google Scholar]
- 31.Kasemkijwattana C, Menetrey J, Bosch P, Somogyi G, Moreland M S, Fu F H, Buranapanitkit B, Watkins S C, Huard J. Clin Orthop. 2000;370:272–285. doi: 10.1097/00003086-200001000-00028. [DOI] [PubMed] [Google Scholar]
- 32.Matsuda H, Koyama H, Sato H, Sawada J, Itakura A, Tanaka A, Matsumoto M, Konno K, Ushio H, Matsuda K. J Exp Med. 1998;187:297–306. doi: 10.1084/jem.187.3.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Casaccia Bennefil P, Kong H, Chao M V. Cell Death Differ. 1998;5:357–364. doi: 10.1038/sj.cdd.4400377. [DOI] [PubMed] [Google Scholar]
- 34.Streuli C. Curr Opin Cell Biol. 1999;11:634–640. doi: 10.1016/s0955-0674(99)00026-5. [DOI] [PubMed] [Google Scholar]
- 35.Salani D, Taraboletti G, Rosano L, Di Castro V, Borsotti P, Giavazzi R, Bagnato A. Am J Pathol. 2000;157:1703–1711. doi: 10.1016/S0002-9440(10)64807-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nishimura F, Terranova V P. J Dent Res. 1996;75:986–992. doi: 10.1177/00220345960750041401. [DOI] [PubMed] [Google Scholar]
- 37.Kanekar S, Borg T K, Terracio L, Carver W. Cell Adhes Commun. 2000;7:513–523. doi: 10.3109/15419060009040308. [DOI] [PubMed] [Google Scholar]
- 38.Carver W, Molano I, Reaves T A, Borg T K, Terracio L. J Cell Physiol. 1995;165:425–437. doi: 10.1002/jcp.1041650224. [DOI] [PubMed] [Google Scholar]
- 39.Sasaki M, Kashima M, Ito T, Watanabe A, Izumiyama N, Sano M, Kagaya M, Shioya T, Miura M. Mediators Inflamm. 2000;9:155–160. doi: 10.1080/09629350020002895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blitstein-Willinger E. Skin Pharmacol. 1991;4:175–182. doi: 10.1159/000210946. [DOI] [PubMed] [Google Scholar]
- 41.Cosgaya J M, Aranda A. J Neurochem. 1995;65:2484–2490. doi: 10.1046/j.1471-4159.1995.65062484.x. [DOI] [PubMed] [Google Scholar]
- 42.Frazier K, Williams S, Kothapalli D, Kappler H, Grotendorst G R. J Invest Dermatol. 1996;107:404–411. doi: 10.1111/1523-1747.ep12363389. [DOI] [PubMed] [Google Scholar]
- 43.Grotendorst G R. Cytokine Growth Factor Rev. 1997;8:171–179. doi: 10.1016/s1359-6101(97)00010-5. [DOI] [PubMed] [Google Scholar]