Abstract
The title compound congestiflorone, C28H32O4, which was isolated from the stem bark of Mesua congestiflora, consists of a benzophenone skeleton with two attached pyran rings to which a cyclohexane ring and a C6 side chain are bonded. The benzene ring is significantly distorted from planarity (r.m.s. deviation = 0.0007 Å) due to the constraints imposed by junctions with the two pyran rings. The cyclohexane ring is in a chair conformation, one pyran ring is in a boat conformation, while the other is a distorted chair. The phenyl and benzene rings make a dihedral angle of 55.85 (9)°. An intramolecular O—H⋯O hydrogen bond is observed. In the crystal, molecules are linked via C—H⋯O interactions.
Related literature
For phytochemical investigations of Mesua congestiflora, see: Awang et al. (2010 ▶); Bala & Seshadri (1971 ▶); Ee et al. (2005b
▶); Bandaranayak et al. (1975 ▶); Morel et al. (1999 ▶); Walia & Mukerjee (1984 ▶). For the biological activity of Congestiflora species, see: Pinto et al. (1994 ▶); Ee et al. (2005a
▶); Mazumder et al. (2004 ▶); Verotta et al. (2004 ▶); Huerta-Reyes et al. (2004 ▶). For related structures, see: Hua et al. (2008 ▶); Liu et al. (2005 ▶). For a description of the Cambridge Structural Database, see Allen (2002 ▶)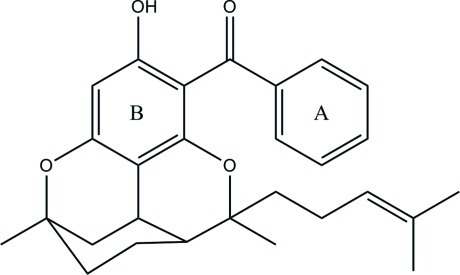
Experimental
Crystal data
C28H32O4
M r = 432.56
Triclinic,
a = 6.2022 (4) Å
b = 7.5220 (4) Å
c = 24.7673 (15) Å
α = 98.410 (5)°
β = 94.425 (5)°
γ = 94.200 (5)°
V = 1135.43 (12) Å3
Z = 2
Cu Kα radiation
μ = 0.66 mm−1
T = 150 K
0.29 × 0.09 × 0.05 mm
Data collection
Oxford Diffraction Gemin area-detector diffractometer
Absorption correction: multi-scan (CrysAlis PRO; Oxford Diffraction, 2006 ▶) T min = 0.942, T max = 0.968
15011 measured reflections
4340 independent reflections
3423 reflections with I > 2σ(I)
R int = 0.034
Refinement
R[F 2 > 2σ(F 2)] = 0.046
wR(F 2) = 0.117
S = 1.00
4321 reflections
289 parameters
H-atom parameters constrained
Δρmax = 0.39 e Å−3
Δρmin = −0.34 e Å−3
Data collection: CrysAlis CCD (Oxford Diffraction, 2006 ▶); cell refinement: CrysAlis CCD; data reduction: CrysAlis RED (Oxford Diffraction, 2006 ▶); program(s) used to solve structure: SIR92 (Altomare et al., 1994 ▶); program(s) used to refine structure: CRYSTALS (Betteridge et al., 2003 ▶); molecular graphics: Mercury (Macrae et al., 2006 ▶); software used to prepare material for publication: CRYSTALS.
Supplementary Material
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S1600536812010756/kp2392sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536812010756/kp2392Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C18—H181⋯O1i | 1.00 | 2.60 | 3.563 (3) | 162 |
| O11—H111⋯O1 | 0.87 | 1.78 | 2.551 (3) | 145 |
Symmetry code: (i) 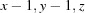 .
.
Acknowledgments
The authors are grateful to the Ministry of Science, Technology and Innovation (MOSTI) for a grant from the escience fund.
supplementary crystallographic information
Comment
Mesua congestiflora is native to Indonesia and is also distributed throughout Borneo, Sarawak. Previous phytochemical investigations on the genus show the existence of xanthones (Bandaranayak et al. 1975; Walia & Mukerjee 1984; Ee et al. 2005b), coumarins (Bandaranayak, Selliah et al. 1975; Morel, Guilet et al. 1999; Awang, Chan et al. 2010), terpenoids (Ee et al. 2005a) and essential oils (Bala & Seshadri 1971). These secondary metabolites have been extensively reported for their biological activities; for instance antifungal(Pinto et al. 1994), anticancer(Ee et al. 2005a), antibacterial (Mazumder, Dastidar et al. 2004; Verotta, Lovaglio et al. 2004), and anti-HIV-1(Huerta-Reyes et al. 2004). However, pharmacognosy and preliminary phytochemical analysis on this species have not been reported before.
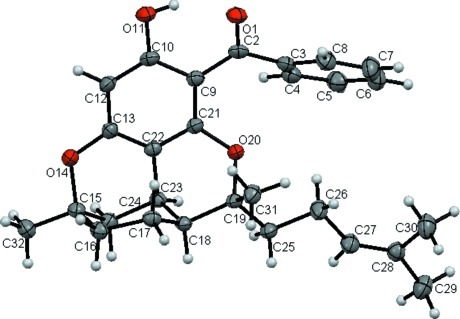
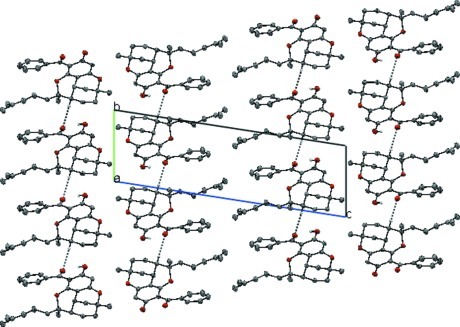
The title compound (I), congestiflorone C28H32O4 skeleton comprises five 6-membered rings and a 1-methylpent-2-enyl side chain (Fig. 1). The skeleton is similar to that of Sumadain A (Hua, Wang et al. 2008) except for the absence of 2 methylene groups next to the carbonyl group. Dihedral angle of those two benzene rings was 55.85 (9)°. The benzene ring (C9—C10—C12—C13—C21—C22) is not planar (the largest deviation from the best least squares plane is 0.082 (2) Å at C22). This departure from planarity of the ring A might be caused by the constraint of two adjacent pyrane rings which adapt a distorted chair conformation and a boat conformation. The cyclohexane ring adapted a chair conformation and the puckering parameter is Q= 0.5635 (18), θ= 166.13 (18)°, Φ2= 93.4 (8)°. The conformations of pyran and cyclohexane rings are comparable to the structure of Sumadain A (Hua, Wang et al. 2008). The orientation of the 1-methylpent-2-enly (C25—C28) side chain with respect to the cyclohexane ring is indicated by the torsion angle of C19—C25—C26—C27 = 177.17 (16)° and C25—C26—C27—C28=144.0 (2)° [169.5 (3)° and 145.4 (4)° respectively in Hua, Wang et al. 2008]. The structure of the molecule exhibits an intramolecular O—H···O hydrogen bond (Table 1). In the crystal, molecules are linked via a intermolecular C—H···O hydrogen bonding in the a,b-plane (Fig. 2).
The cystallographic data of this crystal structure has been deposited at Cambridge Crystallographic Data Center with deposition number CCDC 849099. (Allen, 2002)
Experimental
The stem bark of Mesua congestiflora was collected from the Sri Aman district in Sarawak, Malaysia. The sample (840 g) was milled, air-dried and ground, the powdered sample was extracted with n-hexane. The extract was dried under reduced pressure in a rotary evaporator to yield the hexane extract (5.50 g). Stepwise gradient systems using hexane/chloroform and chloroform/methanol or hexane/ethyl acetate and ethyl acetate/methanol, were applied for the separation and purification of the extract. Congestiflorone, a yellowish crystals with the melting point of 483 K were isolated. This compound was crystallised from slow evaporation of ethyl acetate at room temperature.
Refinement
The H atoms were all located in a difference map, but those attached to carbon atoms were repositioned geometrically. The H atoms were initially refined with soft restraints on the bond lengths and angles to regularize their geometry (C—H in the range 0.93–0.98, N—H in the range 0.86–0.89 N—H to 0.86 O—H = 0.82 Å) and Uiso(H) (in the range 1.2–1.5 times Ueq of the parent atom), after which the positions were refined with riding constraints.
Figures
Fig. 1.
The molecular structure of the title compound shows a 50% probability displacement ellipsoids and the atom-numbering scheme. The intramolecular hydrogen bond O11-H···O1 is observed.
Fig. 2.
The crystal packing of the title compound is viewed along the a axis. H atoms not involved in hydrogen bonds have been omitted for clarity. Hydrohen bonds are shown in dashed lines.
Crystal data
| C28H32O4 | Z = 2 |
| Mr = 432.56 | F(000) = 464 |
| Triclinic, P1 | Dx = 1.265 Mg m−3 |
| Hall symbol: -P 1 | Melting point: 483 K |
| a = 6.2022 (4) Å | Cu Kα radiation, λ = 1.54180 Å |
| b = 7.5220 (4) Å | Cell parameters from 4627 reflections |
| c = 24.7673 (15) Å | θ = 4–71° |
| α = 98.410 (5)° | µ = 0.66 mm−1 |
| β = 94.425 (5)° | T = 150 K |
| γ = 94.200 (5)° | Needle, yellow |
| V = 1135.43 (12) Å3 | 0.29 × 0.09 × 0.05 mm |
Data collection
| Oxford Diffraction Gemin area-detector diffractometer | 4340 independent reflections |
| Radiation source: sealed x-ray tube | 3423 reflections with I > 2σ(I) |
| Graphite monochromator | Rint = 0.034 |
| ω/2θ scans | θmax = 71.2°, θmin = 3.6° |
| Absorption correction: multi-scan (CrysAlis PRO; Oxford Diffraction, 2006) | h = −7→7 |
| Tmin = 0.942, Tmax = 0.968 | k = −9→9 |
| 15011 measured reflections | l = −30→30 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.046 | H-atom parameters constrained |
| wR(F2) = 0.117 | Method = Modified Sheldrick w = 1/[σ2(F2) + ( 0.05P)2 + 0.61P], where P = (max(Fo2,0) + 2Fc2)/3 |
| S = 1.00 | (Δ/σ)max = 0.001 |
| 4321 reflections | Δρmax = 0.39 e Å−3 |
| 289 parameters | Δρmin = −0.34 e Å−3 |
| 0 restraints |
Special details
| Refinement. For this compound, 15011 numbers of reflections were collected and measured during the refinement. Symmetry related reflections were measured more than once and after merging the symmetry equivalent reflections there were only 4340 reflection left. 19 more reflections were filtered, as σ cutoff was set as -3 and (sinθ/x)set to>0.01 (to eliminate reflection measured near the vicinity of beam stop) therefore numbers of reflection reduced to 4321. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| O1 | 1.3916 (2) | 0.62936 (17) | 0.77877 (5) | 0.0320 | |
| C2 | 1.2067 (3) | 0.5510 (2) | 0.76653 (7) | 0.0250 | |
| C3 | 1.1384 (3) | 0.4954 (2) | 0.70701 (7) | 0.0263 | |
| C4 | 0.9367 (3) | 0.5313 (2) | 0.68404 (8) | 0.0291 | |
| C5 | 0.8872 (3) | 0.4939 (3) | 0.62773 (8) | 0.0365 | |
| C6 | 1.0351 (4) | 0.4162 (3) | 0.59461 (8) | 0.0431 | |
| C7 | 1.2346 (4) | 0.3778 (3) | 0.61725 (9) | 0.0447 | |
| C8 | 1.2880 (3) | 0.4204 (3) | 0.67332 (8) | 0.0355 | |
| C9 | 1.0615 (3) | 0.5227 (2) | 0.80880 (7) | 0.0235 | |
| C10 | 1.1140 (3) | 0.6170 (2) | 0.86326 (7) | 0.0255 | |
| O11 | 1.2907 (2) | 0.73655 (17) | 0.87537 (5) | 0.0336 | |
| C12 | 0.9944 (3) | 0.5844 (2) | 0.90629 (7) | 0.0267 | |
| C13 | 0.8146 (3) | 0.4582 (2) | 0.89609 (7) | 0.0239 | |
| O14 | 0.7243 (2) | 0.40329 (16) | 0.93968 (5) | 0.0282 | |
| C15 | 0.5848 (3) | 0.2316 (2) | 0.92940 (7) | 0.0255 | |
| C16 | 0.7221 (3) | 0.0698 (2) | 0.91977 (7) | 0.0263 | |
| C17 | 0.8289 (3) | 0.0419 (2) | 0.86545 (7) | 0.0251 | |
| C18 | 0.6761 (3) | 0.0648 (2) | 0.81520 (7) | 0.0224 | |
| C19 | 0.7858 (3) | 0.0839 (2) | 0.76156 (7) | 0.0235 | |
| O20 | 0.82634 (19) | 0.27776 (16) | 0.75412 (5) | 0.0252 | |
| C21 | 0.8751 (3) | 0.3983 (2) | 0.80136 (7) | 0.0220 | |
| C22 | 0.7405 (3) | 0.3818 (2) | 0.84248 (7) | 0.0222 | |
| C23 | 0.5588 (3) | 0.2361 (2) | 0.82988 (7) | 0.0217 | |
| C24 | 0.4288 (3) | 0.2301 (2) | 0.87914 (7) | 0.0252 | |
| C25 | 0.6276 (3) | 0.0004 (2) | 0.71244 (7) | 0.0263 | |
| C26 | 0.6933 (3) | 0.0294 (3) | 0.65592 (8) | 0.0363 | |
| C27 | 0.5303 (3) | −0.0667 (3) | 0.61092 (8) | 0.0320 | |
| C28 | 0.5695 (3) | −0.1476 (3) | 0.56176 (7) | 0.0294 | |
| C29 | 0.3899 (3) | −0.2423 (3) | 0.52118 (8) | 0.0404 | |
| C30 | 0.7924 (3) | −0.1539 (3) | 0.54199 (9) | 0.0430 | |
| C31 | 1.0059 (3) | 0.0067 (3) | 0.75961 (8) | 0.0304 | |
| C32 | 0.4671 (3) | 0.2328 (3) | 0.98082 (8) | 0.0319 | |
| H41 | 0.8314 | 0.5832 | 0.7073 | 0.0367* | |
| H51 | 0.7488 | 0.5234 | 0.6122 | 0.0454* | |
| H61 | 0.9999 | 0.3875 | 0.5557 | 0.0533* | |
| H71 | 1.3367 | 0.3232 | 0.5942 | 0.0547* | |
| H81 | 1.4295 | 0.3981 | 0.6891 | 0.0457* | |
| H121 | 1.0361 | 0.6440 | 0.9423 | 0.0345* | |
| H161 | 0.8374 | 0.0796 | 0.9503 | 0.0335* | |
| H162 | 0.6217 | −0.0382 | 0.9209 | 0.0328* | |
| H171 | 0.9603 | 0.1294 | 0.8679 | 0.0317* | |
| H172 | 0.8805 | −0.0812 | 0.8599 | 0.0324* | |
| H181 | 0.5662 | −0.0425 | 0.8070 | 0.0291* | |
| H231 | 0.4619 | 0.2584 | 0.7977 | 0.0269* | |
| H241 | 0.3411 | 0.3350 | 0.8846 | 0.0319* | |
| H242 | 0.3299 | 0.1190 | 0.8740 | 0.0317* | |
| H251 | 0.6091 | −0.1324 | 0.7137 | 0.0342* | |
| H252 | 0.4852 | 0.0508 | 0.7176 | 0.0341* | |
| H261 | 0.8365 | −0.0145 | 0.6505 | 0.0461* | |
| H262 | 0.7055 | 0.1603 | 0.6535 | 0.0467* | |
| H271 | 0.3814 | −0.0696 | 0.6200 | 0.0408* | |
| H291 | 0.3915 | −0.1929 | 0.4867 | 0.0632* | |
| H292 | 0.4065 | −0.3713 | 0.5141 | 0.0633* | |
| H293 | 0.2496 | −0.2260 | 0.5358 | 0.0628* | |
| H301 | 0.7949 | −0.1071 | 0.5070 | 0.0677* | |
| H302 | 0.8301 | −0.2777 | 0.5363 | 0.0685* | |
| H303 | 0.9008 | −0.0786 | 0.5679 | 0.0685* | |
| H311 | 1.0584 | 0.0141 | 0.7238 | 0.0482* | |
| H312 | 1.1094 | 0.0772 | 0.7879 | 0.0485* | |
| H313 | 0.9902 | −0.1190 | 0.7658 | 0.0489* | |
| H323 | 0.3689 | 0.1245 | 0.9770 | 0.0491* | |
| H322 | 0.5668 | 0.2357 | 1.0134 | 0.0499* | |
| H321 | 0.3815 | 0.3378 | 0.9856 | 0.0494* | |
| H111 | 1.3748 | 0.7225 | 0.8486 | 0.0530* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| O1 | 0.0270 (7) | 0.0331 (7) | 0.0343 (7) | −0.0087 (6) | 0.0017 (5) | 0.0060 (6) |
| C2 | 0.0252 (9) | 0.0171 (9) | 0.0327 (10) | −0.0010 (7) | 0.0009 (7) | 0.0060 (7) |
| C3 | 0.0282 (9) | 0.0220 (9) | 0.0289 (9) | −0.0045 (7) | 0.0043 (7) | 0.0067 (7) |
| C4 | 0.0316 (10) | 0.0255 (10) | 0.0307 (10) | −0.0008 (8) | 0.0052 (8) | 0.0064 (8) |
| C5 | 0.0369 (11) | 0.0391 (12) | 0.0333 (11) | −0.0020 (9) | −0.0017 (8) | 0.0103 (9) |
| C6 | 0.0479 (13) | 0.0531 (14) | 0.0259 (10) | −0.0068 (11) | 0.0022 (9) | 0.0043 (9) |
| C7 | 0.0419 (12) | 0.0568 (14) | 0.0341 (11) | 0.0006 (10) | 0.0143 (9) | −0.0010 (10) |
| C8 | 0.0297 (10) | 0.0410 (12) | 0.0356 (11) | 0.0000 (9) | 0.0073 (8) | 0.0046 (9) |
| C9 | 0.0252 (9) | 0.0196 (9) | 0.0257 (9) | 0.0003 (7) | 0.0007 (7) | 0.0045 (7) |
| C10 | 0.0263 (9) | 0.0182 (9) | 0.0308 (10) | −0.0024 (7) | −0.0020 (7) | 0.0047 (7) |
| O11 | 0.0342 (7) | 0.0309 (7) | 0.0319 (7) | −0.0133 (6) | −0.0005 (6) | 0.0020 (6) |
| C12 | 0.0331 (10) | 0.0213 (9) | 0.0233 (9) | −0.0015 (8) | −0.0005 (7) | −0.0012 (7) |
| C13 | 0.0263 (9) | 0.0201 (9) | 0.0254 (9) | 0.0025 (7) | 0.0052 (7) | 0.0020 (7) |
| O14 | 0.0326 (7) | 0.0272 (7) | 0.0227 (6) | −0.0052 (5) | 0.0049 (5) | −0.0005 (5) |
| C15 | 0.0246 (9) | 0.0251 (9) | 0.0256 (9) | −0.0033 (7) | 0.0036 (7) | 0.0024 (7) |
| C16 | 0.0254 (9) | 0.0280 (10) | 0.0257 (9) | −0.0002 (7) | −0.0001 (7) | 0.0071 (7) |
| C17 | 0.0241 (9) | 0.0220 (9) | 0.0295 (9) | 0.0022 (7) | 0.0025 (7) | 0.0050 (7) |
| C18 | 0.0211 (8) | 0.0197 (9) | 0.0254 (9) | −0.0023 (7) | 0.0034 (7) | 0.0014 (7) |
| C19 | 0.0224 (9) | 0.0199 (9) | 0.0264 (9) | −0.0036 (7) | 0.0031 (7) | 0.0003 (7) |
| O20 | 0.0300 (7) | 0.0226 (6) | 0.0213 (6) | −0.0055 (5) | 0.0033 (5) | 0.0009 (5) |
| C21 | 0.0234 (8) | 0.0189 (9) | 0.0228 (9) | 0.0008 (7) | −0.0009 (7) | 0.0025 (7) |
| C22 | 0.0224 (8) | 0.0188 (9) | 0.0254 (9) | 0.0026 (7) | 0.0010 (7) | 0.0035 (7) |
| C23 | 0.0191 (8) | 0.0231 (9) | 0.0228 (9) | 0.0004 (7) | 0.0006 (7) | 0.0039 (7) |
| C24 | 0.0220 (9) | 0.0269 (9) | 0.0266 (9) | 0.0012 (7) | 0.0033 (7) | 0.0037 (7) |
| C25 | 0.0247 (9) | 0.0247 (9) | 0.0270 (9) | −0.0038 (7) | 0.0030 (7) | −0.0013 (7) |
| C26 | 0.0315 (10) | 0.0470 (13) | 0.0264 (10) | −0.0084 (9) | 0.0046 (8) | −0.0027 (9) |
| C27 | 0.0267 (9) | 0.0387 (11) | 0.0291 (10) | −0.0007 (8) | 0.0037 (8) | 0.0011 (8) |
| C28 | 0.0323 (10) | 0.0303 (10) | 0.0254 (9) | 0.0032 (8) | 0.0012 (8) | 0.0040 (8) |
| C29 | 0.0416 (12) | 0.0440 (13) | 0.0321 (11) | 0.0039 (10) | −0.0013 (9) | −0.0041 (9) |
| C30 | 0.0398 (12) | 0.0566 (14) | 0.0316 (11) | 0.0061 (10) | 0.0092 (9) | −0.0002 (10) |
| C31 | 0.0249 (9) | 0.0349 (11) | 0.0296 (10) | 0.0009 (8) | 0.0043 (7) | −0.0015 (8) |
| C32 | 0.0332 (10) | 0.0350 (11) | 0.0277 (10) | −0.0017 (8) | 0.0076 (8) | 0.0050 (8) |
Geometric parameters (Å, º)
| O1—C2 | 1.245 (2) | C18—C23 | 1.537 (2) |
| C2—C3 | 1.492 (3) | C18—H181 | 1.004 |
| C2—C9 | 1.461 (2) | C19—O20 | 1.502 (2) |
| C3—C4 | 1.395 (3) | C19—C25 | 1.530 (2) |
| C3—C8 | 1.391 (3) | C19—C31 | 1.523 (2) |
| C4—C5 | 1.388 (3) | O20—C21 | 1.370 (2) |
| C4—H41 | 0.969 | C21—C22 | 1.379 (2) |
| C5—C6 | 1.382 (3) | C22—C23 | 1.494 (2) |
| C5—H51 | 0.967 | C23—C24 | 1.518 (2) |
| C6—C7 | 1.386 (3) | C23—H231 | 1.003 |
| C6—H61 | 0.961 | C24—H241 | 0.990 |
| C7—C8 | 1.387 (3) | C24—H242 | 0.986 |
| C7—H71 | 0.959 | C25—C26 | 1.529 (2) |
| C8—H81 | 0.969 | C25—H251 | 1.002 |
| C9—C10 | 1.431 (2) | C25—H252 | 0.996 |
| C9—C21 | 1.416 (2) | C26—C27 | 1.506 (3) |
| C10—O11 | 1.351 (2) | C26—H261 | 0.982 |
| C10—C12 | 1.385 (2) | C26—H262 | 0.993 |
| O11—H111 | 0.874 | C27—C28 | 1.328 (3) |
| C12—C13 | 1.393 (2) | C27—H271 | 0.967 |
| C12—H121 | 0.945 | C28—C29 | 1.503 (3) |
| C13—O14 | 1.357 (2) | C28—C30 | 1.504 (3) |
| C13—C22 | 1.396 (2) | C29—H291 | 0.981 |
| O14—C15 | 1.479 (2) | C29—H292 | 0.975 |
| C15—C16 | 1.536 (2) | C29—H293 | 0.977 |
| C15—C24 | 1.515 (2) | C30—H301 | 0.982 |
| C15—C32 | 1.515 (2) | C30—H302 | 0.970 |
| C16—C17 | 1.539 (2) | C30—H303 | 0.978 |
| C16—H161 | 0.991 | C31—H311 | 0.976 |
| C16—H162 | 0.992 | C31—H312 | 0.978 |
| C17—C18 | 1.543 (2) | C31—H313 | 0.979 |
| C17—H171 | 1.001 | C32—H323 | 0.969 |
| C17—H172 | 0.996 | C32—H322 | 0.975 |
| C18—C19 | 1.559 (2) | C32—H321 | 0.982 |
| O1—C2—C3 | 116.89 (16) | O20—C19—C31 | 105.61 (13) |
| O1—C2—C9 | 120.99 (16) | C25—C19—C31 | 112.13 (14) |
| C3—C2—C9 | 122.07 (15) | C19—O20—C21 | 115.62 (12) |
| C2—C3—C4 | 121.53 (16) | C9—C21—O20 | 121.81 (15) |
| C2—C3—C8 | 118.50 (16) | C9—C21—C22 | 122.22 (16) |
| C4—C3—C8 | 119.73 (17) | O20—C21—C22 | 115.80 (15) |
| C3—C4—C5 | 119.91 (18) | C13—C22—C21 | 118.67 (16) |
| C3—C4—H41 | 120.2 | C13—C22—C23 | 122.24 (15) |
| C5—C4—H41 | 119.9 | C21—C22—C23 | 116.14 (15) |
| C4—C5—C6 | 120.00 (19) | C18—C23—C22 | 103.24 (13) |
| C4—C5—H51 | 119.1 | C18—C23—C24 | 112.94 (14) |
| C6—C5—H51 | 120.9 | C22—C23—C24 | 110.36 (14) |
| C5—C6—C7 | 120.37 (19) | C18—C23—H231 | 110.6 |
| C5—C6—H61 | 120.2 | C22—C23—H231 | 109.6 |
| C7—C6—H61 | 119.4 | C24—C23—H231 | 109.9 |
| C6—C7—C8 | 119.9 (2) | C23—C24—C15 | 108.64 (14) |
| C6—C7—H71 | 120.2 | C23—C24—H241 | 110.7 |
| C8—C7—H71 | 119.9 | C15—C24—H241 | 109.7 |
| C3—C8—C7 | 120.02 (19) | C23—C24—H242 | 110.5 |
| C3—C8—H81 | 119.9 | C15—C24—H242 | 108.7 |
| C7—C8—H81 | 120.1 | H241—C24—H242 | 108.4 |
| C2—C9—C10 | 119.22 (15) | C19—C25—C26 | 116.47 (15) |
| C2—C9—C21 | 124.97 (16) | C19—C25—H251 | 106.8 |
| C10—C9—C21 | 115.68 (15) | C26—C25—H251 | 108.8 |
| C9—C10—O11 | 120.83 (16) | C19—C25—H252 | 107.8 |
| C9—C10—C12 | 122.15 (16) | C26—C25—H252 | 108.3 |
| O11—C10—C12 | 116.94 (16) | H251—C25—H252 | 108.4 |
| C10—O11—H111 | 109.2 | C25—C26—C27 | 111.43 (16) |
| C10—C12—C13 | 119.00 (16) | C25—C26—H261 | 109.8 |
| C10—C12—H121 | 120.7 | C27—C26—H261 | 109.3 |
| C13—C12—H121 | 120.2 | C25—C26—H262 | 109.7 |
| C12—C13—O14 | 118.03 (15) | C27—C26—H262 | 109.1 |
| C12—C13—C22 | 120.50 (16) | H261—C26—H262 | 107.4 |
| O14—C13—C22 | 121.31 (16) | C26—C27—C28 | 127.51 (18) |
| C13—O14—C15 | 117.10 (13) | C26—C27—H271 | 114.7 |
| O14—C15—C16 | 110.88 (14) | C28—C27—H271 | 117.8 |
| O14—C15—C24 | 109.24 (14) | C27—C28—C29 | 121.68 (18) |
| C16—C15—C24 | 108.97 (14) | C27—C28—C30 | 123.77 (18) |
| O14—C15—C32 | 103.92 (14) | C29—C28—C30 | 114.55 (17) |
| C16—C15—C32 | 112.01 (15) | C28—C29—H291 | 110.1 |
| C24—C15—C32 | 111.74 (15) | C28—C29—H292 | 110.3 |
| C15—C16—C17 | 116.82 (14) | H291—C29—H292 | 109.4 |
| C15—C16—H161 | 109.1 | C28—C29—H293 | 109.9 |
| C17—C16—H161 | 108.3 | H291—C29—H293 | 109.0 |
| C15—C16—H162 | 105.6 | H292—C29—H293 | 108.0 |
| C17—C16—H162 | 108.1 | C28—C30—H301 | 110.0 |
| H161—C16—H162 | 108.7 | C28—C30—H302 | 109.5 |
| C16—C17—C18 | 113.52 (14) | H301—C30—H302 | 108.5 |
| C16—C17—H171 | 109.4 | C28—C30—H303 | 111.4 |
| C18—C17—H171 | 108.8 | H301—C30—H303 | 107.1 |
| C16—C17—H172 | 108.9 | H302—C30—H303 | 110.3 |
| C18—C17—H172 | 109.1 | C19—C31—H311 | 109.0 |
| H171—C17—H172 | 106.9 | C19—C31—H312 | 109.6 |
| C17—C18—C19 | 116.21 (14) | H311—C31—H312 | 108.8 |
| C17—C18—C23 | 108.18 (14) | C19—C31—H313 | 109.2 |
| C19—C18—C23 | 106.99 (13) | H311—C31—H313 | 110.3 |
| C17—C18—H181 | 108.4 | H312—C31—H313 | 109.9 |
| C19—C18—H181 | 107.7 | C15—C32—H323 | 109.1 |
| C23—C18—H181 | 109.2 | C15—C32—H322 | 112.2 |
| C18—C19—O20 | 111.90 (13) | H323—C32—H322 | 108.1 |
| C18—C19—C25 | 108.74 (14) | C15—C32—H321 | 109.6 |
| O20—C19—C25 | 104.37 (13) | H323—C32—H321 | 108.5 |
| C18—C19—C31 | 113.70 (14) | H322—C32—H321 | 109.3 |
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C18—H181···O1i | 1.00 | 2.60 | 3.563 (3) | 162 |
| O11—H111···O1 | 0.87 | 1.78 | 2.551 (3) | 145 |
Symmetry code: (i) x−1, y−1, z.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: KP2392).
References
- Allen, F. H. (2002). Acta Cryst. B58, 380–388. [DOI] [PubMed]
- Altomare, A., Cascarano, G., Giacovazzo, C., Guagliardi, A., Burla, M. C., Polidori, G. & Camalli, M. (1994). J. Appl. Cryst. 27, 435.
- Awang, K., Chan, G., Litaudon, M., Ismail, N. H., Martin, M. T. & Gueritte, F. (2010). Bioorg. Med. Chem. 18, 7873–7877. [DOI] [PubMed]
- Bala, K. R. & Seshadri, T. R. (1971). Phytochemistry, 10, 1131–1134.
- Bandaranayak, W. M., Selliah, S. S. & Sultanbawa, M. U. S. (1975). Phytochemistry, 14, 265–269.
- Betteridge, P. W., Carruthers, J. R., Cooper, R. I., Prout, K. & Watkin, D. J. (2003). J. Appl. Cryst. 36, 1487.
- Ee, G. C. L., Lim, C. K., Cheow, Y. L. & Sukari, M. A. (2005a). Malays. J. Sci. 24, 183–185.
- Ee, G. C. L., Lim, C. K., Rahmat, A. & Lee, H. L. (2005b). Trop. Biomed. 22., 99–102. [PubMed]
- Hua, S.-Z., Wang, X.-B., Luo, J.-G., Wang, J.-S. & Kong, L.-Y. (2008). Tetrahedron Lett. 49, 5658–5661.
- Huerta-Reyes, M., Basualdo Mdel, C., Abe, F., Jimenez-Estrada, M., Soler, C. & Reyes-Chilpa, R. (2004). Biol. Pharm. Bull. 27., 1471–1475. [DOI] [PubMed]
- Liu, K.-Y., Gao, W.-Y., Zhang, T.-J., Chen, H.-X. & Zhou, B. (2005). Acta Cryst. E61, o391–o392.
- Macrae, C. F., Edgington, P. R., McCabe, P., Pidcock, E., Shields, G. P., Taylor, R., Towler, M. & van de Streek, J. (2006). J. Appl. Cryst. 39, 453–457.
- Mazumder, R., Dastidar, S. G., Basu, S. P., Mazumder, A. & Singh, S. K. (2004). Phytother. Res. 18, 824–826. [DOI] [PubMed]
- Morel, C., Guilet, D., Oger, J. M., Seraphin, D., Sevenet, T., Wiart, C., Hadi, A. H. A., Richomme, P. & Bruneton, J. (1999). Phytochemistry, 50., 1243–1247.
- Oxford Diffraction (2006). CrysAlis CCD and CrysAlis RED Oxford Diffraction Ltd, Abingdon, England.
- Pinto, D. C. G., Fuzzati, N., Pazmino, X. C. & Hostettmann, K. (1994). Phytochemistry, 3 . 875-878. [DOI] [PubMed]
- Verotta, L., Lovaglio, E., Vidari, G., Finzi, P. V., Neri, M. G., Raimondi, A., Parapini, S., Taramelli, D., Riva, A. & Bombardelli, E. (2004). Phytochemistry, 65, 2867–2879. [DOI] [PubMed]
- Walia, S. & Mukerjee, S. K. (1984). Phytochemistry, 23, 1816–1817.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S1600536812010756/kp2392sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536812010756/kp2392Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report