Abstract
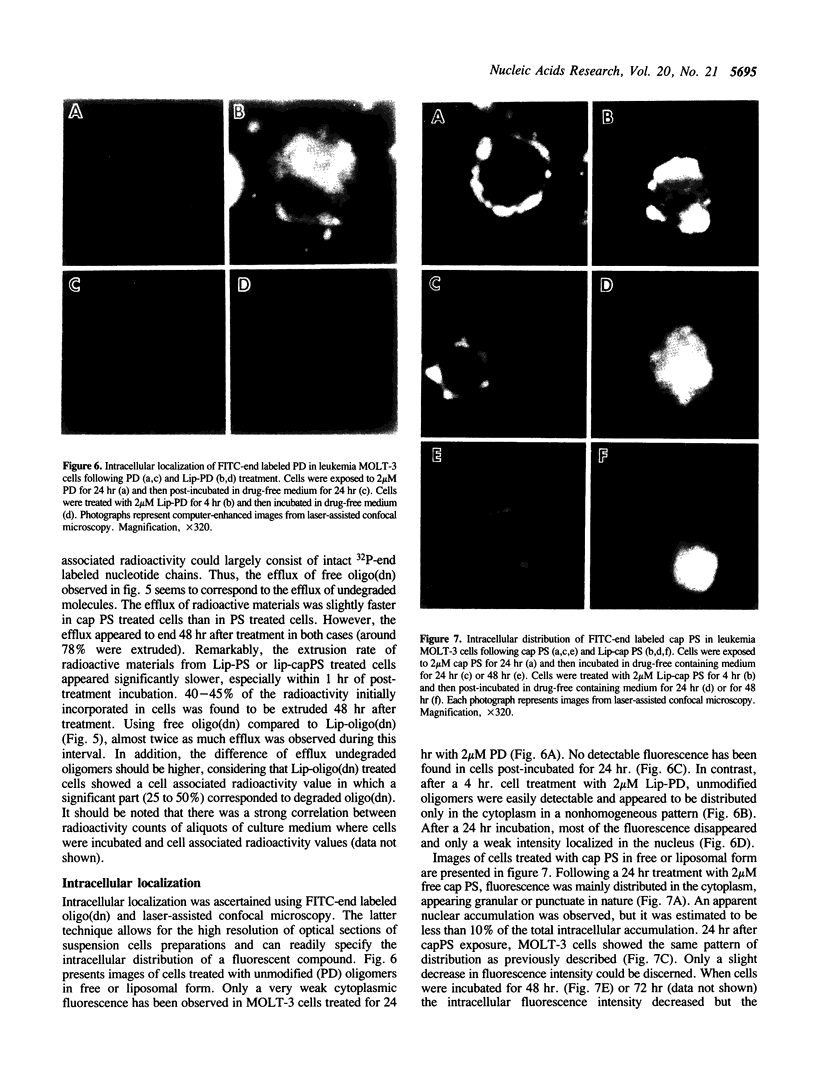
We have studied factors which may effect the intracellular availability of oligonucleotides to achieve antisense activity. 15-20 mer unmodified, phosphorothioate modified and liposomally encapsulated oligodeoxynucleotides have been tested in leukemia MOLT-3 cells. Phosphorothioate analogs penetrated and accumulated intact in cells in contrast to unmodified oligomers, which showed a high instability in cell culture medium. A slow decrease of intracellular concentration of undegraded phosphorothioate oligodeoxynucleotides was observed after cell treatment and could be predominantly explained by a significant efflux transport. Using laser-assisted confocal microscopy we have observed that fluorescein 5-end-labeled phosphorothioate derivatives predominantly distributed in intracytoplasmic endocytic vesicles following cell treatment. The end-capped version of phosphorothioate oligodeoxynucleotides exhibited greater cellular uptake than fully modified analogues while exhibiting similar biological stability. Liposome encapsulation made possible oligomer protection in serum-containing medium and substantially improved cellular accumulation. Furthermore, the efflux rate of oligomer initially introduced within liposomes is 2-fold lower than that observed in cells which have been incubated with free oligonucleotides. Liposomal preparations of oligodeoxynucleotides facilitate release from endocytic vesicles, and thus, cytoplasmic and nuclear localization are observed following cell treatment. Furthermore, intracellular distribution studies demonstrate that intracellular transport of unmodified oligomers is effectively achieved using the liposomal carrier.
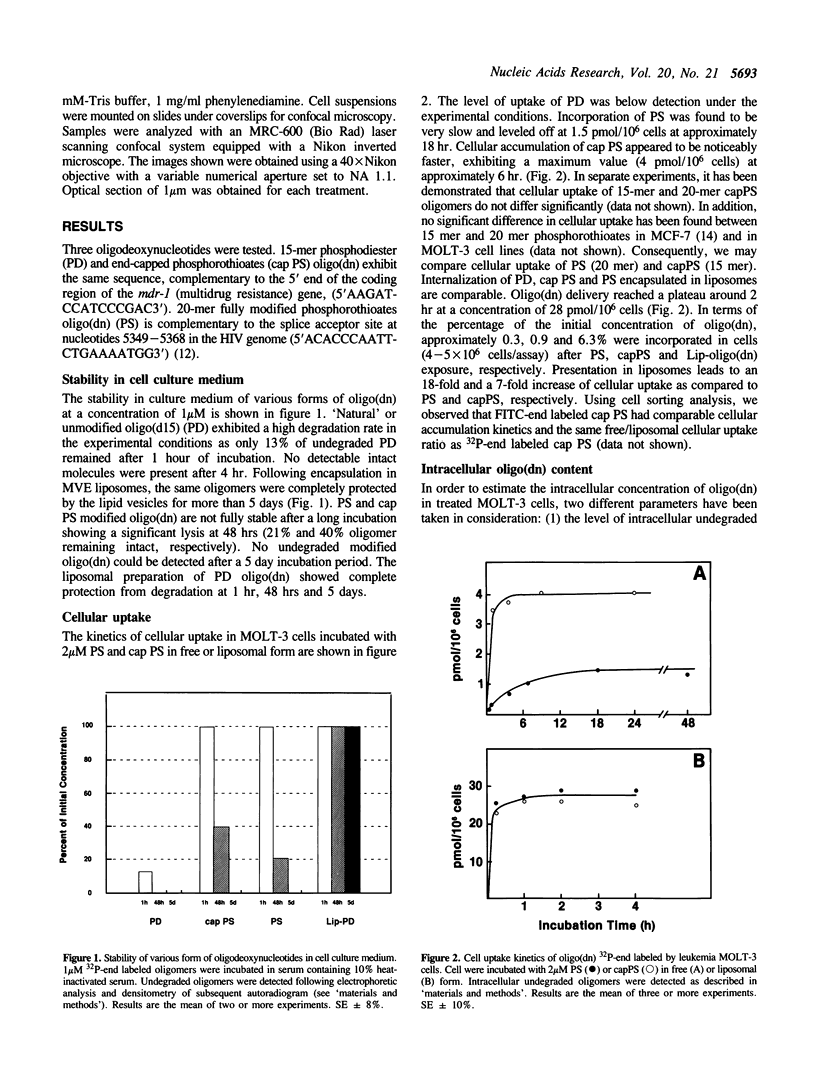
Full text
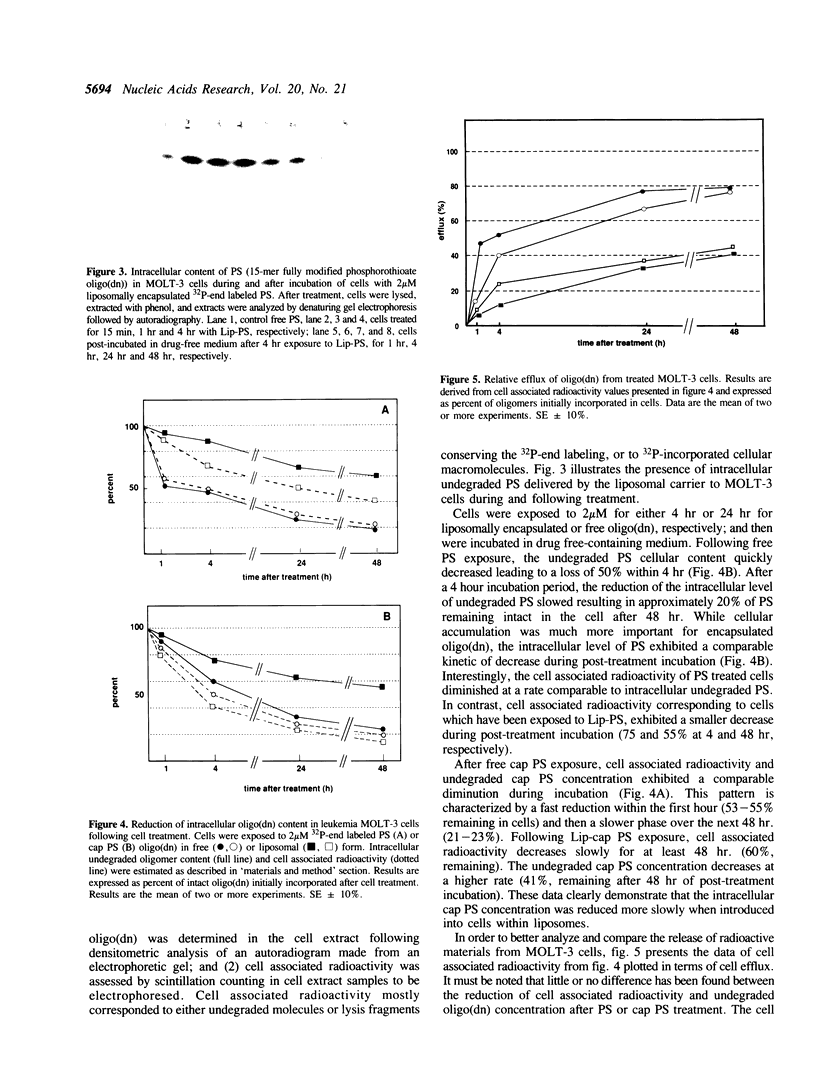
PDF




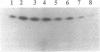
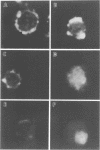
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agrawal S., Temsamani J., Tang J. Y. Pharmacokinetics, biodistribution, and stability of oligodeoxynucleotide phosphorothioates in mice. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7595–7599. doi: 10.1073/pnas.88.17.7595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhtar S., Basu S., Wickstrom E., Juliano R. L. Interactions of antisense DNA oligonucleotide analogs with phospholipid membranes (liposomes). Nucleic Acids Res. 1991 Oct 25;19(20):5551–5559. doi: 10.1093/nar/19.20.5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhtar S., Kole R., Juliano R. L. Stability of antisense DNA oligodeoxynucleotide analogs in cellular extracts and sera. Life Sci. 1991;49(24):1793–1801. doi: 10.1016/0024-3205(91)90480-y. [DOI] [PubMed] [Google Scholar]
- Bayard B., Leserman L. D., Bisbal C., Lebleu B. Antiviral activity in L1210 cells of liposome-encapsulated (2'-5')oligo(adenylate) analogues. Eur J Biochem. 1985 Sep 2;151(2):319–325. doi: 10.1111/j.1432-1033.1985.tb09103.x. [DOI] [PubMed] [Google Scholar]
- Cazenave C., Stein C. A., Loreau N., Thuong N. T., Neckers L. M., Subasinghe C., Hélène C., Cohen J. S., Toulmé J. J. Comparative inhibition of rabbit globin mRNA translation by modified antisense oligodeoxynucleotides. Nucleic Acids Res. 1989 Jun 12;17(11):4255–4273. doi: 10.1093/nar/17.11.4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin D. J., Green G. A., Zon G., Szoka F. C., Jr, Straubinger R. M. Rapid nuclear accumulation of injected oligodeoxyribonucleotides. New Biol. 1990 Dec;2(12):1091–1100. [PubMed] [Google Scholar]
- Crooke R. M. In vitro toxicology and pharmacokinetics of antisense oligonucleotides. Anticancer Drug Des. 1991 Dec;6(6):609–646. [PubMed] [Google Scholar]
- Florini J. R., Ewton D. Z. Highly specific inhibition of IGF-I-stimulated differentiation by an antisense oligodeoxyribonucleotide to myogenin mRNA. No effects on other actions of IGF-T. J Biol Chem. 1990 Aug 15;265(23):13435–13437. [PubMed] [Google Scholar]
- Gao W. Y., Jaroszewski J. W., Cohen J. S., Cheng Y. C. Mechanisms of inhibition of herpes simplex virus type 2 growth by 28-mer phosphorothioate oligodeoxycytidine. J Biol Chem. 1990 Nov 25;265(33):20172–20178. [PubMed] [Google Scholar]
- Harel-Bellan A., Ferris D. K., Vinocour M., Holt J. T., Farrar W. L. Specific inhibition of c-myc protein biosynthesis using an antisense synthetic deoxy-oligonucleotide in human T lymphocytes. J Immunol. 1988 Apr 1;140(7):2431–2435. [PubMed] [Google Scholar]
- Hashiro M., Matsumoto K., Okumura H., Hashimoto K., Yoshikawa K. Growth inhibition of human keratinocytes by antisense c-myc oligomer is not coupled to induction of differentiation. Biochem Biophys Res Commun. 1991 Jan 15;174(1):287–292. doi: 10.1016/0006-291x(91)90518-c. [DOI] [PubMed] [Google Scholar]
- Hélène C., Toulmé J. J. Specific regulation of gene expression by antisense, sense and antigene nucleic acids. Biochim Biophys Acta. 1990 Jun 21;1049(2):99–125. doi: 10.1016/0167-4781(90)90031-v. [DOI] [PubMed] [Google Scholar]
- Iversen P. In vivo studies with phosphorothioate oligonucleotides: pharmacokinetics prologue. Anticancer Drug Des. 1991 Dec;6(6):531–538. [PubMed] [Google Scholar]
- Jaroszewski J. W., Kaplan O., Syi J. L., Sehested M., Faustino P. J., Cohen J. S. Concerning antisense inhibition of the multiple drug resistance gene. Cancer Commun. 1990;2(8):287–294. doi: 10.3727/095535490820874254. [DOI] [PubMed] [Google Scholar]
- Leonetti J. P., Mechti N., Degols G., Gagnor C., Lebleu B. Intracellular distribution of microinjected antisense oligonucleotides. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2702–2706. doi: 10.1073/pnas.88.7.2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManaway M. E., Neckers L. M., Loke S. L., al-Nasser A. A., Redner R. L., Shiramizu B. T., Goldschmidts W. L., Huber B. E., Bhatia K., Magrath I. T. Tumour-specific inhibition of lymphoma growth by an antisense oligodeoxynucleotide. Lancet. 1990 Apr 7;335(8693):808–811. doi: 10.1016/0140-6736(90)90934-w. [DOI] [PubMed] [Google Scholar]
- Miller P. S., McParland K. B., Jayaraman K., Ts'o P. O. Biochemical and biological effects of nonionic nucleic acid methylphosphonates. Biochemistry. 1981 Mar 31;20(7):1874–1880. doi: 10.1021/bi00510a024. [DOI] [PubMed] [Google Scholar]
- Reed J. C., Stein C., Subasinghe C., Haldar S., Croce C. M., Yum S., Cohen J. Antisense-mediated inhibition of BCL2 protooncogene expression and leukemic cell growth and survival: comparisons of phosphodiester and phosphorothioate oligodeoxynucleotides. Cancer Res. 1990 Oct 15;50(20):6565–6570. [PubMed] [Google Scholar]
- Rothenberg M., Johnson G., Laughlin C., Green I., Cradock J., Sarver N., Cohen J. S. Oligodeoxynucleotides as anti-sense inhibitors of gene expression: therapeutic implications. J Natl Cancer Inst. 1989 Oct 18;81(20):1539–1544. doi: 10.1093/jnci/81.20.1539. [DOI] [PubMed] [Google Scholar]
- Sankar S., Cheah K. C., Porter A. G. Antisense oligonucleotide inhibition of encephalomyocarditis virus RNA translation. Eur J Biochem. 1989 Sep 1;184(1):39–45. doi: 10.1111/j.1432-1033.1989.tb14987.x. [DOI] [PubMed] [Google Scholar]
- Sarin P. S., Agrawal S., Civeira M. P., Goodchild J., Ikeuchi T., Zamecnik P. C. Inhibition of acquired immunodeficiency syndrome virus by oligodeoxynucleoside methylphosphonates. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7448–7451. doi: 10.1073/pnas.85.20.7448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw J. P., Kent K., Bird J., Fishback J., Froehler B. Modified deoxyoligonucleotides stable to exonuclease degradation in serum. Nucleic Acids Res. 1991 Feb 25;19(4):747–750. doi: 10.1093/nar/19.4.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thierry A. R., Dritschilo A. Liposomal delivery of antisense oligodeoxynucleotides. Application to the inhibition of the multidrug resistance in cancer cells. Ann N Y Acad Sci. 1992 Oct 28;660:300–302. doi: 10.1111/j.1749-6632.1992.tb21093.x. [DOI] [PubMed] [Google Scholar]
- Tidd D. M., Warenius H. M. Partial protection of oncogene, anti-sense oligodeoxynucleotides against serum nuclease degradation using terminal methylphosphonate groups. Br J Cancer. 1989 Sep;60(3):343–350. doi: 10.1038/bjc.1989.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickstrom E. L., Bacon T. A., Gonzalez A., Freeman D. L., Lyman G. H., Wickstrom E. Human promyelocytic leukemia HL-60 cell proliferation and c-myc protein expression are inhibited by an antisense pentadecadeoxynucleotide targeted against c-myc mRNA. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1028–1032. doi: 10.1073/pnas.85.4.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf T. M., Jennings C. G., Rebagliati M., Melton D. A. The stability, toxicity and effectiveness of unmodified and phosphorothioate antisense oligodeoxynucleotides in Xenopus oocytes and embryos. Nucleic Acids Res. 1990 Apr 11;18(7):1763–1769. doi: 10.1093/nar/18.7.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakubov L. A., Deeva E. A., Zarytova V. F., Ivanova E. M., Ryte A. S., Yurchenko L. V., Vlassov V. V. Mechanism of oligonucleotide uptake by cells: involvement of specific receptors? Proc Natl Acad Sci U S A. 1989 Sep;86(17):6454–6458. doi: 10.1073/pnas.86.17.6454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamecnik P. C., Goodchild J., Taguchi Y., Sarin P. S. Inhibition of replication and expression of human T-cell lymphotropic virus type III in cultured cells by exogenous synthetic oligonucleotides complementary to viral RNA. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4143–4146. doi: 10.1073/pnas.83.12.4143. [DOI] [PMC free article] [PubMed] [Google Scholar]