Abstract
The genes encoding the endonuclease and the methylase of the PvuI restriction and modification system were cloned in E.coli and characterized. The genes were adjacent in tandem orientation spanning a distance of 2200 bases. The PvuI endonuclease was a single polypeptide with a calculated molecular weight of 27,950 daltons. The endonuclease was easily detectable when the gene was expressed from its endogenous promotor and present on a low copy plasmid, but expression was considerably enhanced when the endonuclease gene was placed under the control of a strong promoter on a high copy plasmid. The methylase did not completely protect plasmid DNA from R.PvuI digestion until the methylase gene was placed under lac promotor control in a multicopy plasmid. In the absence of the M.PvuI methylase, expression of the R.PvuI endonuclease from the lac promotor on a multicopy plasmid was not lethal to wild type E.coli, but was lethal in a temperature-sensitive ligase mutant at the non-permissive temperature. Moreover, induction of the R.PvuI endonuclease under lambda pL promotor control resulted in complete digestion of the E.coli chromosome by R.PvuI.
Full text
PDF

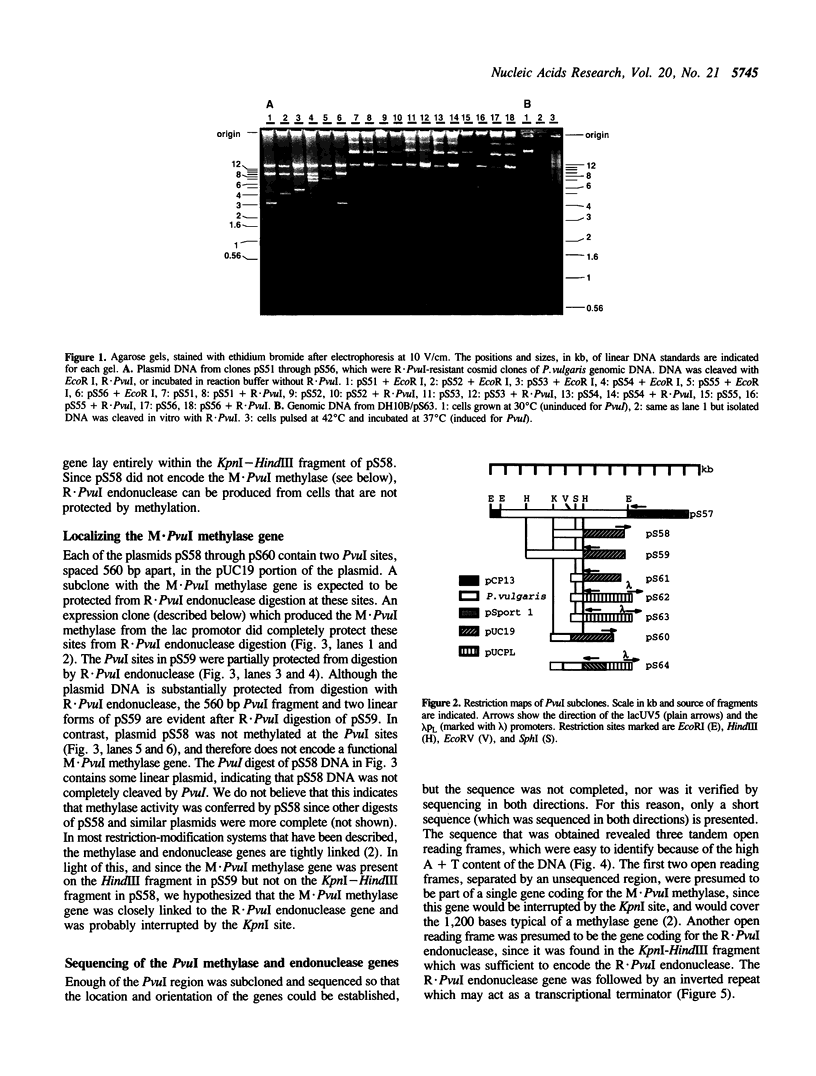
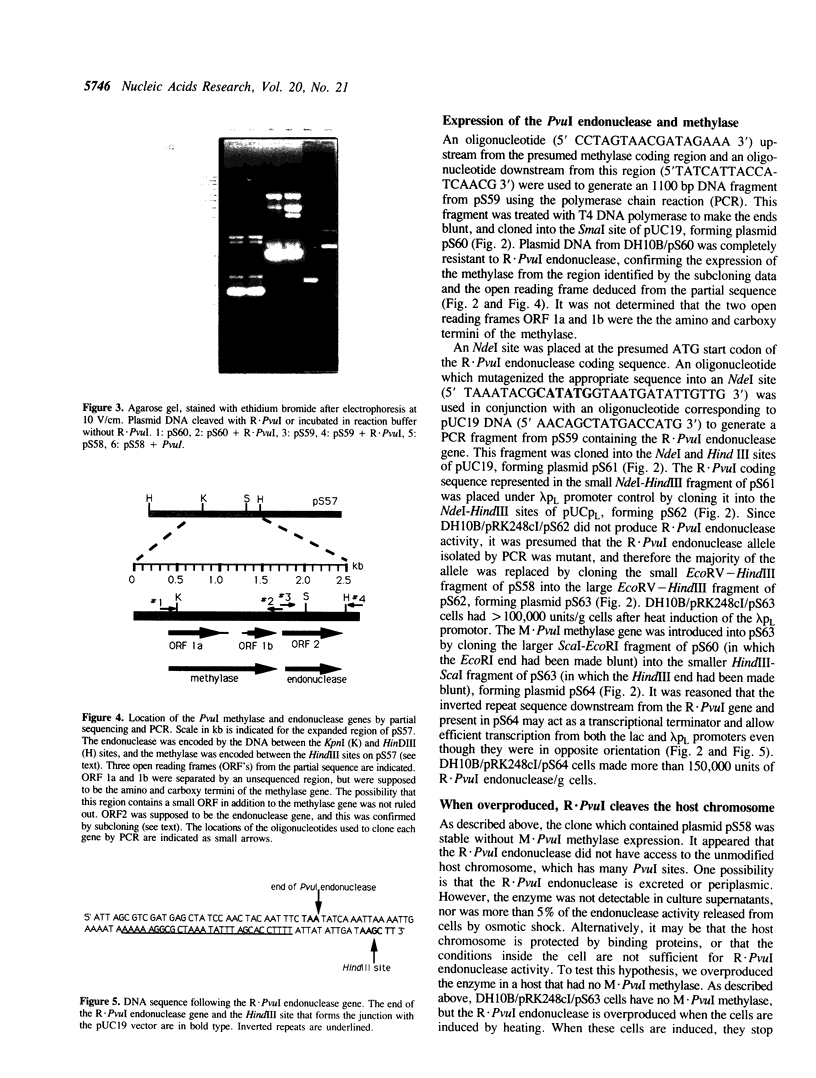

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barany F. A genetic system for isolation and characterization of TaqI restriction endonuclease mutants. Gene. 1987;56(1):13–27. doi: 10.1016/0378-1119(87)90154-5. [DOI] [PubMed] [Google Scholar]
- Bernard H. U., Remaut E., Hershfield M. V., Das H. K., Helinski D. R., Yanofsky C., Franklin N. Construction of plasmid cloning vehicles that promote gene expression from the bacteriophage lambda pL promoter. Gene. 1979 Jan;5(1):59–76. doi: 10.1016/0378-1119(79)90092-1. [DOI] [PubMed] [Google Scholar]
- Chatterjee D. K., Fujimura R. K., Campbell J. H., Gerard G. F. Cloning and overexpression of the gene encoding bacteriophage T5 DNA polymerase. Gene. 1991 Jan 2;97(1):13–19. doi: 10.1016/0378-1119(91)90004-u. [DOI] [PubMed] [Google Scholar]
- Cheng S. C., Modrich P. Positive-selection cloning vehicle useful for overproduction of hybrid proteins. J Bacteriol. 1983 May;154(2):1005–1008. doi: 10.1128/jb.154.2.1005-1008.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordonnier C., Bernardi G. Localization of E. coli endonuclease I. Biochem Biophys Res Commun. 1965 Sep 8;20(5):555–559. doi: 10.1016/0006-291x(65)90434-1. [DOI] [PubMed] [Google Scholar]
- Darzins A., Chakrabarty A. M. Cloning of genes controlling alginate biosynthesis from a mucoid cystic fibrosis isolate of Pseudomonas aeruginosa. J Bacteriol. 1984 Jul;159(1):9–18. doi: 10.1128/jb.159.1.9-18.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingeras T. R., Brooks J. E. Cloned restriction/modification system from Pseudomonas aeruginosa. Proc Natl Acad Sci U S A. 1983 Jan;80(2):402–406. doi: 10.1073/pnas.80.2.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingeras T. R., Greenough L., Schildkraut I., Roberts R. J. Two new restriction endonucleases from Proteus vulgaris. Nucleic Acids Res. 1981 Sep 25;9(18):4525–4536. doi: 10.1093/nar/9.18.4525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitman J., Zinder N. D., Model P. Repair of the Escherichia coli chromosome after in vivo scission by the EcoRI endonuclease. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2281–2285. doi: 10.1073/pnas.86.7.2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunnen K. D., Barsomian J. M., Camp R. R., Card C. O., Chen S. Z., Croft R., Looney M. C., Meda M. M., Moran L. S., Nwankwo D. O. Cloning type-II restriction and modification genes. Gene. 1988 Dec 25;74(1):25–32. doi: 10.1016/0378-1119(88)90242-9. [DOI] [PubMed] [Google Scholar]
- Mann M. B., Rao R. N., Smith H. O. Cloning of restriction and modification genes in E. coli: the HbaII system from Haemophilus haemolyticus. Gene. 1978 Apr;3(2):97–112. doi: 10.1016/0378-1119(78)90054-9. [DOI] [PubMed] [Google Scholar]
- Neu H. C., Heppel L. A. The release of enzymes from Escherichia coli by osmotic shock and during the formation of spheroplasts. J Biol Chem. 1965 Sep;240(9):3685–3692. [PubMed] [Google Scholar]
- Roberts R. J. Restriction enzymes and their isoschizomers. Nucleic Acids Res. 1990 Apr 25;18 (Suppl):2331–2365. doi: 10.1093/nar/18.suppl.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoner B., Kelly S., Smith H. O. The nucleotide sequence of the HhaII restriction and modification genes from Haemophilus haemolyticus. Gene. 1983 Oct;24(2-3):227–236. doi: 10.1016/0378-1119(83)90083-5. [DOI] [PubMed] [Google Scholar]
- Szomolányi E., Kiss A., Venetianer P. Cloning the modification methylase gene of Bacillus sphaericus R in Escherichia coli. Gene. 1980 Aug;10(3):219–225. doi: 10.1016/0378-1119(80)90051-7. [DOI] [PubMed] [Google Scholar]
- Wilson G. G. Organization of restriction-modification systems. Nucleic Acids Res. 1991 May 25;19(10):2539–2566. doi: 10.1093/nar/19.10.2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]