Abstract
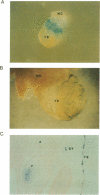
We have investigated the developmental and tissue specific expression of the human embryonic zeta-globin gene in transgenic mice. A construct containing 550 bp of zeta-globin 5' flanking region, fused to a beta-galactosidase (lacZ) reporter gene and linked to the locus control region (LCR)-like alpha positive regulatory element (alpha PRE) was employed for the production of transgenic mice. Firstly, we compared the number of live born transgenic mice containing this construct to the number of live born transgenic mice containing the entire zeta-globin gene linked to the alpha PRE or the beta LCR. Data showed that 12% of mice generated from eggs injected with zeta-promoter/lacZ/alpha PRE DNA were transgenic compared to only 2% of mice generated from eggs injected with the entire zeta-globin gene linked to the alpha PRE or the beta LCR. The reduced number of live born transgenic mice containing the latter constructs suggests that death of transgenic embryos, possibly due to thalassaemia, may be occurring. X-gal staining of whole embryos containing the lacZ gene revealed that zeta-globin promoter activity was most pronounced at 8.5-9.5 days of development and was restricted to erythroid cells. By 15 days of development, no zeta-globin promoter activity was detected. These results suggest that the alpha PRE can direct high level expression from the zeta-globin promoter and that sequences required for the correct tissue and developmental specific expression of the human zeta-globin gene are present within 550 bp's of 5' flanking region. Sequences within the body of the zeta-globin gene or 3' of the cap site do not appear to be necessary for correct zeta-globin developmental regulation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albitar M., Katsumata M., Liebhaber S. A. Human alpha-globin genes demonstrate autonomous developmental regulation in transgenic mice. Mol Cell Biol. 1991 Jul;11(7):3786–3794. doi: 10.1128/mcb.11.7.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Deisseroth A., Hendrick D. Activation of phenotypic expression of human globin genes from nonerythroid cells by chromosome-dependent transfer to tetraploid mouse erythroleukemia cells. Proc Natl Acad Sci U S A. 1979 May;76(5):2185–2189. doi: 10.1073/pnas.76.5.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester W. C., Novak U., Gelinas R., Groudine M. Molecular analysis of the human beta-globin locus activation region. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5439–5443. doi: 10.1073/pnas.86.14.5439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester W. C., Novak U., Gelinas R., Groudine M. Molecular analysis of the human beta-globin locus activation region. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5439–5443. doi: 10.1073/pnas.86.14.5439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosveld F., van Assendelft G. B., Greaves D. R., Kollias G. Position-independent, high-level expression of the human beta-globin gene in transgenic mice. Cell. 1987 Dec 24;51(6):975–985. doi: 10.1016/0092-8674(87)90584-8. [DOI] [PubMed] [Google Scholar]
- Hanscombe O., Vidal M., Kaeda J., Luzzatto L., Greaves D. R., Grosveld F. High-level, erythroid-specific expression of the human alpha-globin gene in transgenic mice and the production of human hemoglobin in murine erythrocytes. Genes Dev. 1989 Oct;3(10):1572–1581. doi: 10.1101/gad.3.10.1572. [DOI] [PubMed] [Google Scholar]
- Herbomel P., Bourachot B., Yaniv M. Two distinct enhancers with different cell specificities coexist in the regulatory region of polyoma. Cell. 1984 Dec;39(3 Pt 2):653–662. doi: 10.1016/0092-8674(84)90472-0. [DOI] [PubMed] [Google Scholar]
- Higgs D. R., Wood W. G., Jarman A. P., Sharpe J., Lida J., Pretorius I. M., Ayyub H. A major positive regulatory region located far upstream of the human alpha-globin gene locus. Genes Dev. 1990 Sep;4(9):1588–1601. doi: 10.1101/gad.4.9.1588. [DOI] [PubMed] [Google Scholar]
- Jarman A. P., Wood W. G., Sharpe J. A., Gourdon G., Ayyub H., Higgs D. R. Characterization of the major regulatory element upstream of the human alpha-globin gene cluster. Mol Cell Biol. 1991 Sep;11(9):4679–4689. doi: 10.1128/mcb.11.9.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazazian H. H., Jr, Woodhead A. P. Hemoglobin A synthesis in the developing fetus. N Engl J Med. 1973 Jul 12;289(2):58–62. doi: 10.1056/NEJM197307122890202. [DOI] [PubMed] [Google Scholar]
- Kollias G., Wrighton N., Hurst J., Grosveld F. Regulated expression of human A gamma-, beta-, and hybrid gamma beta-globin genes in transgenic mice: manipulation of the developmental expression patterns. Cell. 1986 Jul 4;46(1):89–94. doi: 10.1016/0092-8674(86)90862-7. [DOI] [PubMed] [Google Scholar]
- Magram J., Chada K., Costantini F. Developmental regulation of a cloned adult beta-globin gene in transgenic mice. Nature. 1985 May 23;315(6017):338–340. doi: 10.1038/315338a0. [DOI] [PubMed] [Google Scholar]
- Morley B. J., Abbott C. A., Sharpe J. A., Lida J., Chan-Thomas P. S., Wood W. G. A single beta-globin locus control region element (5' hypersensitive site 2) is sufficient for developmental regulation of human globin genes in transgenic mice. Mol Cell Biol. 1992 May;12(5):2057–2066. doi: 10.1128/mcb.12.5.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ney P. A., Sorrentino B. P., McDonagh K. T., Nienhuis A. W. Tandem AP-1-binding sites within the human beta-globin dominant control region function as an inducible enhancer in erythroid cells. Genes Dev. 1990 Jun;4(6):993–1006. doi: 10.1101/gad.4.6.993. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D., Brinster R. L. Germ-line transformation of mice. Annu Rev Genet. 1986;20:465–499. doi: 10.1146/annurev.ge.20.120186.002341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papayannopoulou T., Brice M., Stamatoyannopoulos G. Analysis of human hemoglobin switching in MEL x human fetal erythroid cell hybrids. Cell. 1986 Aug 1;46(3):469–476. doi: 10.1016/0092-8674(86)90667-7. [DOI] [PubMed] [Google Scholar]
- Peschle C., Mavilio F., Carè A., Migliaccio G., Migliaccio A. R., Salvo G., Samoggia P., Petti S., Guerriero R., Marinucci M. Haemoglobin switching in human embryos: asynchrony of zeta----alpha and epsilon----gamma-globin switches in primitive and definite erythropoietic lineage. Nature. 1985 Jan 17;313(5999):235–238. doi: 10.1038/313235a0. [DOI] [PubMed] [Google Scholar]
- Pondel M. D., George M., Proudfoot N. J. The LCR-like alpha-globin positive regulatory element functions as an enhancer in transiently transfected cells during erythroid differentiation. Nucleic Acids Res. 1992 Jan 25;20(2):237–243. doi: 10.1093/nar/20.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan T. M., Behringer R. R., Martin N. C., Townes T. M., Palmiter R. D., Brinster R. L. A single erythroid-specific DNase I super-hypersensitive site activates high levels of human beta-globin gene expression in transgenic mice. Genes Dev. 1989 Mar;3(3):314–323. doi: 10.1101/gad.3.3.314. [DOI] [PubMed] [Google Scholar]
- Sorrentino B., Ney P., Bodine D., Nienhius A. W. A 46 base pair enhancer sequence within the locus activating region is required for induced expression of the gamma-globin gene during erythroid differentiation. Nucleic Acids Res. 1990 May 11;18(9):2721–2731. doi: 10.1093/nar/18.9.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangler E. A., Andrews K. A., Rubin E. M. Developmental regulation of the human zeta globin gene in transgenic mice. Nucleic Acids Res. 1990 Dec 11;18(23):7093–7097. doi: 10.1093/nar/18.23.7093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot D., Collis P., Antoniou M., Vidal M., Grosveld F., Greaves D. R. A dominant control region from the human beta-globin locus conferring integration site-independent gene expression. Nature. 1989 Mar 23;338(6213):352–355. doi: 10.1038/338352a0. [DOI] [PubMed] [Google Scholar]
- Talbot D., Grosveld F. The 5'HS2 of the globin locus control region enhances transcription through the interaction of a multimeric complex binding at two functionally distinct NF-E2 binding sites. EMBO J. 1991 Jun;10(6):1391–1398. doi: 10.1002/j.1460-2075.1991.tb07659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townes T. M., Lingrel J. B., Chen H. Y., Brinster R. L., Palmiter R. D. Erythroid-specific expression of human beta-globin genes in transgenic mice. EMBO J. 1985 Jul;4(7):1715–1723. doi: 10.1002/j.1460-2075.1985.tb03841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas P., Vickers M. A., Simmons D. L., Ayyub H., Craddock C. F., Higgs D. R. Cis-acting sequences regulating expression of the human alpha-globin cluster lie within constitutively open chromatin. Cell. 1992 May 29;69(5):781–793. doi: 10.1016/0092-8674(92)90290-s. [DOI] [PubMed] [Google Scholar]
- Watt P., Lamb P., Squire L., Proudfoot N. A factor binding GATAAG confers tissue specificity on the promoter of the human zeta-globin gene. Nucleic Acids Res. 1990 Mar 25;18(6):1339–1350. doi: 10.1093/nar/18.6.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitelaw E., Tsai S. F., Hogben P., Orkin S. H. Regulated expression of globin chains and the erythroid transcription factor GATA-1 during erythropoiesis in the developing mouse. Mol Cell Biol. 1990 Dec;10(12):6596–6606. doi: 10.1128/mcb.10.12.6596. [DOI] [PMC free article] [PubMed] [Google Scholar]