Abstract
In the title compound, C36H50N4O2, the two pyrrolidine rings have envelope conformations. The conformation of the macrocycle is stabilized by N—H⋯N hydrogen bonds and a C—H⋯N interaction. The benzoyl ring is inclined to an adjacent pyrrole ring by 6.76 (9)°, with a centroid-to-centroid distance of 3.6285 (10) Å. In the crystal, apart from a C—H⋯O and a C—H⋯π interaction, molecules are linked via an N—H⋯O hydrogen bond, leading to the formation of helical chains propagating along [010].
Related literature
For the heterogeneous catalytic hydrogenation of meso-octamethylcalix[4]pyrrole, which gave meso-octamethylcalix[2]pyrrole[2]pyrrolidine, see: Blangy et al. (2009 ▶). For the N-acylation of pyrrolidines using substituted benzoyl chlorides, see: Journot et al. (2012a
▶); Zhang et al. (2009 ▶). For the synthesis and reactivity of the title compound, see: Journot & Neier (2012 ▶). For the crystal structures of similar compounds, see: Journot et al. (2012b
▶,c
▶,d
▶,e
▶)
Experimental
Crystal data
C36H50N4O2
M r = 570.80
Monoclinic,
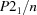
a = 10.3150 (4) Å
b = 11.8104 (5) Å
c = 26.1856 (10) Å
β = 98.629 (3)°
V = 3153.9 (2) Å3
Z = 4
Mo Kα radiation
μ = 0.08 mm−1
T = 173 K
0.40 × 0.39 × 0.39 mm
Data collection
Stoe IPDS II diffractometer
Absorption correction: multi-scan (MULABS in PLATON; Spek, 2009 ▶) T min = 0.893, T max = 1.000
33803 measured reflections
5943 independent reflections
4470 reflections with I > 2σ(I)
R int = 0.063
Refinement
R[F 2 > 2σ(F 2)] = 0.045
wR(F 2) = 0.097
S = 1.03
5943 reflections
393 parameters
H atoms treated by a mixture of independent and constrained refinement
Δρmax = 0.20 e Å−3
Δρmin = −0.17 e Å−3
Data collection: X-AREA (Stoe & Cie, 2009 ▶); cell refinement: X-AREA; data reduction: X-RED32 (Stoe & Cie, 2009 ▶); program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: PLATON (Spek, 2009 ▶); software used to prepare material for publication: SHELXL97, PLATON and publCIF (Westrip, 2010 ▶).
Supplementary Material
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S1600536812008008/aa2052sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536812008008/aa2052Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536812008008/aa2052Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
Cg1 is the centroid of the pyrrole ring N2/C3/C4/C25/C26 and Cg2 is the centroid of the benzene ring C30–C35.
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N2—H2A⋯N3 | 0.88 | 2.57 | 3.0671 (18) | 117 |
| N4—H4A⋯N3 | 0.88 | 2.33 | 2.8759 (18) | 121 |
| C28—H28B⋯N4 | 0.98 | 2.59 | 3.561 (2) | 171 |
| C28—H28B⋯Cg1 | 0.98 | 2.45 | 3.3632 (18) | 155 |
| N3—H3N⋯O1i | 0.924 (18) | 2.283 (18) | 3.1401 (17) | 154.0 (15) |
| C20—H20B⋯O1i | 0.98 | 2.56 | 3.530 (2) | 170 |
| C15—H15A⋯Cg2i | 0.98 | 2.85 | 3.7176 (19) | 148 |
Symmetry code: (i)  .
.
Acknowledgments
HSE thanks the staff of the XRD Application Laboratory, CSEM, Neuchâtel, for access to the X-ray diffraction equipment.
supplementary crystallographic information
Comment
We have recently reported the access to new macrocycles by heterogeneous catalytic hydrogenation of meso-octamethylcalix[4]pyrrole, which gave meso-octamethylcalix[2]pyrrole[2]pyrrolidine (1 in Fig. 3) (Blangy et al., 2009). It was decided to investigate the nucleophilicity of this new macrocycle, which showed interesting reactivity (Journot & Neier, 2012), by reacting different substituted benzoyl chlorides with the macrocycle under smooth conditions (Journot et al., 2012a; Zhang et al., 2009). Herein, we report on the synthesis and crystal structure of the title 4-methoxybenzoyl derivative, one of five compounds that have been studied by X-ray diffraction analysis (Journot et al., 2012b,c,d,e).
The molecular structure of the title compound is given in Fig. 1. The two pyrrolidine rings (N1,C1,C12–C14) and (N3,C6,C7,C21,C22) have envelope conformations with, respectively, atoms C14 and C7 as the flaps. The conformation of the macrocycle is stabilized by intramolecular N—H···N hydrogen bonds involving atom N3 and the two pyrrole H atoms, H2 and H4 (Table 1). The benzoyl ring (C30–C35) is inclined to the pyrrole ring (N2,C3,C4,C25,C26) by 6.76 (9)°, with a centroid-to-centroid distance of 3.6285 (10) Å. The methyl group C28 is also in close contact with the pyrrole ring (N4,C9,C10,C17,C18), with a short C28—H28A···N4 interaction and a C28—H28A···centroid distance of 3.3632 (18) Å (Table 1).
In the crystal, molecules are linked via an N—H···O hydrogen bond, involving the N3 pyrrolidine H atom (H3N) and the benzoyl O atom (O1), leading to the formation of helical chains propagating along [010] (Fig. 2 and Table 1). The same O atom is involved in a C—H···O contact with methyl group C20. A C—H···π interaction is also observed, involving the methyl group C15 and the benzoyl ring (C30–C35) (see Table 1).
The overall geometry and crystal packing is very similar to that reported for the 4-chlorobenzoyl derivative (Journot et al., 2012b), and the 4-nitrobenzoyl (Journot et al., 2012d) and 4-methylbenzoyl (Journot et al., 2012e) derivatives. The benzoyl derivative (Journot et al., 2012c) crystallized in the trigonal space group R3, as a partial (0.25H2O) hydrate, and forms hydrogen bonded chains propagating along [001].
Experimental
General procedure for the N-acylation of meso-octamethylcalix[2]pyrrolidino[2]pyrrole (1) is illustrated in Fig. 3. The full details of this synthesis will be reported elsewhere (Journot & Neier, 2012). The title amide 3e was prepared, according to the general procedure, from 100 mg of 1 (0.23 mmol), 4-methoxybenzoyl chloride (2e, 64.93 µl, 0.48 mmol), potassium carbonate (70 mg, 0.48 mmol) in THF (5 ml) and ACN (2.5 ml). The residue was purified by column chromatography (SiO2, CH2Cl2/MeOH, 97/3) to yield 117.0 mg (90%) of colourless crystals of the title compound (3e). Melting point: 488 K. HRMS calcd. for C36H50N4O2+H+ 571.4007, found 571.3993. Further synthetic and spectroscopic data has been reported elsewhere (Journot & Neier, 2012).
Refinement
The NH H atoms were located in a difference electron-density map. H atom H3N was freely refined, while the other NH H atoms and the C-bound H atoms were included in calculated positions and treated as riding atoms: N—H = 0.88 Å, C—H = 0.95 Å for CH-allyl and CH-aromatic H atoms, and 1.00, 0.99 and 0.98 Å, for methine, methylene and methyl H atoms, respectively, with Uiso(H) = k × Ueq(C, N), where k = 1.5 for CH3 H atoms, and = 1.2 for the other H atoms.
Figures
Fig. 1.
A view of the molecular structure of the title compound, with the numbering scheme and displacement ellipsoids drawn at the 50% probability level. The N—H···N hydrogen bonds are shown as dashed lines (see Table 1 for details; the C-bound H atoms have been omitted for clarity).
Fig. 2.
A view along the a axis of the crystal packing of the title compound. The N—H···N and N—H···O hydrogen bonds are shown as dashed lines (see Table 1 for details; the C-bound H atoms have been omitted for clarity).
Fig. 3.
The general procedure for the N-acylation of meso-octamethylcalix[2]pyrrolidino[2]pyrrole (1).
Crystal data
| C36H50N4O2 | F(000) = 1240 |
| Mr = 570.80 | Dx = 1.202 Mg m−3 |
| Monoclinic, P21/n | Melting point: 488 K |
| Hall symbol: -P 2yn | Mo Kα radiation, λ = 0.71073 Å |
| a = 10.3150 (4) Å | Cell parameters from 22254 reflections |
| b = 11.8104 (5) Å | θ = 1.6–26.1° |
| c = 26.1856 (10) Å | µ = 0.08 mm−1 |
| β = 98.629 (3)° | T = 173 K |
| V = 3153.9 (2) Å3 | Block, colourless |
| Z = 4 | 0.40 × 0.39 × 0.39 mm |
Data collection
| Stoe IPDS II diffractometer | 5943 independent reflections |
| Radiation source: fine-focus sealed tube | 4470 reflections with I > 2σ(I) |
| Graphite monochromator | Rint = 0.063 |
| φ and ω scans | θmax = 25.6°, θmin = 1.6° |
| Absorption correction: multi-scan (MULABS in PLATON; Spek, 2009) | h = −12→12 |
| Tmin = 0.893, Tmax = 1.000 | k = −14→14 |
| 33803 measured reflections | l = −31→31 |
Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.045 | H atoms treated by a mixture of independent and constrained refinement |
| wR(F2) = 0.097 | w = 1/[σ2(Fo2) + (0.0447P)2 + 0.4659P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.03 | (Δ/σ)max < 0.001 |
| 5943 reflections | Δρmax = 0.20 e Å−3 |
| 393 parameters | Δρmin = −0.17 e Å−3 |
| 0 restraints | Extinction correction: SHELXL97 (Sheldrick, 2008), Fc*=kFc[1+0.001xFc2λ3/sin(2θ)]-1/4 |
| Primary atom site location: structure-invariant direct methods | Extinction coefficient: 0.0016 (3) |
Special details
| Geometry. Bond distances, angles etc. have been calculated using the rounded fractional coordinates. All su's are estimated from the variances of the (full) variance-covariance matrix. The cell e.s.d.'s are taken into account in the estimation of distances, angles and torsion angles |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R-factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| O1 | 0.20707 (11) | 0.58944 (10) | 0.24279 (4) | 0.0303 (4) | |
| O2 | 0.54533 (13) | 0.47589 (14) | 0.07206 (6) | 0.0546 (5) | |
| N1 | 0.04328 (12) | 0.64660 (11) | 0.17968 (5) | 0.0219 (4) | |
| N2 | 0.14268 (12) | 0.82620 (11) | 0.10133 (5) | 0.0241 (4) | |
| N3 | 0.17847 (13) | 1.07140 (11) | 0.13942 (5) | 0.0240 (4) | |
| N4 | −0.01564 (12) | 0.97053 (11) | 0.19335 (5) | 0.0220 (4) | |
| C1 | −0.03465 (15) | 0.60651 (14) | 0.13026 (6) | 0.0251 (5) | |
| C2 | −0.05119 (16) | 0.69079 (14) | 0.08382 (6) | 0.0258 (5) | |
| C3 | 0.08118 (16) | 0.73569 (13) | 0.07453 (6) | 0.0245 (5) | |
| C4 | 0.25883 (15) | 0.85139 (14) | 0.08367 (6) | 0.0255 (5) | |
| C5 | 0.34568 (16) | 0.94944 (15) | 0.10384 (6) | 0.0282 (5) | |
| C6 | 0.26805 (16) | 1.06197 (14) | 0.10030 (6) | 0.0275 (5) | |
| C7 | 0.07249 (16) | 1.14824 (14) | 0.11697 (6) | 0.0259 (5) | |
| C8 | −0.04038 (16) | 1.16205 (14) | 0.14923 (6) | 0.0262 (5) | |
| C9 | −0.09460 (15) | 1.05203 (13) | 0.16707 (6) | 0.0245 (5) | |
| C10 | −0.08901 (15) | 0.88939 (13) | 0.21376 (6) | 0.0227 (5) | |
| C11 | −0.02631 (15) | 0.79183 (13) | 0.24618 (6) | 0.0236 (5) | |
| C12 | −0.04177 (15) | 0.67167 (14) | 0.22005 (6) | 0.0233 (5) | |
| C13 | −0.18076 (16) | 0.64202 (14) | 0.19360 (7) | 0.0279 (5) | |
| C14 | −0.16030 (16) | 0.56400 (15) | 0.14901 (7) | 0.0306 (5) | |
| C15 | 0.11827 (16) | 0.81902 (14) | 0.26463 (7) | 0.0288 (5) | |
| C16 | −0.09630 (18) | 0.78299 (16) | 0.29437 (7) | 0.0347 (6) | |
| C17 | −0.21771 (16) | 0.92137 (14) | 0.20026 (7) | 0.0283 (5) | |
| C18 | −0.22099 (16) | 1.02167 (14) | 0.17079 (7) | 0.0292 (5) | |
| C19 | −0.15049 (17) | 1.22845 (15) | 0.11586 (7) | 0.0346 (6) | |
| C20 | 0.01016 (17) | 1.23347 (14) | 0.19778 (7) | 0.0308 (5) | |
| C21 | 0.04096 (17) | 1.10045 (15) | 0.06251 (6) | 0.0316 (6) | |
| C22 | 0.17762 (17) | 1.08058 (16) | 0.04826 (6) | 0.0334 (6) | |
| C23 | 0.41022 (16) | 0.93032 (15) | 0.16006 (7) | 0.0322 (6) | |
| C24 | 0.45482 (18) | 0.96246 (18) | 0.06999 (7) | 0.0414 (7) | |
| C25 | 0.27112 (17) | 0.77462 (15) | 0.04554 (7) | 0.0312 (5) | |
| C26 | 0.16092 (17) | 0.70252 (15) | 0.03980 (6) | 0.0301 (5) | |
| C27 | −0.10938 (17) | 0.61989 (16) | 0.03625 (7) | 0.0340 (6) | |
| C28 | −0.14476 (16) | 0.78916 (14) | 0.08942 (6) | 0.0288 (5) | |
| C29 | 0.16336 (15) | 0.59792 (13) | 0.19642 (6) | 0.0234 (5) | |
| C30 | 0.25177 (15) | 0.56260 (13) | 0.15833 (6) | 0.0240 (5) | |
| C31 | 0.23655 (17) | 0.46683 (14) | 0.12715 (6) | 0.0294 (5) | |
| C32 | 0.33205 (17) | 0.43414 (15) | 0.09790 (7) | 0.0331 (5) | |
| C33 | 0.44455 (17) | 0.49856 (17) | 0.09941 (7) | 0.0346 (6) | |
| C34 | 0.46181 (17) | 0.59379 (16) | 0.13061 (7) | 0.0360 (6) | |
| C35 | 0.36688 (16) | 0.62456 (15) | 0.15988 (7) | 0.0295 (5) | |
| C36 | 0.5385 (2) | 0.3742 (2) | 0.04287 (10) | 0.0684 (10) | |
| H1 | 0.01140 | 0.53830 | 0.11920 | 0.0300* | |
| H2A | 0.11210 | 0.86280 | 0.12620 | 0.0290* | |
| H3N | 0.2223 (17) | 1.0984 (15) | 0.1704 (7) | 0.031 (5)* | |
| H4A | 0.07050 | 0.97030 | 0.19670 | 0.0260* | |
| H6 | 0.33220 | 1.12590 | 0.10560 | 0.0330* | |
| H7 | 0.11240 | 1.22470 | 0.11410 | 0.0310* | |
| H12 | −0.01830 | 0.61490 | 0.24830 | 0.0280* | |
| H13A | −0.23050 | 0.60290 | 0.21790 | 0.0340* | |
| H13B | −0.22910 | 0.71110 | 0.18060 | 0.0340* | |
| H14A | −0.23590 | 0.56850 | 0.12090 | 0.0370* | |
| H14B | −0.14980 | 0.48450 | 0.16090 | 0.0370* | |
| H15A | 0.12560 | 0.89470 | 0.28010 | 0.0430* | |
| H15B | 0.16670 | 0.81680 | 0.23520 | 0.0430* | |
| H15C | 0.15500 | 0.76290 | 0.29040 | 0.0430* | |
| H16A | −0.18870 | 0.76360 | 0.28360 | 0.0520* | |
| H16B | −0.09020 | 0.85580 | 0.31260 | 0.0520* | |
| H16C | −0.05430 | 0.72400 | 0.31750 | 0.0520* | |
| H17 | −0.29180 | 0.88260 | 0.20920 | 0.0340* | |
| H18 | −0.29770 | 1.06130 | 0.15610 | 0.0350* | |
| H19A | −0.19130 | 1.18050 | 0.08740 | 0.0520* | |
| H19B | −0.11360 | 1.29620 | 0.10190 | 0.0520* | |
| H19C | −0.21670 | 1.25100 | 0.13710 | 0.0520* | |
| H20A | 0.03940 | 1.30760 | 0.18710 | 0.0460* | |
| H20B | 0.08370 | 1.19430 | 0.21850 | 0.0460* | |
| H20C | −0.06070 | 1.24370 | 0.21840 | 0.0460* | |
| H21A | −0.00950 | 1.15530 | 0.03880 | 0.0380* | |
| H21B | −0.00900 | 1.02880 | 0.06210 | 0.0380* | |
| H22A | 0.17790 | 1.01320 | 0.02580 | 0.0400* | |
| H22B | 0.20670 | 1.14710 | 0.02990 | 0.0400* | |
| H23A | 0.46920 | 0.86500 | 0.16170 | 0.0480* | |
| H23B | 0.34220 | 0.91600 | 0.18170 | 0.0480* | |
| H23C | 0.46040 | 0.99780 | 0.17270 | 0.0480* | |
| H24A | 0.41530 | 0.97620 | 0.03410 | 0.0620* | |
| H24B | 0.50730 | 0.89300 | 0.07190 | 0.0620* | |
| H24C | 0.51120 | 1.02650 | 0.08250 | 0.0620* | |
| H25 | 0.34190 | 0.77060 | 0.02620 | 0.0370* | |
| H26 | 0.14470 | 0.64160 | 0.01600 | 0.0360* | |
| H27A | −0.19780 | 0.59470 | 0.04030 | 0.0510* | |
| H27B | −0.05360 | 0.55370 | 0.03330 | 0.0510* | |
| H27C | −0.11360 | 0.66620 | 0.00500 | 0.0510* | |
| H28A | −0.23220 | 0.75930 | 0.09200 | 0.0430* | |
| H28B | −0.11220 | 0.83240 | 0.12060 | 0.0430* | |
| H28C | −0.14980 | 0.83870 | 0.05920 | 0.0430* | |
| H31 | 0.15910 | 0.42270 | 0.12580 | 0.0350* | |
| H32 | 0.32010 | 0.36810 | 0.07700 | 0.0400* | |
| H34 | 0.53920 | 0.63800 | 0.13190 | 0.0430* | |
| H35 | 0.38040 | 0.68940 | 0.18150 | 0.0350* | |
| H36A | 0.53140 | 0.30950 | 0.06570 | 0.1030* | |
| H36B | 0.46140 | 0.37640 | 0.01600 | 0.1030* | |
| H36C | 0.61790 | 0.36640 | 0.02680 | 0.1030* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| O1 | 0.0324 (6) | 0.0370 (7) | 0.0197 (6) | 0.0040 (5) | −0.0016 (5) | 0.0026 (5) |
| O2 | 0.0369 (8) | 0.0764 (11) | 0.0524 (9) | 0.0096 (7) | 0.0125 (7) | −0.0272 (8) |
| N1 | 0.0231 (7) | 0.0226 (7) | 0.0193 (7) | −0.0005 (5) | 0.0006 (5) | −0.0018 (5) |
| N2 | 0.0261 (7) | 0.0275 (7) | 0.0185 (7) | 0.0031 (6) | 0.0032 (5) | −0.0056 (6) |
| N3 | 0.0263 (7) | 0.0262 (7) | 0.0191 (7) | 0.0004 (6) | 0.0023 (6) | −0.0022 (6) |
| N4 | 0.0183 (6) | 0.0238 (7) | 0.0238 (7) | 0.0013 (5) | 0.0029 (5) | 0.0001 (6) |
| C1 | 0.0265 (8) | 0.0230 (8) | 0.0240 (8) | 0.0003 (7) | −0.0025 (7) | −0.0062 (7) |
| C2 | 0.0280 (9) | 0.0263 (9) | 0.0214 (8) | 0.0024 (7) | −0.0017 (7) | −0.0041 (7) |
| C3 | 0.0302 (9) | 0.0246 (8) | 0.0167 (8) | 0.0041 (7) | −0.0029 (6) | −0.0010 (6) |
| C4 | 0.0249 (8) | 0.0299 (9) | 0.0218 (8) | 0.0063 (7) | 0.0034 (7) | −0.0011 (7) |
| C5 | 0.0252 (9) | 0.0344 (10) | 0.0258 (9) | −0.0004 (7) | 0.0069 (7) | −0.0030 (7) |
| C6 | 0.0312 (9) | 0.0286 (9) | 0.0235 (9) | −0.0054 (7) | 0.0072 (7) | −0.0011 (7) |
| C7 | 0.0317 (9) | 0.0209 (8) | 0.0246 (9) | 0.0003 (7) | 0.0022 (7) | 0.0027 (7) |
| C8 | 0.0294 (9) | 0.0235 (9) | 0.0248 (9) | 0.0037 (7) | 0.0016 (7) | −0.0005 (7) |
| C9 | 0.0246 (8) | 0.0233 (8) | 0.0251 (8) | 0.0044 (6) | 0.0017 (7) | −0.0021 (7) |
| C10 | 0.0243 (8) | 0.0229 (8) | 0.0218 (8) | −0.0018 (6) | 0.0061 (6) | −0.0042 (6) |
| C11 | 0.0253 (8) | 0.0240 (9) | 0.0213 (8) | −0.0026 (7) | 0.0032 (6) | −0.0011 (7) |
| C12 | 0.0245 (8) | 0.0242 (9) | 0.0213 (8) | −0.0029 (6) | 0.0043 (6) | 0.0020 (6) |
| C13 | 0.0253 (9) | 0.0263 (9) | 0.0324 (9) | −0.0053 (7) | 0.0049 (7) | −0.0018 (7) |
| C14 | 0.0286 (9) | 0.0269 (9) | 0.0344 (10) | −0.0055 (7) | −0.0013 (7) | −0.0040 (8) |
| C15 | 0.0313 (9) | 0.0249 (9) | 0.0279 (9) | −0.0021 (7) | −0.0034 (7) | −0.0005 (7) |
| C16 | 0.0452 (11) | 0.0346 (10) | 0.0260 (9) | −0.0029 (8) | 0.0114 (8) | −0.0014 (8) |
| C17 | 0.0219 (8) | 0.0280 (9) | 0.0359 (10) | −0.0022 (7) | 0.0072 (7) | −0.0056 (7) |
| C18 | 0.0226 (8) | 0.0276 (9) | 0.0358 (10) | 0.0047 (7) | −0.0007 (7) | −0.0057 (8) |
| C19 | 0.0362 (10) | 0.0335 (10) | 0.0335 (10) | 0.0100 (8) | 0.0031 (8) | 0.0022 (8) |
| C20 | 0.0350 (10) | 0.0261 (9) | 0.0316 (9) | 0.0012 (7) | 0.0062 (8) | −0.0026 (7) |
| C21 | 0.0395 (10) | 0.0329 (10) | 0.0206 (9) | 0.0040 (8) | −0.0009 (7) | 0.0039 (7) |
| C22 | 0.0447 (11) | 0.0330 (10) | 0.0234 (9) | 0.0016 (8) | 0.0079 (8) | 0.0022 (7) |
| C23 | 0.0262 (9) | 0.0362 (10) | 0.0325 (10) | 0.0019 (7) | −0.0010 (7) | −0.0056 (8) |
| C24 | 0.0326 (10) | 0.0535 (13) | 0.0407 (11) | −0.0045 (9) | 0.0139 (8) | −0.0092 (9) |
| C25 | 0.0326 (9) | 0.0353 (10) | 0.0269 (9) | 0.0059 (8) | 0.0084 (7) | −0.0044 (8) |
| C26 | 0.0395 (10) | 0.0283 (9) | 0.0221 (9) | 0.0047 (8) | 0.0029 (7) | −0.0066 (7) |
| C27 | 0.0366 (10) | 0.0365 (10) | 0.0260 (9) | 0.0009 (8) | −0.0047 (7) | −0.0080 (8) |
| C28 | 0.0295 (9) | 0.0296 (10) | 0.0255 (9) | 0.0043 (7) | −0.0016 (7) | 0.0000 (7) |
| C29 | 0.0263 (8) | 0.0195 (8) | 0.0235 (9) | −0.0004 (6) | 0.0007 (7) | 0.0003 (6) |
| C30 | 0.0264 (8) | 0.0233 (8) | 0.0208 (8) | 0.0046 (7) | −0.0015 (6) | 0.0032 (7) |
| C31 | 0.0317 (9) | 0.0258 (9) | 0.0294 (9) | 0.0020 (7) | 0.0004 (7) | 0.0000 (7) |
| C32 | 0.0380 (10) | 0.0311 (9) | 0.0281 (9) | 0.0110 (8) | −0.0023 (8) | −0.0077 (8) |
| C33 | 0.0280 (9) | 0.0461 (11) | 0.0290 (10) | 0.0137 (8) | 0.0023 (7) | −0.0056 (8) |
| C34 | 0.0249 (9) | 0.0429 (11) | 0.0396 (11) | 0.0006 (8) | 0.0032 (8) | −0.0080 (9) |
| C35 | 0.0265 (9) | 0.0312 (9) | 0.0292 (9) | 0.0032 (7) | −0.0013 (7) | −0.0064 (7) |
| C36 | 0.0559 (14) | 0.0877 (19) | 0.0634 (16) | 0.0183 (13) | 0.0151 (12) | −0.0374 (14) |
Geometric parameters (Å, º)
| O1—C29 | 1.2343 (19) | C34—C35 | 1.380 (2) |
| O2—C33 | 1.374 (2) | C1—H1 | 1.0000 |
| O2—C36 | 1.420 (3) | C6—H6 | 1.0000 |
| N1—C1 | 1.494 (2) | C7—H7 | 1.0000 |
| N1—C12 | 1.501 (2) | C12—H12 | 1.0000 |
| N1—C29 | 1.376 (2) | C13—H13A | 0.9900 |
| N2—C3 | 1.380 (2) | C13—H13B | 0.9900 |
| N2—C4 | 1.380 (2) | C14—H14A | 0.9900 |
| N3—C6 | 1.483 (2) | C14—H14B | 0.9900 |
| N3—C7 | 1.474 (2) | C15—H15A | 0.9800 |
| N4—C9 | 1.377 (2) | C15—H15B | 0.9800 |
| N4—C10 | 1.378 (2) | C15—H15C | 0.9800 |
| N2—H2A | 0.8800 | C16—H16A | 0.9800 |
| N3—H3N | 0.924 (18) | C16—H16B | 0.9800 |
| N4—H4A | 0.8800 | C16—H16C | 0.9800 |
| C1—C2 | 1.561 (2) | C17—H17 | 0.9500 |
| C1—C14 | 1.538 (2) | C18—H18 | 0.9500 |
| C2—C3 | 1.518 (2) | C19—H19A | 0.9800 |
| C2—C27 | 1.546 (2) | C19—H19B | 0.9800 |
| C2—C28 | 1.531 (2) | C19—H19C | 0.9800 |
| C3—C26 | 1.372 (2) | C20—H20A | 0.9800 |
| C4—C5 | 1.510 (2) | C20—H20B | 0.9800 |
| C4—C25 | 1.369 (2) | C20—H20C | 0.9800 |
| C5—C23 | 1.539 (2) | C21—H21A | 0.9900 |
| C5—C6 | 1.547 (2) | C21—H21B | 0.9900 |
| C5—C24 | 1.542 (2) | C22—H22A | 0.9900 |
| C6—C22 | 1.547 (2) | C22—H22B | 0.9900 |
| C7—C8 | 1.546 (2) | C23—H23A | 0.9800 |
| C7—C21 | 1.523 (2) | C23—H23B | 0.9800 |
| C8—C20 | 1.549 (2) | C23—H23C | 0.9800 |
| C8—C9 | 1.516 (2) | C24—H24A | 0.9800 |
| C8—C19 | 1.540 (2) | C24—H24B | 0.9800 |
| C9—C18 | 1.370 (2) | C24—H24C | 0.9800 |
| C10—C11 | 1.517 (2) | C25—H25 | 0.9500 |
| C10—C17 | 1.375 (2) | C26—H26 | 0.9500 |
| C11—C16 | 1.549 (2) | C27—H27A | 0.9800 |
| C11—C12 | 1.573 (2) | C27—H27B | 0.9800 |
| C11—C15 | 1.531 (2) | C27—H27C | 0.9800 |
| C12—C13 | 1.536 (2) | C28—H28A | 0.9800 |
| C13—C14 | 1.527 (3) | C28—H28B | 0.9800 |
| C17—C18 | 1.411 (2) | C28—H28C | 0.9800 |
| C21—C22 | 1.530 (2) | C31—H31 | 0.9500 |
| C25—C26 | 1.410 (3) | C32—H32 | 0.9500 |
| C29—C30 | 1.508 (2) | C34—H34 | 0.9500 |
| C30—C35 | 1.390 (2) | C35—H35 | 0.9500 |
| C30—C31 | 1.390 (2) | C36—H36A | 0.9800 |
| C31—C32 | 1.390 (2) | C36—H36B | 0.9800 |
| C32—C33 | 1.383 (3) | C36—H36C | 0.9800 |
| C33—C34 | 1.386 (3) | ||
| C33—O2—C36 | 117.72 (16) | C11—C12—H12 | 107.00 |
| C1—N1—C12 | 112.15 (12) | C13—C12—H12 | 107.00 |
| C1—N1—C29 | 119.01 (13) | C12—C13—H13A | 111.00 |
| C12—N1—C29 | 116.83 (12) | C12—C13—H13B | 111.00 |
| C3—N2—C4 | 110.65 (13) | C14—C13—H13A | 111.00 |
| C6—N3—C7 | 105.81 (12) | C14—C13—H13B | 111.00 |
| C9—N4—C10 | 111.22 (13) | H13A—C13—H13B | 109.00 |
| C4—N2—H2A | 125.00 | C1—C14—H14A | 111.00 |
| C3—N2—H2A | 125.00 | C1—C14—H14B | 111.00 |
| C6—N3—H3N | 111.0 (11) | C13—C14—H14A | 111.00 |
| C7—N3—H3N | 111.9 (11) | C13—C14—H14B | 111.00 |
| C10—N4—H4A | 124.00 | H14A—C14—H14B | 109.00 |
| C9—N4—H4A | 124.00 | C11—C15—H15A | 109.00 |
| N1—C1—C14 | 101.34 (12) | C11—C15—H15B | 109.00 |
| N1—C1—C2 | 117.02 (13) | C11—C15—H15C | 109.00 |
| C2—C1—C14 | 117.27 (13) | H15A—C15—H15B | 109.00 |
| C1—C2—C28 | 113.95 (13) | H15A—C15—H15C | 110.00 |
| C1—C2—C3 | 110.60 (13) | H15B—C15—H15C | 109.00 |
| C1—C2—C27 | 105.44 (13) | C11—C16—H16A | 109.00 |
| C27—C2—C28 | 108.30 (13) | C11—C16—H16B | 109.00 |
| C3—C2—C27 | 108.06 (13) | C11—C16—H16C | 110.00 |
| C3—C2—C28 | 110.20 (13) | H16A—C16—H16B | 109.00 |
| C2—C3—C26 | 130.52 (15) | H16A—C16—H16C | 109.00 |
| N2—C3—C26 | 106.43 (14) | H16B—C16—H16C | 109.00 |
| N2—C3—C2 | 123.03 (14) | C10—C17—H17 | 126.00 |
| C5—C4—C25 | 130.40 (15) | C18—C17—H17 | 126.00 |
| N2—C4—C5 | 123.19 (14) | C9—C18—H18 | 126.00 |
| N2—C4—C25 | 106.37 (14) | C17—C18—H18 | 126.00 |
| C4—C5—C6 | 111.30 (13) | C8—C19—H19A | 109.00 |
| C6—C5—C23 | 109.22 (13) | C8—C19—H19B | 109.00 |
| C4—C5—C23 | 111.77 (14) | C8—C19—H19C | 110.00 |
| C4—C5—C24 | 108.75 (14) | H19A—C19—H19B | 110.00 |
| C6—C5—C24 | 107.21 (14) | H19A—C19—H19C | 109.00 |
| C23—C5—C24 | 108.44 (14) | H19B—C19—H19C | 109.00 |
| N3—C6—C22 | 104.00 (13) | C8—C20—H20A | 109.00 |
| N3—C6—C5 | 113.18 (13) | C8—C20—H20B | 110.00 |
| C5—C6—C22 | 114.39 (13) | C8—C20—H20C | 109.00 |
| N3—C7—C8 | 114.92 (13) | H20A—C20—H20B | 110.00 |
| N3—C7—C21 | 100.76 (13) | H20A—C20—H20C | 110.00 |
| C8—C7—C21 | 118.57 (14) | H20B—C20—H20C | 109.00 |
| C7—C8—C20 | 108.51 (13) | C7—C21—H21A | 111.00 |
| C9—C8—C19 | 109.67 (13) | C7—C21—H21B | 111.00 |
| C7—C8—C9 | 114.89 (13) | C22—C21—H21A | 111.00 |
| C7—C8—C19 | 107.19 (13) | C22—C21—H21B | 111.00 |
| C9—C8—C20 | 107.94 (13) | H21A—C21—H21B | 109.00 |
| C19—C8—C20 | 108.49 (14) | C6—C22—H22A | 111.00 |
| N4—C9—C8 | 122.37 (14) | C6—C22—H22B | 111.00 |
| N4—C9—C18 | 106.23 (14) | C21—C22—H22A | 111.00 |
| C8—C9—C18 | 130.20 (15) | C21—C22—H22B | 111.00 |
| N4—C10—C17 | 105.94 (14) | H22A—C22—H22B | 109.00 |
| N4—C10—C11 | 122.17 (13) | C5—C23—H23A | 110.00 |
| C11—C10—C17 | 131.82 (15) | C5—C23—H23B | 110.00 |
| C12—C11—C16 | 105.33 (13) | C5—C23—H23C | 110.00 |
| C10—C11—C15 | 109.30 (13) | H23A—C23—H23B | 109.00 |
| C15—C11—C16 | 107.95 (13) | H23A—C23—H23C | 109.00 |
| C10—C11—C12 | 115.78 (13) | H23B—C23—H23C | 109.00 |
| C10—C11—C16 | 107.19 (13) | C5—C24—H24A | 109.00 |
| C12—C11—C15 | 110.90 (13) | C5—C24—H24B | 109.00 |
| N1—C12—C13 | 104.01 (12) | C5—C24—H24C | 109.00 |
| N1—C12—C11 | 116.90 (13) | H24A—C24—H24B | 110.00 |
| C11—C12—C13 | 115.38 (13) | H24A—C24—H24C | 109.00 |
| C12—C13—C14 | 104.81 (13) | H24B—C24—H24C | 109.00 |
| C1—C14—C13 | 105.49 (14) | C4—C25—H25 | 126.00 |
| C10—C17—C18 | 108.32 (15) | C26—C25—H25 | 126.00 |
| C9—C18—C17 | 108.28 (15) | C3—C26—H26 | 126.00 |
| C7—C21—C22 | 102.13 (13) | C25—C26—H26 | 126.00 |
| C6—C22—C21 | 105.24 (13) | C2—C27—H27A | 109.00 |
| C4—C25—C26 | 108.45 (15) | C2—C27—H27B | 110.00 |
| C3—C26—C25 | 108.11 (15) | C2—C27—H27C | 110.00 |
| O1—C29—N1 | 121.72 (14) | H27A—C27—H27B | 109.00 |
| N1—C29—C30 | 120.64 (13) | H27A—C27—H27C | 110.00 |
| O1—C29—C30 | 117.41 (14) | H27B—C27—H27C | 109.00 |
| C31—C30—C35 | 117.79 (15) | C2—C28—H28A | 109.00 |
| C29—C30—C35 | 115.71 (14) | C2—C28—H28B | 109.00 |
| C29—C30—C31 | 125.98 (14) | C2—C28—H28C | 109.00 |
| C30—C31—C32 | 121.51 (16) | H28A—C28—H28B | 110.00 |
| C31—C32—C33 | 119.49 (16) | H28A—C28—H28C | 109.00 |
| O2—C33—C32 | 125.09 (17) | H28B—C28—H28C | 109.00 |
| O2—C33—C34 | 115.15 (16) | C30—C31—H31 | 119.00 |
| C32—C33—C34 | 119.75 (17) | C32—C31—H31 | 119.00 |
| C33—C34—C35 | 120.14 (17) | C31—C32—H32 | 120.00 |
| C30—C35—C34 | 121.29 (16) | C33—C32—H32 | 120.00 |
| N1—C1—H1 | 107.00 | C33—C34—H34 | 120.00 |
| C2—C1—H1 | 107.00 | C35—C34—H34 | 120.00 |
| C14—C1—H1 | 107.00 | C30—C35—H35 | 119.00 |
| N3—C6—H6 | 108.00 | C34—C35—H35 | 119.00 |
| C5—C6—H6 | 108.00 | O2—C36—H36A | 109.00 |
| C22—C6—H6 | 108.00 | O2—C36—H36B | 109.00 |
| N3—C7—H7 | 107.00 | O2—C36—H36C | 109.00 |
| C8—C7—H7 | 107.00 | H36A—C36—H36B | 109.00 |
| C21—C7—H7 | 107.00 | H36A—C36—H36C | 109.00 |
| N1—C12—H12 | 107.00 | H36B—C36—H36C | 110.00 |
| C36—O2—C33—C32 | −4.9 (3) | C24—C5—C6—N3 | −167.61 (13) |
| C36—O2—C33—C34 | 174.72 (18) | C24—C5—C6—C22 | 73.50 (17) |
| C12—N1—C1—C2 | 107.23 (15) | N3—C6—C22—C21 | 0.44 (17) |
| C12—N1—C1—C14 | −21.59 (16) | C5—C6—C22—C21 | 124.39 (15) |
| C29—N1—C1—C2 | −111.23 (16) | N3—C7—C8—C9 | −48.77 (18) |
| C29—N1—C1—C14 | 119.95 (14) | N3—C7—C8—C19 | −170.90 (13) |
| C1—N1—C12—C11 | −127.09 (14) | N3—C7—C8—C20 | 72.12 (17) |
| C1—N1—C12—C13 | 1.36 (16) | C21—C7—C8—C9 | 70.41 (19) |
| C29—N1—C12—C11 | 90.47 (17) | C21—C7—C8—C19 | −51.73 (19) |
| C29—N1—C12—C13 | −141.08 (14) | C21—C7—C8—C20 | −168.70 (14) |
| C1—N1—C29—O1 | −149.49 (15) | N3—C7—C21—C22 | −43.89 (15) |
| C1—N1—C29—C30 | 36.2 (2) | C8—C7—C21—C22 | −170.18 (14) |
| C12—N1—C29—O1 | −9.7 (2) | C7—C8—C9—N4 | 52.8 (2) |
| C12—N1—C29—C30 | 175.94 (13) | C7—C8—C9—C18 | −141.52 (18) |
| C4—N2—C3—C2 | −177.68 (14) | C19—C8—C9—N4 | 173.56 (14) |
| C4—N2—C3—C26 | 0.71 (18) | C19—C8—C9—C18 | −20.7 (2) |
| C3—N2—C4—C5 | 177.31 (14) | C20—C8—C9—N4 | −68.43 (19) |
| C3—N2—C4—C25 | −0.66 (18) | C20—C8—C9—C18 | 97.3 (2) |
| C7—N3—C6—C5 | −153.56 (13) | N4—C9—C18—C17 | 0.74 (19) |
| C7—N3—C6—C22 | −28.83 (16) | C8—C9—C18—C17 | −166.71 (16) |
| C6—N3—C7—C8 | 174.58 (13) | N4—C10—C11—C12 | −109.38 (16) |
| C6—N3—C7—C21 | 45.89 (15) | N4—C10—C11—C15 | 16.7 (2) |
| C10—N4—C9—C8 | 168.50 (14) | N4—C10—C11—C16 | 133.45 (15) |
| C10—N4—C9—C18 | −0.16 (18) | C17—C10—C11—C12 | 74.0 (2) |
| C9—N4—C10—C11 | −177.86 (14) | C17—C10—C11—C15 | −159.91 (17) |
| C9—N4—C10—C17 | −0.49 (18) | C17—C10—C11—C16 | −43.2 (2) |
| N1—C1—C2—C3 | 52.15 (18) | N4—C10—C17—C18 | 0.94 (19) |
| N1—C1—C2—C27 | 168.72 (13) | C11—C10—C17—C18 | 177.95 (16) |
| N1—C1—C2—C28 | −72.64 (18) | C10—C11—C12—N1 | 75.88 (17) |
| C14—C1—C2—C3 | 172.90 (14) | C10—C11—C12—C13 | −46.87 (19) |
| C14—C1—C2—C27 | −70.53 (17) | C15—C11—C12—N1 | −49.38 (18) |
| C14—C1—C2—C28 | 48.11 (19) | C15—C11—C12—C13 | −172.14 (14) |
| N1—C1—C14—C13 | 33.50 (16) | C16—C11—C12—N1 | −165.92 (13) |
| C2—C1—C14—C13 | −95.17 (16) | C16—C11—C12—C13 | 71.33 (17) |
| C1—C2—C3—N2 | −83.99 (18) | N1—C12—C13—C14 | 19.90 (16) |
| C1—C2—C3—C26 | 98.0 (2) | C11—C12—C13—C14 | 149.28 (13) |
| C27—C2—C3—N2 | 161.07 (15) | C12—C13—C14—C1 | −33.92 (17) |
| C27—C2—C3—C26 | −16.9 (2) | C10—C17—C18—C9 | −1.1 (2) |
| C28—C2—C3—N2 | 42.9 (2) | C7—C21—C22—C6 | 26.63 (17) |
| C28—C2—C3—C26 | −135.07 (18) | C4—C25—C26—C3 | 0.09 (19) |
| N2—C3—C26—C25 | −0.48 (18) | O1—C29—C30—C31 | 108.99 (19) |
| C2—C3—C26—C25 | 177.74 (16) | O1—C29—C30—C35 | −62.4 (2) |
| N2—C4—C5—C6 | −53.7 (2) | N1—C29—C30—C31 | −76.4 (2) |
| N2—C4—C5—C23 | 68.7 (2) | N1—C29—C30—C35 | 112.15 (17) |
| N2—C4—C5—C24 | −171.63 (15) | C29—C30—C31—C32 | −171.98 (16) |
| C25—C4—C5—C6 | 123.70 (19) | C35—C30—C31—C32 | −0.7 (2) |
| C25—C4—C5—C23 | −113.9 (2) | C29—C30—C35—C34 | 173.61 (16) |
| C25—C4—C5—C24 | 5.8 (2) | C31—C30—C35—C34 | 1.4 (3) |
| N2—C4—C25—C26 | 0.34 (19) | C30—C31—C32—C33 | −0.5 (3) |
| C5—C4—C25—C26 | −177.43 (16) | C31—C32—C33—O2 | −179.42 (17) |
| C4—C5—C6—N3 | 73.58 (16) | C31—C32—C33—C34 | 1.0 (3) |
| C4—C5—C6—C22 | −45.31 (18) | O2—C33—C34—C35 | −179.91 (18) |
| C23—C5—C6—N3 | −50.31 (18) | C32—C33—C34—C35 | −0.3 (3) |
| C23—C5—C6—C22 | −169.19 (14) | C33—C34—C35—C30 | −1.0 (3) |
Hydrogen-bond geometry (Å, º)
Cg1 is the centroid of the pyrrole ring N2/C3/C4/C25/C26 and Cg2 is the centroid of the benzene ring C30–C35.
| D—H···A | D—H | H···A | D···A | D—H···A |
| N2—H2A···N3 | 0.88 | 2.57 | 3.0671 (18) | 117 |
| N4—H4A···N3 | 0.88 | 2.33 | 2.8759 (18) | 121 |
| C28—H28B···N4 | 0.98 | 2.59 | 3.561 (2) | 171 |
| C28—H28B···Cg1 | 0.98 | 2.45 | 3.3632 (18) | 155 |
| N3—H3N···O1i | 0.924 (18) | 2.283 (18) | 3.1401 (17) | 154.0 (15) |
| C20—H20B···O1i | 0.98 | 2.56 | 3.530 (2) | 170 |
| C15—H15A···Cg2i | 0.98 | 2.85 | 3.7176 (19) | 148 |
Symmetry code: (i) −x+1/2, y+1/2, −z+1/2.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: AA2052).
References
- Blangy, V., Heiss, C., Khlebnikov, V., Letondor, C., Stoeckli-Evans, H. & Neier, R. (2009). Angew. Chem. Int. Ed. 2009, 48, 1688–1691. [DOI] [PubMed]
- Journot, G. & Neier, R. (2012). In preparation.
- Journot, G., Neier, R. & Stoeckli-Evans, H. (2012a). Acta Cryst. C68, o119–o122. [DOI] [PubMed]
- Journot, G., Neier, R. & Stoeckli-Evans, H. (2012b). Acta Cryst. E68, o976–o977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Journot, G., Neier, R. & Stoeckli-Evans, H. (2012c). Private communication (deposition number CCDC-866917). CCDC, Cambridge, England.
- Journot, G., Neier, R. & Stoeckli-Evans, H. (2012d). Private communication (deposition number CCDC-866918). CCDC, Cambridge, England.
- Journot, G., Neier, R. & Stoeckli-Evans, H. (2012e). Private communication (deposition number CCDC-866919). CCDC, Cambridge, England.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Spek, A. L. (2009). Acta Cryst. D65, 148–155. [DOI] [PMC free article] [PubMed]
- Stoe & Cie (2009). X-AREA and X-RED32 Stoe & Cie GmbH, Darmstadt, Germany.
- Westrip, S. P. (2010). J. Appl. Cryst. 43, 920–925.
- Zhang, L., Wang, X.-J., Wang, J., Grinberg, N., Krishnamurthy, D. K. & Senanayake, C. H. (2009). Tetrahedron Lett. 50, 2964–2966.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S1600536812008008/aa2052sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536812008008/aa2052Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536812008008/aa2052Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report





