Abstract
In the title hydrated molecular salt, C38H52N4 2+·2Br−·H2O, the central benzene ring of the dication makes dihedral angles of 89.47 (13) and 72.69 (12)° with the pendant benzimidazol-3-ium rings. The conformations of the octyl side chains are completely different. In the crystal, the components are linked by O—H⋯Br, C—H⋯Br and C—H⋯O hydrogen bonds into a two-dimensional network lying parallel to the ac plane. Aromatic π–π stacking interactions are also observed [shortest centroid-to-centroid separation = 3.5047 (16) Å].
Related literature
For related structures, see: Haque et al. (2012 ▶); Iqbal et al. (2012 ▶); Haque et al. (2011 ▶). For the stability of the temperature controller used for the data collection, see: Cosier & Glazer (1986 ▶).
Experimental
Crystal data
C38H52N4 2+·2Br−·H2O
M r = 742.67
Triclinic,
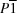
a = 8.7203 (4) Å
b = 14.9342 (12) Å
c = 16.4090 (8) Å
α = 115.598 (3)°
β = 104.638 (2)°
γ = 92.358 (3)°
V = 1836.77 (19) Å3
Z = 2
Mo Kα radiation
μ = 2.24 mm−1
T = 100 K
0.42 × 0.32 × 0.24 mm
Data collection
Bruker SMART APEXII CCD diffractometer
Absorption correction: multi-scan (SADABS; Bruker, 2009 ▶) T min = 0.451, T max = 0.614
44354 measured reflections
10659 independent reflections
8816 reflections with I > 2σ(I)
R int = 0.047
Refinement
R[F 2 > 2σ(F 2)] = 0.052
wR(F 2) = 0.161
S = 1.05
10659 reflections
416 parameters
H atoms treated by a mixture of independent and constrained refinement
Δρmax = 2.62 e Å−3
Δρmin = −1.68 e Å−3
Data collection: APEX2 (Bruker, 2009 ▶); cell refinement: SAINT (Bruker, 2009 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXTL (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXTL; molecular graphics: SHELXTL; software used to prepare material for publication: SHELXTL and PLATON (Spek, 2009 ▶).
Supplementary Material
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S1600536812008331/hb6650sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536812008331/hb6650Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536812008331/hb6650Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| O1W—H1W1⋯Br1 | 0.83 (5) | 2.50 (5) | 3.326 (3) | 173 (4) |
| O1W—H2W1⋯Br2 | 0.83 (5) | 2.52 (5) | 3.343 (3) | 177 (5) |
| C7—H7A⋯Br1 | 0.95 | 2.69 | 3.569 (3) | 154 |
| C15—H15A⋯Br1 | 0.99 | 2.92 | 3.672 (3) | 134 |
| C16—H16A⋯Br1 | 0.95 | 2.79 | 3.594 (3) | 143 |
| C2—H2A⋯Br2i | 0.95 | 2.81 | 3.698 (3) | 155 |
| C4—H4A⋯O1Wii | 0.95 | 2.49 | 3.218 (4) | 133 |
| C8—H8A⋯Br1iii | 0.99 | 2.80 | 3.779 (3) | 169 |
| C8—H8B⋯Br2iii | 0.99 | 2.71 | 3.655 (3) | 159 |
| C19—H19A⋯Br2iv | 0.95 | 2.84 | 3.770 (3) | 167 |
| C21—H21A⋯Br2v | 0.95 | 2.90 | 3.785 (3) | 155 |
| C31—H31A⋯Br2v | 0.99 | 2.87 | 3.786 (3) | 154 |
Symmetry codes: (i) 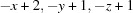 ; (ii)
; (ii) 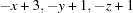 ; (iii)
; (iii) 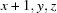 ; (iv)
; (iv) 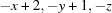 ; (v)
; (v) 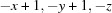 .
.
Acknowledgments
RAH thanks the Universiti Sains Malaysia (USM) for Research University (RU) grants Nos. 1001/PKI MIA/811157 and 1001/PKIMIA/823082. MAI is grateful to (IPS) USM for financial support [a fellowship, grant No. USM.IPS/JWT/1/19 (JLD 6), and a research attachment fund, grant No. P-KM0018/10(R)-308/AIPS/415401]. HKF and SA thank the Universiti Sains Malaysia (USM) for Research University Grant No. 1001/PFIZIK/811160. SA also thanks the Malaysian government and USM for an award through the Academic Staff Training Scheme (ASTS).
supplementary crystallographic information
Comment
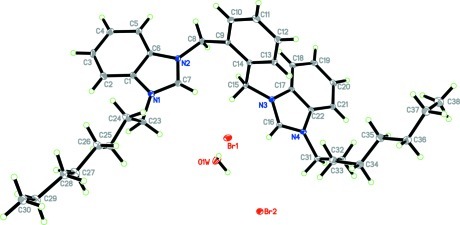
As a part of our ongoing studies, we have previously reported crystal structures of ortho-xylyl linked bis-benzimidazolium salts with ethyl (Haque et al., 2012), propyl (Iqbal et al., 2012), and heptyl (Haque et al., 2011) substitutions. In this paper we describe single-crystal X-ray diffraction study of the title compound, (I) (Fig. 1).
Bond lengths and angles are comparable to the related structure (Haque et al., 2011). The central benzene (C9–C14) ring makes dihedral angles of 89.47 (13) and 72.69 (12)° with the terminal 1H-benzo[d]imidazol-3-ium (N1/N2/C1–C7) and (N3/N4/C16–C22) rings, respectively.
The crystal structure is shown in Fig. 2. The cations, anions and water molecules are linked by intermolecular O—H···Br, C—H···Br and C—H···O hydrogen bonds (Table 1) into a two-dimensional network parallel to the ac plane. π–π interactions of Cg1···Cg3 = 3.5622 (18) Å (symmetry code: 3 - x, 1 - y, 1 - z), Cg3···Cg3 = 3.7158 (18) Å (symmetry code: 3 - x, 1 - y, 1 - z), Cg2···Cg4 = 3.6206 (17) Å (symmetry code: 2 - x, 1 - y, -z) and Cg4···Cg4 = 3.5047 (16) Å (symmetry code: 2 - x, 1 - y, -z) further stabilized the structure. [Cg1, Cg2, Cg3 and Cg4 is the centroid of the N1/N2/C1/C6/C7, N3/N4/C16/C17/C22, C1–C6 and C17–C22 rings, respectively].
Experimental
A mixture of benzimidazole (5.90 g, 50 mmol) and finely ground potassium hydroxide (4.50 g, 80 mmol) in 50 ml of DMSO was stirred at room temperature (27–28 °C) for 30 min. 1-Bromoctane (8.70 ml, 50 mmol) was added drop-wise into this consistently stirred mixture with further stirring for 2 h at the same temperature. The mixture was then poured into water (700 ml) and was extracted by chloroform (5 × 30 ml). The extract was dried by filtering through five plies of Whatman filter papers. This process was repeated twice to collect crystal a clear solution which was evaporated under reduced pressure to get N-octylbenzimidazole (1) as a thick yellowish fluid. Furthermore, a mixture of 1 (4.04 g, 20 mmol) and 1,2-bis(bromomethyl)benzene (2.64 g, 10 mmol) in 1,4-dioxane (50 ml) was refluxed at 100 °C for 18 h. After cooling the reaction mixture to room temperature, the desired compound (2.2Br) appeared as white crystalline powder. The salt was filtered and washed by fresh 1,4-dioxane (3 × 5 ml), dried at room temperature for 24 h. The product was collected as white crystalline powder (7.42 g, 97.76%). Saturated solution of 2.2Br in methanol (0.5 ml) was exposed to diethyl ether vapours (vapour diffusion) at room temperature to get colourless blocks of (I). Single crystals were also obtained by slow evaporation of saturated solution of 2.2Br in MeOH/CH3CN (70:30) and by evaporating saturated solution of title compound in d6-DMSO at room temperature.
Refinement
The H atoms of the water molecule were located in a difference Fourier map and refined freely [O—H = 0.84 (5) and 0.83 (5) Å]. All the other H atoms were positioned geometrically [C—H = 0.95–0.99 Å] and refined using a riding model with Uiso(H) = 1.2 or 1.5Ueq(C). A rotating group model was applied to the methyl groups.
Figures
Fig. 1.
The molecular structure of the title compound, showing 30% probability displacement ellipsoids.
Fig. 2.
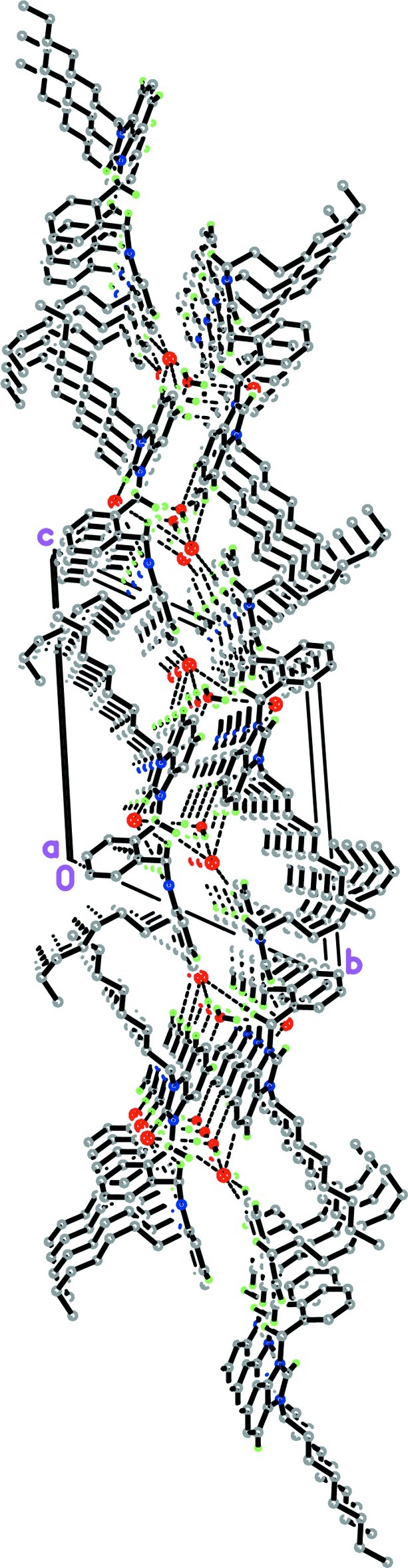
The crystal packing of the title compound. Those H atoms not involved in the intermolecular interactions (dashed lines) have been omitted for clarity.
Crystal data
| C38H52N42+·2Br−·H2O | Z = 2 |
| Mr = 742.67 | F(000) = 776 |
| Triclinic, P1 | Dx = 1.343 Mg m−3 |
| Hall symbol: -P 1 | Mo Kα radiation, λ = 0.71073 Å |
| a = 8.7203 (4) Å | Cell parameters from 9861 reflections |
| b = 14.9342 (12) Å | θ = 2.5–33.3° |
| c = 16.4090 (8) Å | µ = 2.24 mm−1 |
| α = 115.598 (3)° | T = 100 K |
| β = 104.638 (2)° | Block, colourless |
| γ = 92.358 (3)° | 0.42 × 0.32 × 0.24 mm |
| V = 1836.77 (19) Å3 |
Data collection
| Bruker SMART APEXII CCD diffractometer | 10659 independent reflections |
| Radiation source: fine-focus sealed tube | 8816 reflections with I > 2σ(I) |
| Graphite monochromator | Rint = 0.047 |
| φ and ω scans | θmax = 30.0°, θmin = 1.5° |
| Absorption correction: multi-scan (SADABS; Bruker, 2009) | h = −11→12 |
| Tmin = 0.451, Tmax = 0.614 | k = −20→20 |
| 44354 measured reflections | l = −23→23 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.052 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.161 | H atoms treated by a mixture of independent and constrained refinement |
| S = 1.05 | w = 1/[σ2(Fo2) + (0.1139P)2 + 0.460P] where P = (Fo2 + 2Fc2)/3 |
| 10659 reflections | (Δ/σ)max < 0.001 |
| 416 parameters | Δρmax = 2.62 e Å−3 |
| 0 restraints | Δρmin = −1.68 e Å−3 |
Special details
| Experimental. The crystal was placed in the cold stream of an Oxford Cryosystems Cobra open-flow nitrogen cryostat (Cosier & Glazer, 1986) operating at 100.0 (1) K. |
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R-factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Br1 | 0.86202 (3) | 0.228144 (19) | 0.215445 (18) | 0.01929 (9) | |
| Br2 | 0.53091 (3) | 0.518157 (19) | 0.194634 (17) | 0.01747 (9) | |
| N1 | 1.3021 (3) | 0.32869 (16) | 0.42917 (15) | 0.0159 (4) | |
| N2 | 1.4239 (3) | 0.31561 (16) | 0.32400 (14) | 0.0140 (4) | |
| N3 | 1.0154 (2) | 0.33691 (15) | 0.05850 (14) | 0.0137 (4) | |
| N4 | 0.7768 (3) | 0.34636 (15) | −0.01797 (14) | 0.0136 (4) | |
| C1 | 1.4628 (3) | 0.37063 (18) | 0.47927 (17) | 0.0150 (5) | |
| C2 | 1.5453 (3) | 0.4119 (2) | 0.57555 (18) | 0.0198 (5) | |
| H2A | 1.4928 | 0.4172 | 0.6212 | 0.024* | |
| C3 | 1.7092 (4) | 0.4446 (2) | 0.60039 (18) | 0.0215 (5) | |
| H3A | 1.7708 | 0.4731 | 0.6652 | 0.026* | |
| C4 | 1.7875 (3) | 0.4373 (2) | 0.53320 (19) | 0.0201 (5) | |
| H4A | 1.9001 | 0.4607 | 0.5539 | 0.024* | |
| C5 | 1.7050 (3) | 0.39691 (19) | 0.43801 (18) | 0.0173 (5) | |
| H5A | 1.7574 | 0.3923 | 0.3925 | 0.021* | |
| C6 | 1.5401 (3) | 0.36325 (18) | 0.41248 (16) | 0.0138 (4) | |
| C7 | 1.2832 (3) | 0.29643 (19) | 0.33687 (18) | 0.0157 (5) | |
| H7A | 1.1844 | 0.2646 | 0.2877 | 0.019* | |
| C8 | 1.4578 (3) | 0.30028 (19) | 0.23524 (16) | 0.0157 (5) | |
| H8A | 1.5693 | 0.2873 | 0.2398 | 0.019* | |
| H8B | 1.4510 | 0.3631 | 0.2289 | 0.019* | |
| C9 | 1.3448 (3) | 0.21446 (18) | 0.14737 (16) | 0.0139 (4) | |
| C10 | 1.3942 (3) | 0.1203 (2) | 0.11448 (18) | 0.0183 (5) | |
| H10A | 1.4934 | 0.1124 | 0.1491 | 0.022* | |
| C11 | 1.3001 (3) | 0.0386 (2) | 0.03209 (19) | 0.0205 (5) | |
| H11A | 1.3348 | −0.0248 | 0.0105 | 0.025* | |
| C12 | 1.1557 (3) | 0.04955 (19) | −0.01872 (18) | 0.0184 (5) | |
| H12A | 1.0908 | −0.0065 | −0.0750 | 0.022* | |
| C13 | 1.1051 (3) | 0.14323 (19) | 0.01275 (17) | 0.0172 (5) | |
| H13A | 1.0057 | 0.1503 | −0.0224 | 0.021* | |
| C14 | 1.1991 (3) | 0.22640 (18) | 0.09525 (16) | 0.0138 (4) | |
| C15 | 1.1448 (3) | 0.32824 (19) | 0.13064 (17) | 0.0153 (5) | |
| H15A | 1.1069 | 0.3398 | 0.1859 | 0.018* | |
| H15B | 1.2381 | 0.3816 | 0.1523 | 0.018* | |
| C16 | 0.8599 (3) | 0.33075 (18) | 0.05340 (17) | 0.0146 (4) | |
| H16A | 0.8150 | 0.3172 | 0.0948 | 0.018* | |
| C17 | 1.0361 (3) | 0.35837 (17) | −0.01316 (16) | 0.0134 (4) | |
| C18 | 1.1739 (3) | 0.37683 (19) | −0.03566 (18) | 0.0169 (5) | |
| H18A | 1.2774 | 0.3728 | −0.0023 | 0.020* | |
| C19 | 1.1505 (3) | 0.40147 (19) | −0.10989 (18) | 0.0184 (5) | |
| H19A | 1.2409 | 0.4149 | −0.1278 | 0.022* | |
| C20 | 0.9973 (3) | 0.40719 (19) | −0.15933 (18) | 0.0176 (5) | |
| H20A | 0.9874 | 0.4241 | −0.2098 | 0.021* | |
| C21 | 0.8599 (3) | 0.38897 (18) | −0.13704 (17) | 0.0160 (5) | |
| H21A | 0.7563 | 0.3930 | −0.1705 | 0.019* | |
| C22 | 0.8836 (3) | 0.36425 (17) | −0.06195 (17) | 0.0134 (4) | |
| C23 | 1.1749 (3) | 0.3223 (2) | 0.47208 (19) | 0.0192 (5) | |
| H23A | 1.0686 | 0.3131 | 0.4270 | 0.023* | |
| H23B | 1.1887 | 0.3865 | 0.5298 | 0.023* | |
| C24 | 1.1778 (4) | 0.2361 (2) | 0.4980 (2) | 0.0234 (6) | |
| H24A | 1.2903 | 0.2349 | 0.5290 | 0.028* | |
| H24B | 1.1358 | 0.1715 | 0.4394 | 0.028* | |
| C25 | 1.0773 (4) | 0.2460 (2) | 0.56425 (19) | 0.0210 (5) | |
| H25A | 1.1296 | 0.3050 | 0.6264 | 0.025* | |
| H25B | 0.9699 | 0.2586 | 0.5379 | 0.025* | |
| C26 | 1.0565 (4) | 0.1530 (2) | 0.57879 (19) | 0.0216 (5) | |
| H26A | 0.9883 | 0.0966 | 0.5187 | 0.026* | |
| H26B | 1.1631 | 0.1337 | 0.5946 | 0.026* | |
| C27 | 0.9807 (4) | 0.1681 (2) | 0.65654 (19) | 0.0222 (5) | |
| H27A | 0.8804 | 0.1954 | 0.6448 | 0.027* | |
| H27B | 1.0552 | 0.2187 | 0.7180 | 0.027* | |
| C28 | 0.9415 (3) | 0.0714 (2) | 0.66330 (18) | 0.0199 (5) | |
| H28A | 0.8535 | 0.0253 | 0.6060 | 0.024* | |
| H28B | 1.0372 | 0.0380 | 0.6637 | 0.024* | |
| C29 | 0.8916 (4) | 0.0871 (2) | 0.75032 (19) | 0.0229 (5) | |
| H29A | 0.7910 | 0.1156 | 0.7480 | 0.028* | |
| H29B | 0.9761 | 0.1364 | 0.8079 | 0.028* | |
| C30 | 0.8650 (4) | −0.0110 (2) | 0.7569 (2) | 0.0238 (6) | |
| H30A | 0.8340 | 0.0025 | 0.8140 | 0.036* | |
| H30B | 0.9647 | −0.0391 | 0.7600 | 0.036* | |
| H30C | 0.7793 | −0.0594 | 0.7008 | 0.036* | |
| C31 | 0.6030 (3) | 0.34999 (19) | −0.04349 (18) | 0.0159 (5) | |
| H31A | 0.5853 | 0.4046 | −0.0619 | 0.019* | |
| H31B | 0.5656 | 0.3663 | 0.0127 | 0.019* | |
| C32 | 0.5035 (3) | 0.25096 (19) | −0.12457 (18) | 0.0177 (5) | |
| H32A | 0.5543 | 0.2282 | −0.1761 | 0.021* | |
| H32B | 0.3949 | 0.2629 | −0.1498 | 0.021* | |
| C33 | 0.4862 (3) | 0.1670 (2) | −0.09634 (19) | 0.0215 (5) | |
| H33A | 0.4456 | 0.1920 | −0.0406 | 0.026* | |
| H33B | 0.5938 | 0.1501 | −0.0773 | 0.026* | |
| C34 | 0.3727 (3) | 0.0710 (2) | −0.17547 (19) | 0.0219 (5) | |
| H34A | 0.3552 | 0.0241 | −0.1494 | 0.026* | |
| H34B | 0.2673 | 0.0886 | −0.1977 | 0.026* | |
| C35 | 0.4358 (3) | 0.0177 (2) | −0.2595 (2) | 0.0230 (5) | |
| H35A | 0.5445 | 0.0044 | −0.2365 | 0.028* | |
| H35B | 0.4461 | 0.0630 | −0.2882 | 0.028* | |
| C36 | 0.3284 (3) | −0.0821 (2) | −0.3359 (2) | 0.0237 (6) | |
| H36A | 0.3069 | −0.1240 | −0.3057 | 0.028* | |
| H36B | 0.2241 | −0.0678 | −0.3645 | 0.028* | |
| C37 | 0.4014 (4) | −0.1421 (2) | −0.4146 (2) | 0.0252 (6) | |
| H37A | 0.5020 | −0.1603 | −0.3866 | 0.030* | |
| H37B | 0.3257 | −0.2055 | −0.4604 | 0.030* | |
| C38 | 0.4385 (4) | −0.0865 (3) | −0.4677 (2) | 0.0323 (7) | |
| H38A | 0.4655 | −0.1332 | −0.5238 | 0.048* | |
| H38B | 0.5297 | −0.0315 | −0.4263 | 0.048* | |
| H38C | 0.3441 | −0.0587 | −0.4872 | 0.048* | |
| O1W | 0.8891 (3) | 0.47541 (18) | 0.29021 (17) | 0.0315 (5) | |
| H1W1 | 0.877 (5) | 0.414 (3) | 0.275 (3) | 0.043 (12)* | |
| H2W1 | 0.802 (6) | 0.488 (3) | 0.267 (3) | 0.040 (11)* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Br1 | 0.01356 (14) | 0.02374 (15) | 0.02573 (15) | 0.00419 (10) | 0.00812 (10) | 0.01460 (11) |
| Br2 | 0.01520 (14) | 0.02324 (14) | 0.01864 (14) | 0.00351 (10) | 0.00706 (10) | 0.01260 (11) |
| N1 | 0.0155 (10) | 0.0182 (10) | 0.0157 (9) | 0.0032 (8) | 0.0068 (8) | 0.0082 (8) |
| N2 | 0.0130 (10) | 0.0168 (9) | 0.0127 (9) | 0.0020 (7) | 0.0034 (8) | 0.0074 (8) |
| N3 | 0.0114 (9) | 0.0164 (10) | 0.0144 (9) | 0.0023 (7) | 0.0043 (8) | 0.0080 (8) |
| N4 | 0.0114 (9) | 0.0153 (9) | 0.0155 (9) | 0.0029 (7) | 0.0063 (8) | 0.0070 (8) |
| C1 | 0.0155 (11) | 0.0147 (11) | 0.0154 (11) | 0.0032 (9) | 0.0051 (9) | 0.0070 (9) |
| C2 | 0.0247 (14) | 0.0188 (12) | 0.0164 (11) | 0.0058 (10) | 0.0081 (10) | 0.0073 (10) |
| C3 | 0.0241 (14) | 0.0207 (12) | 0.0127 (11) | 0.0042 (10) | −0.0013 (10) | 0.0051 (10) |
| C4 | 0.0154 (12) | 0.0200 (12) | 0.0207 (12) | 0.0018 (9) | 0.0018 (10) | 0.0077 (10) |
| C5 | 0.0134 (11) | 0.0195 (12) | 0.0172 (11) | 0.0015 (9) | 0.0027 (9) | 0.0080 (10) |
| C6 | 0.0140 (11) | 0.0137 (10) | 0.0118 (10) | 0.0022 (8) | 0.0023 (9) | 0.0051 (9) |
| C7 | 0.0144 (11) | 0.0180 (11) | 0.0164 (11) | 0.0028 (9) | 0.0049 (9) | 0.0092 (9) |
| C8 | 0.0138 (11) | 0.0224 (12) | 0.0121 (10) | 0.0011 (9) | 0.0037 (9) | 0.0093 (9) |
| C9 | 0.0112 (11) | 0.0190 (11) | 0.0126 (10) | 0.0015 (9) | 0.0045 (9) | 0.0077 (9) |
| C10 | 0.0169 (12) | 0.0237 (12) | 0.0200 (12) | 0.0073 (10) | 0.0079 (10) | 0.0134 (10) |
| C11 | 0.0256 (14) | 0.0194 (12) | 0.0203 (12) | 0.0074 (10) | 0.0110 (11) | 0.0098 (10) |
| C12 | 0.0208 (13) | 0.0163 (11) | 0.0157 (11) | 0.0012 (9) | 0.0063 (10) | 0.0050 (9) |
| C13 | 0.0156 (12) | 0.0204 (12) | 0.0142 (11) | 0.0028 (9) | 0.0028 (9) | 0.0076 (10) |
| C14 | 0.0125 (11) | 0.0173 (11) | 0.0126 (10) | 0.0038 (9) | 0.0044 (9) | 0.0073 (9) |
| C15 | 0.0125 (11) | 0.0171 (11) | 0.0136 (11) | 0.0019 (9) | 0.0010 (9) | 0.0062 (9) |
| C16 | 0.0138 (11) | 0.0158 (11) | 0.0149 (10) | 0.0027 (9) | 0.0057 (9) | 0.0068 (9) |
| C17 | 0.0137 (11) | 0.0123 (10) | 0.0132 (10) | 0.0019 (8) | 0.0056 (9) | 0.0040 (8) |
| C18 | 0.0143 (12) | 0.0171 (11) | 0.0178 (11) | 0.0031 (9) | 0.0068 (9) | 0.0056 (9) |
| C19 | 0.0181 (12) | 0.0166 (11) | 0.0202 (12) | 0.0017 (9) | 0.0108 (10) | 0.0056 (10) |
| C20 | 0.0229 (13) | 0.0164 (11) | 0.0167 (11) | 0.0051 (9) | 0.0104 (10) | 0.0078 (9) |
| C21 | 0.0190 (12) | 0.0165 (11) | 0.0136 (11) | 0.0061 (9) | 0.0055 (9) | 0.0075 (9) |
| C22 | 0.0139 (11) | 0.0131 (10) | 0.0135 (10) | 0.0032 (8) | 0.0068 (9) | 0.0047 (9) |
| C23 | 0.0191 (12) | 0.0223 (12) | 0.0219 (12) | 0.0056 (10) | 0.0129 (10) | 0.0113 (10) |
| C24 | 0.0286 (15) | 0.0213 (12) | 0.0278 (14) | 0.0077 (11) | 0.0175 (12) | 0.0127 (11) |
| C25 | 0.0256 (14) | 0.0196 (12) | 0.0229 (13) | 0.0050 (10) | 0.0144 (11) | 0.0103 (10) |
| C26 | 0.0257 (14) | 0.0201 (12) | 0.0219 (13) | 0.0040 (10) | 0.0130 (11) | 0.0089 (10) |
| C27 | 0.0271 (14) | 0.0201 (12) | 0.0210 (12) | 0.0032 (10) | 0.0128 (11) | 0.0078 (10) |
| C28 | 0.0225 (13) | 0.0207 (12) | 0.0177 (12) | 0.0036 (10) | 0.0085 (10) | 0.0086 (10) |
| C29 | 0.0261 (14) | 0.0243 (13) | 0.0204 (12) | 0.0028 (11) | 0.0119 (11) | 0.0095 (11) |
| C30 | 0.0219 (14) | 0.0300 (14) | 0.0252 (13) | 0.0052 (11) | 0.0102 (11) | 0.0160 (12) |
| C31 | 0.0106 (11) | 0.0194 (11) | 0.0178 (11) | 0.0044 (9) | 0.0052 (9) | 0.0078 (9) |
| C32 | 0.0134 (11) | 0.0213 (12) | 0.0188 (12) | 0.0032 (9) | 0.0049 (9) | 0.0095 (10) |
| C33 | 0.0212 (13) | 0.0232 (13) | 0.0211 (12) | 0.0013 (10) | 0.0056 (10) | 0.0115 (11) |
| C34 | 0.0183 (13) | 0.0229 (13) | 0.0231 (13) | 0.0003 (10) | 0.0068 (10) | 0.0093 (11) |
| C35 | 0.0210 (13) | 0.0214 (13) | 0.0246 (13) | −0.0001 (10) | 0.0090 (11) | 0.0080 (11) |
| C36 | 0.0204 (13) | 0.0223 (13) | 0.0286 (14) | 0.0033 (10) | 0.0074 (11) | 0.0119 (11) |
| C37 | 0.0259 (15) | 0.0211 (13) | 0.0260 (14) | 0.0049 (11) | 0.0061 (12) | 0.0093 (11) |
| C38 | 0.0359 (18) | 0.0339 (16) | 0.0298 (15) | 0.0072 (14) | 0.0134 (13) | 0.0148 (13) |
| O1W | 0.0215 (11) | 0.0275 (12) | 0.0366 (13) | −0.0002 (9) | −0.0042 (9) | 0.0141 (10) |
Geometric parameters (Å, º)
| N1—C7 | 1.338 (3) | C23—C24 | 1.519 (4) |
| N1—C1 | 1.390 (3) | C23—H23A | 0.9900 |
| N1—C23 | 1.476 (3) | C23—H23B | 0.9900 |
| N2—C7 | 1.337 (3) | C24—C25 | 1.525 (4) |
| N2—C6 | 1.395 (3) | C24—H24A | 0.9900 |
| N2—C8 | 1.482 (3) | C24—H24B | 0.9900 |
| N3—C16 | 1.334 (3) | C25—C26 | 1.518 (4) |
| N3—C17 | 1.395 (3) | C25—H25A | 0.9900 |
| N3—C15 | 1.469 (3) | C25—H25B | 0.9900 |
| N4—C16 | 1.331 (3) | C26—C27 | 1.518 (4) |
| N4—C22 | 1.395 (3) | C26—H26A | 0.9900 |
| N4—C31 | 1.477 (3) | C26—H26B | 0.9900 |
| C1—C2 | 1.393 (3) | C27—C28 | 1.527 (4) |
| C1—C6 | 1.394 (3) | C27—H27A | 0.9900 |
| C2—C3 | 1.387 (4) | C27—H27B | 0.9900 |
| C2—H2A | 0.9500 | C28—C29 | 1.522 (4) |
| C3—C4 | 1.406 (4) | C28—H28A | 0.9900 |
| C3—H3A | 0.9500 | C28—H28B | 0.9900 |
| C4—C5 | 1.380 (4) | C29—C30 | 1.528 (4) |
| C4—H4A | 0.9500 | C29—H29A | 0.9900 |
| C5—C6 | 1.397 (3) | C29—H29B | 0.9900 |
| C5—H5A | 0.9500 | C30—H30A | 0.9800 |
| C7—H7A | 0.9500 | C30—H30B | 0.9800 |
| C8—C9 | 1.508 (3) | C30—H30C | 0.9800 |
| C8—H8A | 0.9900 | C31—C32 | 1.523 (3) |
| C8—H8B | 0.9900 | C31—H31A | 0.9900 |
| C9—C10 | 1.402 (4) | C31—H31B | 0.9900 |
| C9—C14 | 1.407 (3) | C32—C33 | 1.527 (4) |
| C10—C11 | 1.386 (4) | C32—H32A | 0.9900 |
| C10—H10A | 0.9500 | C32—H32B | 0.9900 |
| C11—C12 | 1.383 (4) | C33—C34 | 1.532 (4) |
| C11—H11A | 0.9500 | C33—H33A | 0.9900 |
| C12—C13 | 1.400 (4) | C33—H33B | 0.9900 |
| C12—H12A | 0.9500 | C34—C35 | 1.519 (4) |
| C13—C14 | 1.396 (3) | C34—H34A | 0.9900 |
| C13—H13A | 0.9500 | C34—H34B | 0.9900 |
| C14—C15 | 1.520 (3) | C35—C36 | 1.531 (4) |
| C15—H15A | 0.9900 | C35—H35A | 0.9900 |
| C15—H15B | 0.9900 | C35—H35B | 0.9900 |
| C16—H16A | 0.9500 | C36—C37 | 1.529 (4) |
| C17—C18 | 1.393 (3) | C36—H36A | 0.9900 |
| C17—C22 | 1.397 (3) | C36—H36B | 0.9900 |
| C18—C19 | 1.389 (4) | C37—C38 | 1.515 (4) |
| C18—H18A | 0.9500 | C37—H37A | 0.9900 |
| C19—C20 | 1.405 (4) | C37—H37B | 0.9900 |
| C19—H19A | 0.9500 | C38—H38A | 0.9800 |
| C20—C21 | 1.387 (4) | C38—H38B | 0.9800 |
| C20—H20A | 0.9500 | C38—H38C | 0.9800 |
| C21—C22 | 1.403 (3) | O1W—H1W1 | 0.84 (5) |
| C21—H21A | 0.9500 | O1W—H2W1 | 0.83 (5) |
| C7—N1—C1 | 108.6 (2) | C23—C24—H24A | 109.2 |
| C7—N1—C23 | 126.4 (2) | C25—C24—H24A | 109.2 |
| C1—N1—C23 | 125.0 (2) | C23—C24—H24B | 109.2 |
| C7—N2—C6 | 108.2 (2) | C25—C24—H24B | 109.2 |
| C7—N2—C8 | 128.7 (2) | H24A—C24—H24B | 107.9 |
| C6—N2—C8 | 122.9 (2) | C26—C25—C24 | 113.0 (2) |
| C16—N3—C17 | 108.5 (2) | C26—C25—H25A | 109.0 |
| C16—N3—C15 | 125.8 (2) | C24—C25—H25A | 109.0 |
| C17—N3—C15 | 125.6 (2) | C26—C25—H25B | 109.0 |
| C16—N4—C22 | 108.3 (2) | C24—C25—H25B | 109.0 |
| C16—N4—C31 | 126.3 (2) | H25A—C25—H25B | 107.8 |
| C22—N4—C31 | 125.4 (2) | C27—C26—C25 | 113.4 (2) |
| N1—C1—C2 | 131.5 (2) | C27—C26—H26A | 108.9 |
| N1—C1—C6 | 106.4 (2) | C25—C26—H26A | 108.9 |
| C2—C1—C6 | 122.1 (2) | C27—C26—H26B | 108.9 |
| C3—C2—C1 | 115.6 (2) | C25—C26—H26B | 108.9 |
| C3—C2—H2A | 122.2 | H26A—C26—H26B | 107.7 |
| C1—C2—H2A | 122.2 | C26—C27—C28 | 113.4 (2) |
| C2—C3—C4 | 122.4 (2) | C26—C27—H27A | 108.9 |
| C2—C3—H3A | 118.8 | C28—C27—H27A | 108.9 |
| C4—C3—H3A | 118.8 | C26—C27—H27B | 108.9 |
| C5—C4—C3 | 121.7 (3) | C28—C27—H27B | 108.9 |
| C5—C4—H4A | 119.2 | H27A—C27—H27B | 107.7 |
| C3—C4—H4A | 119.2 | C29—C28—C27 | 114.5 (2) |
| C4—C5—C6 | 116.2 (2) | C29—C28—H28A | 108.6 |
| C4—C5—H5A | 121.9 | C27—C28—H28A | 108.6 |
| C6—C5—H5A | 121.9 | C29—C28—H28B | 108.6 |
| C1—C6—N2 | 106.8 (2) | C27—C28—H28B | 108.6 |
| C1—C6—C5 | 122.0 (2) | H28A—C28—H28B | 107.6 |
| N2—C6—C5 | 131.1 (2) | C28—C29—C30 | 112.1 (2) |
| N2—C7—N1 | 110.0 (2) | C28—C29—H29A | 109.2 |
| N2—C7—H7A | 125.0 | C30—C29—H29A | 109.2 |
| N1—C7—H7A | 125.0 | C28—C29—H29B | 109.2 |
| N2—C8—C9 | 113.9 (2) | C30—C29—H29B | 109.2 |
| N2—C8—H8A | 108.8 | H29A—C29—H29B | 107.9 |
| C9—C8—H8A | 108.8 | C29—C30—H30A | 109.5 |
| N2—C8—H8B | 108.8 | C29—C30—H30B | 109.5 |
| C9—C8—H8B | 108.8 | H30A—C30—H30B | 109.5 |
| H8A—C8—H8B | 107.7 | C29—C30—H30C | 109.5 |
| C10—C9—C14 | 119.5 (2) | H30A—C30—H30C | 109.5 |
| C10—C9—C8 | 117.3 (2) | H30B—C30—H30C | 109.5 |
| C14—C9—C8 | 123.1 (2) | N4—C31—C32 | 112.6 (2) |
| C11—C10—C9 | 120.8 (2) | N4—C31—H31A | 109.1 |
| C11—C10—H10A | 119.6 | C32—C31—H31A | 109.1 |
| C9—C10—H10A | 119.6 | N4—C31—H31B | 109.1 |
| C12—C11—C10 | 119.9 (2) | C32—C31—H31B | 109.1 |
| C12—C11—H11A | 120.0 | H31A—C31—H31B | 107.8 |
| C10—C11—H11A | 120.0 | C31—C32—C33 | 113.9 (2) |
| C11—C12—C13 | 120.0 (2) | C31—C32—H32A | 108.8 |
| C11—C12—H12A | 120.0 | C33—C32—H32A | 108.8 |
| C13—C12—H12A | 120.0 | C31—C32—H32B | 108.8 |
| C14—C13—C12 | 120.7 (2) | C33—C32—H32B | 108.8 |
| C14—C13—H13A | 119.6 | H32A—C32—H32B | 107.7 |
| C12—C13—H13A | 119.6 | C32—C33—C34 | 113.6 (2) |
| C13—C14—C9 | 119.0 (2) | C32—C33—H33A | 108.9 |
| C13—C14—C15 | 121.2 (2) | C34—C33—H33A | 108.9 |
| C9—C14—C15 | 119.7 (2) | C32—C33—H33B | 108.9 |
| N3—C15—C14 | 113.37 (19) | C34—C33—H33B | 108.9 |
| N3—C15—H15A | 108.9 | H33A—C33—H33B | 107.7 |
| C14—C15—H15A | 108.9 | C35—C34—C33 | 113.3 (2) |
| N3—C15—H15B | 108.9 | C35—C34—H34A | 108.9 |
| C14—C15—H15B | 108.9 | C33—C34—H34A | 108.9 |
| H15A—C15—H15B | 107.7 | C35—C34—H34B | 108.9 |
| N4—C16—N3 | 110.3 (2) | C33—C34—H34B | 108.9 |
| N4—C16—H16A | 124.9 | H34A—C34—H34B | 107.7 |
| N3—C16—H16A | 124.9 | C34—C35—C36 | 113.6 (2) |
| C18—C17—N3 | 131.4 (2) | C34—C35—H35A | 108.8 |
| C18—C17—C22 | 122.3 (2) | C36—C35—H35A | 108.8 |
| N3—C17—C22 | 106.2 (2) | C34—C35—H35B | 108.8 |
| C19—C18—C17 | 115.9 (2) | C36—C35—H35B | 108.8 |
| C19—C18—H18A | 122.0 | H35A—C35—H35B | 107.7 |
| C17—C18—H18A | 122.0 | C37—C36—C35 | 113.8 (2) |
| C18—C19—C20 | 122.0 (2) | C37—C36—H36A | 108.8 |
| C18—C19—H19A | 119.0 | C35—C36—H36A | 108.8 |
| C20—C19—H19A | 119.0 | C37—C36—H36B | 108.8 |
| C21—C20—C19 | 122.3 (2) | C35—C36—H36B | 108.8 |
| C21—C20—H20A | 118.9 | H36A—C36—H36B | 107.7 |
| C19—C20—H20A | 118.9 | C38—C37—C36 | 114.1 (3) |
| C20—C21—C22 | 115.7 (2) | C38—C37—H37A | 108.7 |
| C20—C21—H21A | 122.1 | C36—C37—H37A | 108.7 |
| C22—C21—H21A | 122.1 | C38—C37—H37B | 108.7 |
| N4—C22—C17 | 106.7 (2) | C36—C37—H37B | 108.7 |
| N4—C22—C21 | 131.4 (2) | H37A—C37—H37B | 107.6 |
| C17—C22—C21 | 121.8 (2) | C37—C38—H38A | 109.5 |
| N1—C23—C24 | 112.5 (2) | C37—C38—H38B | 109.5 |
| N1—C23—H23A | 109.1 | H38A—C38—H38B | 109.5 |
| C24—C23—H23A | 109.1 | C37—C38—H38C | 109.5 |
| N1—C23—H23B | 109.1 | H38A—C38—H38C | 109.5 |
| C24—C23—H23B | 109.1 | H38B—C38—H38C | 109.5 |
| H23A—C23—H23B | 107.8 | H1W1—O1W—H2W1 | 108 (4) |
| C23—C24—C25 | 112.0 (2) | ||
| C7—N1—C1—C2 | −177.9 (3) | C9—C14—C15—N3 | 165.0 (2) |
| C23—N1—C1—C2 | 2.6 (4) | C22—N4—C16—N3 | −0.3 (3) |
| C7—N1—C1—C6 | 0.7 (3) | C31—N4—C16—N3 | −177.1 (2) |
| C23—N1—C1—C6 | −178.8 (2) | C17—N3—C16—N4 | 0.4 (3) |
| N1—C1—C2—C3 | 178.2 (3) | C15—N3—C16—N4 | 177.2 (2) |
| C6—C1—C2—C3 | −0.2 (4) | C16—N3—C17—C18 | 176.6 (3) |
| C1—C2—C3—C4 | 0.2 (4) | C15—N3—C17—C18 | −0.3 (4) |
| C2—C3—C4—C5 | 0.1 (4) | C16—N3—C17—C22 | −0.3 (3) |
| C3—C4—C5—C6 | −0.5 (4) | C15—N3—C17—C22 | −177.2 (2) |
| N1—C1—C6—N2 | −0.9 (3) | N3—C17—C18—C19 | −176.5 (2) |
| C2—C1—C6—N2 | 177.9 (2) | C22—C17—C18—C19 | 0.0 (4) |
| N1—C1—C6—C5 | −178.9 (2) | C17—C18—C19—C20 | −0.1 (4) |
| C2—C1—C6—C5 | −0.1 (4) | C18—C19—C20—C21 | 0.2 (4) |
| C7—N2—C6—C1 | 0.8 (3) | C19—C20—C21—C22 | −0.1 (4) |
| C8—N2—C6—C1 | 175.7 (2) | C16—N4—C22—C17 | 0.1 (3) |
| C7—N2—C6—C5 | 178.5 (3) | C31—N4—C22—C17 | 177.0 (2) |
| C8—N2—C6—C5 | −6.5 (4) | C16—N4—C22—C21 | −176.8 (2) |
| C4—C5—C6—C1 | 0.5 (4) | C31—N4—C22—C21 | 0.0 (4) |
| C4—C5—C6—N2 | −177.0 (2) | C18—C17—C22—N4 | −177.1 (2) |
| C6—N2—C7—N1 | −0.3 (3) | N3—C17—C22—N4 | 0.1 (3) |
| C8—N2—C7—N1 | −174.9 (2) | C18—C17—C22—C21 | 0.2 (4) |
| C1—N1—C7—N2 | −0.3 (3) | N3—C17—C22—C21 | 177.4 (2) |
| C23—N1—C7—N2 | 179.2 (2) | C20—C21—C22—N4 | 176.5 (2) |
| C7—N2—C8—C9 | −29.6 (4) | C20—C21—C22—C17 | −0.1 (3) |
| C6—N2—C8—C9 | 156.5 (2) | C7—N1—C23—C24 | 101.5 (3) |
| N2—C8—C9—C10 | −94.4 (3) | C1—N1—C23—C24 | −79.1 (3) |
| N2—C8—C9—C14 | 89.2 (3) | N1—C23—C24—C25 | 165.2 (2) |
| C14—C9—C10—C11 | −0.9 (4) | C23—C24—C25—C26 | 170.9 (2) |
| C8—C9—C10—C11 | −177.4 (2) | C24—C25—C26—C27 | 170.5 (3) |
| C9—C10—C11—C12 | 0.0 (4) | C25—C26—C27—C28 | 173.2 (2) |
| C10—C11—C12—C13 | 0.4 (4) | C26—C27—C28—C29 | 170.3 (2) |
| C11—C12—C13—C14 | 0.1 (4) | C27—C28—C29—C30 | −176.1 (2) |
| C12—C13—C14—C9 | −0.9 (4) | C16—N4—C31—C32 | −98.8 (3) |
| C12—C13—C14—C15 | −179.4 (2) | C22—N4—C31—C32 | 84.9 (3) |
| C10—C9—C14—C13 | 1.4 (4) | N4—C31—C32—C33 | 73.6 (3) |
| C8—C9—C14—C13 | 177.6 (2) | C31—C32—C33—C34 | 174.3 (2) |
| C10—C9—C14—C15 | 179.9 (2) | C32—C33—C34—C35 | 66.7 (3) |
| C8—C9—C14—C15 | −3.8 (4) | C33—C34—C35—C36 | 176.1 (2) |
| C16—N3—C15—C14 | 102.7 (3) | C34—C35—C36—C37 | −173.0 (2) |
| C17—N3—C15—C14 | −80.9 (3) | C35—C36—C37—C38 | −59.2 (4) |
| C13—C14—C15—N3 | −16.5 (3) |
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| O1W—H1W1···Br1 | 0.83 (5) | 2.50 (5) | 3.326 (3) | 173 (4) |
| O1W—H2W1···Br2 | 0.83 (5) | 2.52 (5) | 3.343 (3) | 177 (5) |
| C7—H7A···Br1 | 0.95 | 2.69 | 3.569 (3) | 154 |
| C15—H15A···Br1 | 0.99 | 2.92 | 3.672 (3) | 134 |
| C16—H16A···Br1 | 0.95 | 2.79 | 3.594 (3) | 143 |
| C2—H2A···Br2i | 0.95 | 2.81 | 3.698 (3) | 155 |
| C4—H4A···O1Wii | 0.95 | 2.49 | 3.218 (4) | 133 |
| C8—H8A···Br1iii | 0.99 | 2.80 | 3.779 (3) | 169 |
| C8—H8B···Br2iii | 0.99 | 2.71 | 3.655 (3) | 159 |
| C19—H19A···Br2iv | 0.95 | 2.84 | 3.770 (3) | 167 |
| C21—H21A···Br2v | 0.95 | 2.90 | 3.785 (3) | 155 |
| C31—H31A···Br2v | 0.99 | 2.87 | 3.786 (3) | 154 |
Symmetry codes: (i) −x+2, −y+1, −z+1; (ii) −x+3, −y+1, −z+1; (iii) x+1, y, z; (iv) −x+2, −y+1, −z; (v) −x+1, −y+1, −z.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: HB6650).
References
- Bruker (2009). SADABS, APEX2 and SAINT Bruker AXS Inc., Madison, Wisconsin, USA.
- Cosier, J. & Glazer, A. M. (1986). J. Appl. Cryst. 19, 105–107.
- Haque, R. A., Iqbal, M. A., Budagumpi, S., Hemamalini, M. & Fun, H.-K. (2012). Acta Cryst. E68, o573. [DOI] [PMC free article] [PubMed]
- Haque, R. A., Iqbal, M. A., Hemamalini, M. & Fun, H.-K. (2011). Acta Cryst. E67, o1814–o1815. [DOI] [PMC free article] [PubMed]
- Iqbal, M. A., Haque, R. A., Fun, H.-K. & Chia, T. S. (2012). Acta Cryst. E68, o466–o467. [DOI] [PMC free article] [PubMed]
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Spek, A. L. (2009). Acta Cryst. D65, 148–155. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S1600536812008331/hb6650sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536812008331/hb6650Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536812008331/hb6650Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report