Abstract
Tissue remodeling often reflects alterations in local mechanical conditions and manifests as an integrated response among the different cell types that share, and thus cooperatively manage, an extracellular matrix. Here we examine how two different cell types, one that undergoes the stress and the other that primarily remodels the matrix, might communicate a mechanical stress by using airway cells as a representative in vitro system. Normal stress is imposed on bronchial epithelial cells in the presence of unstimulated lung fibroblasts. We show that (i) mechanical stress can be communicated from stressed to unstressed cells to elicit a remodeling response, and (ii) the integrated response of two cell types to mechanical stress mimics key features of airway remodeling seen in asthma: namely, an increase in production of fibronectin, collagen types III and V, and matrix metalloproteinase type 9 (MMP-9) (relative to tissue inhibitor of metalloproteinase-1, TIMP-1). These observations provide a paradigm to use in understanding the management of mechanical forces on the tissue level.
Keywords: airway wall remodeling, airway epithelium, asthma, lung fibroblasts
In the past two decades, it has been well established that many cells are sensitive to mechanical forces and can change their phenotype and surrounding extracellular matrix (ECM) in response to changes in their mechanical environment. However, it has not been established how mechanical forces are managed on a tissue level, in which different cell types share a common ECM and may alter their mechanical environment by inducing other cells to remodel the ECM.
The airway wall is an example of a mechanically active tissue (e.g., bronchoconstriction) composed of multiple cell types that share a common extracellular matrix. It is known that patients with poorly controlled asthma develop structural changes in the airway wall: the subepithelial layers significantly thicken (1–4) and there is deposition of collagen types III and V, among other fibrous proteins, beneath the basement membrane (4, 5). The major consequences of airway remodeling are altered tissue mechanics and airway hyperresponsiveness (6).
The remodeling process in asthma has been attributed to the effects of the inflammatory response that characterizes the disease, but this sequence of events has only been inferentially established. We propose an alternative hypothesis for airway wall remodeling in asthma, namely that the mechanical stress associated with bronchoconstriction that is imposed on bronchial epithelial cells could, itself, stimulate and amplify airway wall remodeling in the absence of inflammatory agents. Specifically, we hypothesize that, when airway epithelial cells are subjected to mechanical stress, they act in concert with subepithelial fibroblasts to modulate the fibrous structure of the airway in response to this mechanical stress.
This hypothesis, if true, would provide two key insights. First, it would challenge the idea that airway wall remodeling in asthma is solely the consequence of inflammation (although the two schemes are not mutually exclusive). This idea would significantly modify our current understanding of the underlying disease process of asthma and might ultimately change the way the disease is treated. Second, it would support a new paradigm in which mechanically induced matrix remodeling may be carried out not directly by the cells stimulated (here, the airway epithelium), but by communication of the mechanical force from the stimulated cell to a responder cell (here, subepithelial fibroblasts). This cell–cell communication is not necessarily a one-way process, and the presence of the responder cells may also influence how the stimulated cells are affected by and respond to the mechanical stress. This paradigm of cells managing their mechanical environment in a shared extracellular matrix builds on the observations that: (i) cells can directly remodel their local matrix in response to a mechanical stress (7, 8) and (ii) epithelial–fibroblast communication is critical in the regulation of matrix production in the lung (9, 10). The experiments reported herein provide direct evidence of such mechanically regulated cell–cell interactions and ECM regulation in vitro.
We have previously shown in rat tracheal epithelial cells that normal stresses in the range of those produced in the epithelial layer of buckled airways can elicit gene up-regulation of transforming growth factor-β, endothelin-1, and early growth response-1 (Egr-1) (11). Thus airway epithelial cells respond to normal stress by regulating the expression of several genes that have been associated with inflammation and remodeling in asthma. In the work reported here, we use a unique coculture system of mechanically stressed human bronchial epithelial cells (HBECs) and unstressed human lung fibroblasts (HLFs) to investigate whether the two cell types communicate with each other in response to the mechanical stress in a way that is relevant to matrix remodeling. The mechanical stress was applied in the form of a hydrostatic pressure difference across the epithelial cell layer to mimic the normal stresses that develop in the folds of a buckled airway (12). Cocultured fibroblasts were not exposed to a mechanical stimulus but were in contact with the epithelial layer via soluble mediators. Because the two cell types are separated mechanically but not biochemically (i.e., in contact through medium), this system enables the isolation of stress to one cell type and thus permits controlled investigations into cell–cell communication.
As response indicators, we evaluated the synthesis of total fibrous proteins as well as specific collagen types and their relevant inhibitors. These included collagen types I, III, and V, which are the primary types produced during asthma-related airway wall remodeling (5, 13–15), particularly type III. We also examined the presence of matrix metalloproteinase-9 (MMP-9) along with its inhibitor, tissue inhibitor of metalloproteinase-1 (TIMP-1). Both of these have been strongly associated with asthma, and their ratio has been correlated with severity of airway wall remodeling (16, 17) and corticosteroid responsiveness (16, 18) in asthmatics.
To validate the direct response of the epithelial cells to this mechanical stimulus, we evaluated HBEC production of Egr-1 and fibronectin (FN) protein after increasing transepithelial pressure. Egr-1 is a mechanically responsive gene (11, 19, 20) that encodes a transcription factor capable of modulating a number of genes that regulate fibroblast recruitment, proliferation, and activation [e.g., transforming growth factor-β, platelet-derived growth factor-A, FN, and tumor necrosis factor-α (21, 22)]. Fibronectin was chosen as a response indicator for a number of reasons. The importance of FN in asthma is well documented; increased levels of the protein are found in airway liquid of asthmatics (23–25) and fibronectin receptor expression is increased in the airway epithelial cells of asthmatics as well (26). Most relevant to the work presented here, FN release by bronchial epithelial cells has been shown to induce fibroblast proliferation and chemotaxis in culture (27).
Materials and Methods
Cell Lines.
Normal HBECs were obtained from Clonetics (San Diego) and cultured according to conditions previously described (28). For each experiment, passage-2 cells were expanded on plastic in supplemented bronchial epithelial growth medium (Clonetics) and then plated onto circular (25-mm diameter), uncoated 0.4-μm porous culture inserts (transwell clears, Costar) at 100,000 cells/well. They were fed apically as well as basally with a 1:1 mixture of supplemented bronchial epithelial growth medium and DMEM (GIBCO) until they reached confluence (6–8 days), at which time an air-liquid interface was established at the apical surface. This was maintained with daily feeding for 15–20 days (21–28 days total), at which time a considerable fraction of the cells had differentiated into ciliated and mucus-secreting cells, as is typical of a normal airway wall (29).
Normal HLFs (CCL-186, American Type Culture Collection) were plated onto tissue culture treated 6-well plates (Costar) and cultured in Eagle's modified essential medium supplemented with 10% calf serum and 1% penicillin/streptomycin (all from GIBCO). Passage-4 cells were seeded at 5,000 cells/well and maintained for 15 days beyond confluence (21 days total), at which time they had formed a densely packed monolayer and produced a sparse net of matrix proteins (specifically examined by immunofluorescence were fibronectin and type I collagen).
Experimental Setup.
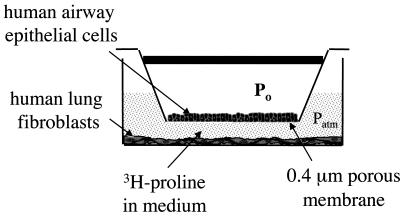
Twelve hours before the experiment, the HLFs were rinsed in PBS and 2 ml of unsupplemented, serum-free minimal media (bronchial epithelial growth medium/DMEM), spiked with either 3 μCi/ml [3H]proline or 3 μCi/ml [3H]thymidine (New England Nuclear), was added to each well. At this time the inserts containing HBECs were also rinsed in PBS and transferred to coculture with the fibroblasts. The inserts were capped and connected in parallel to an air pressure reservoir as shown in Fig. 1. This setup allowed an elevated air pressure to be applied on the apical surface of the epithelial cells whereas the basal side, as well as the fibroblasts, were exposed to medium at atmospheric pressure.
Figure 1.
Schematic diagram of one well in the experimental setup. For each experiment, several of these wells are connected in parallel to a pressure reservoir with humid incubator air (5% CO2 and 20% O2).
At t = 0, the appropriate wells were subjected to a transmural pressure of 0, 10, 20, 30, or 40 cm H2O (7.4–29.4 mmHg; 1 mmHg = 133 Pa), for a duration of 1, 2, or 4 h (and in some cases longer). When the pressure was returned to atmospheric conditions, the cells were maintained in coculture for a total of 24 h from t = 0 before harvesting and processing. The only exceptions were in those wells evaluated for Egr-1 and FN production by epithelial cells; in that case, the epithelial cells were rinsed and either (i) lysed for Western blot analysis, or (ii) fixed in 4% paraformaldehyde for immunohistochemical analysis, after 30 min (Egr-1) or 4 h (FN). These time points were chosen because preliminary studies revealed that maximal Egr-1 protein was detected at 30 min after the onset of stimulation and FN at 4 h. Controls included each cell type alone and epithelial cells stimulated with phorbol 12-myristate 13-acetate (PMA, Sigma) as a positive control. In addition, another control was added to ascertain the ability of elevated hydrostatic pressure alone to elicit a response in cultured epithelial cells. For this, a pressure-tight chamber was used to impose an air pressure of 30 cm H2O to the entire system. Finally, we repeated the experiments by using conditioned medium rather than coculture. In those experiments, the epithelial cells (which were not placed in coculture with the fibroblasts) were subjected to a transmembrane pressure of 0, 20, or 40 cm H2O for 4 h, and after an additional 4 h at no stress (to allow sufficient time for gene transcription and protein release), the medium was transferred to the fibroblasts.
At the conclusion of the experiment (24 h from onset of pressure), medium from each well was immediately transferred to a tube containing a mixture of protease inhibitors (2.5 mM EDTA/200 μM PMSF/1 mM N-ethylmaleimide; all from Sigma) and kept at 4°C to preserve the proteins for later analysis. Also, those wells containing [3H]thymidine were rinsed twice in ice-cold PBS, treated with 6% trichloroacetic acid (TCA) overnight at −20°C, rinsed again in PBS, and solubilized in 200 mM NaOH before evaluating in a scintillation counter (Beckman Coulter). This was performed only on fibroblast cell layers. Each experimental condition was performed five times for statistical significance.
Evaluation of Matrix Remodeling Activity.
Synthesis of total fibrous protein during the experiment was estimated from the incorporation of [3H]proline into TCA-precipitated proteins in the medium (30). From each well, 1.0 ml of medium was precipitated overnight in 15% TCA at 4°C, washed twice in 10% TCA, and resuspended in 0.5 M NaOH. Samples were then added to scintillation fluid and the total amount of 3H was determined by scintillation spectroscopy (Beckman Coulter).
MMP-9, TIMP-1, soluble FN, and collagen types I, III, and V were measured by Western blot analysis. Media samples from three wells were pooled together and concentrated (Centricon 3, Millipore) approximately 10-fold. All antibodies were mouse monoclonals and were obtained from Oncogene Science. Horseradish peroxidase-conjugated goat anti-mouse IgG (Dako) was used as a secondary antibody, and detection was achieved with chemiluminescence (ECL, Amersham Pharmacia). Optical densities of the resulting exposures were analyzed by using standard densitometry.
Evidence for Epithelial Response.
To assure that our mechanical stimulus elicited a direct epithelial cell response, a rabbit polyclonal anti-Egr-1 antibody (Santa Cruz Biotechnology) and a mouse monoclonal anti-fibronectin antibody (Transduction Laboratories, Lexington, KY) were used for immunostaining of epithelial layers and Western blots of pooled epithelial cell lysates. Samples were first permeabilized in 0.03% triton and blocked in goat or horse serum (for Egr-1 and FN, respectively) before incubating overnight at 4°C in the primary antibody. Biotin-conjugated goat anti-rabbit or horse anti-mouse IgGs (Dako) were used as secondary antibodies, and fluorescein-conjugated avidin (Vector Laboratories) was used for detection. Western blot analyses of cell lysates were performed as described for the collagens above, by using horseradish peroxidase-conjugated goat anti-rabbit or goat anti-mouse IgGs as secondary antibodies (Dako).
Results
Mechanical Stimulation of HBECs Induces Egr-1 and FN Synthesis.
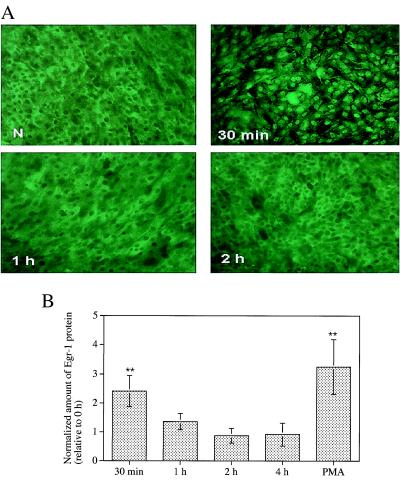
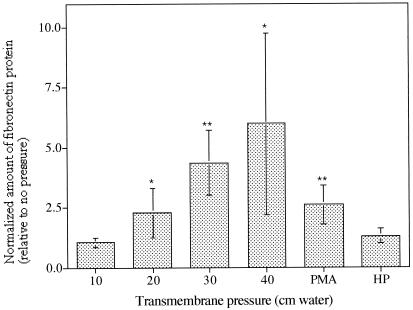
The ability of HBECs to respond directly to the mechanical stimulus was established by using Egr-1 and FN protein production as response indicators. Transient Egr-1 protein expression peaked (P < 0.005, ANOVA) after 30 min of either transmembrane pressure or PMA stimulation (Fig. 2 A and B) and returned to baseline levels at 2 h, consistent with its role as an immediate early response gene. Cell-associated FN was increased in stimulated HBECs after 2 h in a pressure-dependent manner (P = 0.01, ANOVA; Fig. 3) and continued to be detectable at 6 and 8 h, but if more than 2 h, stimulation time did not affect protein levels (data not shown). For both molecules, hydrostatic pressure alone did not elicit any significant changes in protein levels (data not shown), demonstrating the need for a transepithelial pressure difference to elicit signal transduction in this system. It should be noted that the inserts were rigid and no membrane strain was detected in the pressurized wells in TEM sections.
Figure 2.
(A) Induction of Egr-1 protein in HBECs by transmembrane pressure of 30 cm H2O. Unstimulated cells (N) are compared to cells fixed after 0.5, 1, and 2 h of mechanical stimulation. (B) The relative amounts of Egr-1 protein detected in HBEC lysates by Western blot after the application of 30 cm H2O transmembrane pressure for 30 min, 1 h, 2 h, or 4 h, or after 30 min stimulation with PMA (mean ± SD, n = 5). Densitometry was used to quantify protein levels relative to the unstimulated cells (**, P < 0.005 in comparison to no pressure using ANOVA).
Figure 3.
The effect of transepithelial pressure on the expression of FN proteins from lysed epithelial cells by using Western blot analysis. The densitometry results shown were calculated for pressure magnitudes of 10, 20, 30, and 40 cm H2O, PMA stimulation, and a uniform hydrostatic pressure (HP) of 30 cm H2O (applied to both sides of the membrane) for 4 h, relative to protein levels in unstimulated cells. For all data shown, significance was tested for each sample relative to the unstimulated cells (*, P < 0.05 and **, P < 0.005, ANOVA); furthermore, the dose dependence was significant from 0 to 40 cm H2O (P = 0.01).
Mechanical Stimulation of HBECs Regulates Collagen Production by Cocultured HLFs but Not HLF Proliferation.
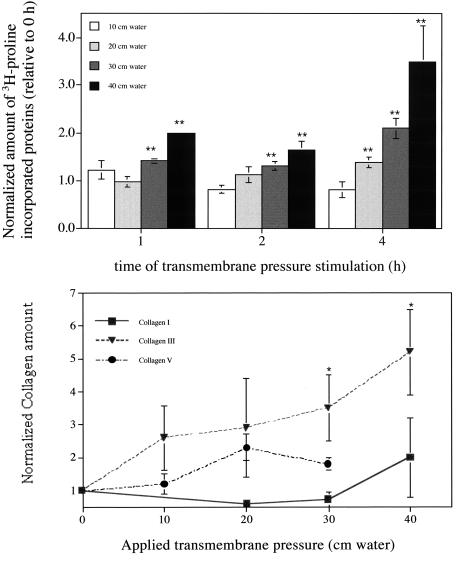
Fibroblasts responded to epithelial cell stimulation (with pressure levels at or above 20 cm H2O) by increasing collagen synthesis, as evidenced by 3H-labeled, TCA-precipitated proteins released into the media (Fig. 4A). This response was both strongly pressure- and time-dependent: Using ANOVA, each time group (e.g., 20 cm H2O for 0, 1, 2, and 4 h) gave P values < 5 × 10−5 and each pressure group (e.g., 2-h stimulation at 0, 20, 30, and 40 cm H2O) gave P values < 5 × 10−6; no significant differences were seen at 10 cm H2O relative to no pressure. Neither PMA stimulation of epithelial cells nor hydrostatic pressures of 30–40 cm H2O were able to induce any detectable changes in collagen production. In unstressed epithelial-only control wells, the amount of newly synthesized collagen that we detected averaged 0.30 ± 0.06 (avg. ± SD) relative to that from unstressed cocultured wells, and 0.6 ± 0.1 for stressed (P = 40 cm H2O) epithelial-only wells relative to the same control; both were significantly different from their coculture counterparts (P < 0.005, ANOVA). In fibroblast-only wells, we could not detect any significant difference in collagen synthesis from unstressed cocultured samples.
Figure 4.
(A) Effect of normal stress on the neosynthesis of fibrous proteins: magnitude (normalized to the unstimulated cocultures) vs. time (h) for transmembrane pressures of 10, 20, 30, or 40 cm H2O. Both a time dependence was seen for 20, 30, and 40 cm H2O (P < 10−5, ANOVA) as well as a pressure magnitude dependence for 1-, 2-, and 4-h stimulation (P < 10−6). (B) The effect of transepithelial pressure (4-h stimulation) on the presence of collagen types I (■), III (▴), and V (●) protein in pooled, concentrated media samples by using Western blot analysis.
Of the specific collagen types examined by Western blot, type III increased dramatically from baseline at pressures above 10 cm H2O (Fig. 4B). Very little collagen of types I, III, or V was seen in epithelial-only wells, and no significant changes were seen with pressure in this group.
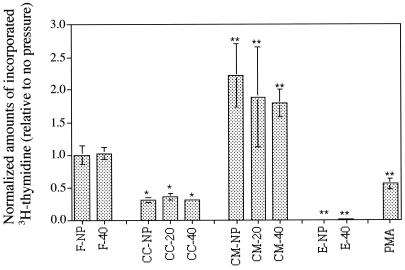
We found that mechanical stimulation of HBECs did not, however, affect the incorporation of [3H]thymidine (31) into cocultured HLFs (Fig. 5). These levels of incorporation were actually one-third of that in fibroblast-only controls and 6–7 times less than in samples treated with epithelia-conditioned media. At each level of pressure, the differences between conditioned media and coculture was significant (P < 0.05 in all cases using ANOVA) as well as in conditioned media vs. fibroblasts alone and coculture vs. fibroblasts alone. However, there was no difference between unstressed and stressed conditions for any of the systems.
Figure 5.
Fibroblast proliferation under various experimental conditions as measured by incorporation of [3H]thymidine. The levels are normalized to fibroblast-only samples. Significance was calculated relative to fibroblast-only samples (with no pressure) and was calculated by using ANOVA. CC, coculture; CM, conditioned media; F, fibroblast only samples; E, epithelial-only samples; PMA, PMA-stimulated (coculture); NP, no pressure; and 20 or 40, applied transmembrane pressure of 20 or 40 cm H2O.
MMP-9 and TIMP-1 Production Is Regulated by Mechanical Stress and Cell–Cell Communication.
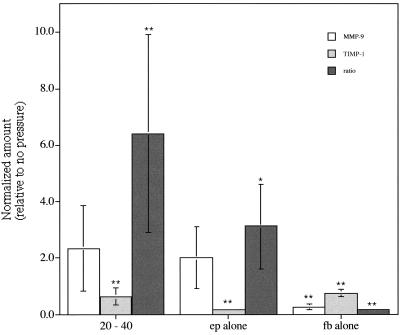
Epithelial cells also contributed to an overall remodeling response seen in the medium by regulating MMP-9 and TIMP-1 ratios. Overall, MMP-9 increased approximately twofold when stresses above 20 cm H2O were applied, whereas TIMP-1 decreased with stress, leading to a significant (P < 0.05, ANOVA) increase in their ratio (MMP-9/TIMP-1) with stress (Fig. 6). This response did not significantly depend on stress magnitude for levels above 10 cm H2O. Epithelial-only wells, both stimulated and unstimulated, had a MMP-9/TIMP-1 ratio level of approximately three times that found in unstimulated cocultures, whereas small amounts of either protein were seen in stimulated and unstimulated fibroblast-only wells, compared to unstressed coculture wells.
Figure 6.
Average protein levels of MMP-9, TIMP-1, and their ratio MMP-9/TIMP-1 in concentrated medium samples after 4-h stimulation as detected by Western blot (*, P < 0.05 and **, P < 0.005, ANOVA). Average values represent normalized amounts of protein relative to unstressed coculture conditions. Average values were taken from all samples stimulated with 20, 30, or 40 cm H2O because no statistically significant differences were detected among these levels.
Discussion
In this work, we demonstrate that mechanical stress on human airway epithelial cells elicits a matrix remodeling response in unstressed, cocultured lung fibroblasts through soluble signals. This effect was magnitude-dependent. Furthermore, collagen type III was the most markedly increased of the three types investigated (I, III, and V), which is consistent with asthma-associated airway wall thickening (5, 13–15). In fact, the entire integrated response—collagen synthesis, increased HBEC production of fibronectin, and increased MMP-9/TIMP-1 ratio—mimics many facets of the fibrotic response observed in patients with asthma, yet in the absence of inflammatory cells.
Our fibroblast proliferation results also highlight the fact that epithelial–fibroblast interactions in the lung are complex and that the presence of each cell type affects the response of the other. Previous studies by other investigators have shown that adult lung epithelial cells may enhance lung fibroblast growth in vitro by using conditioned medium (32–34), direct contact (35), or contact through a collagen gel (34) and that lung fibroblast-conditioned medium can enhance lung epithelial cell growth (35–38). However, it also has been shown that epithelial cells can suppress fibroblast growth when cultured with a filter and only medium between the two cell types (35), although this has not always been the case (33). Our findings corroborate these results: We saw an inhibitory effect of cocultured HBECs, without direct contact, on fibroblast proliferation and the opposite effect of conditioned media from HBECs. On the other hand, mechanical stress did not affect the ability of the HBECs to modulate HLF proliferation under either condition, demonstrating that at least in this system, fibroblast proliferation is regulated by interaction between the two cell types, but not by the mechanical stress used here.
The protease–anti-protease system most commonly associated with asthma is MMP-9, which degrades collagen type IV (basement membrane), and its inhibitor TIMP-1. They are secreted together by bronchial epithelial cells, bind in a 1:1 ratio, and can be modulated by inflammatory mediators and cytokines (16–18, 39–41). A high MMP-9/TIMP-1 ratio in bronchoalveolar lavage fluid indicates collagen degradation, inflammation, and poor prognosis, whereas higher TIMP-1 correlates with tissue repair and fibrosis. In our system, the MMP-9/TIMP-1 ratio was greater than one, and was increased when the HBECs were mechanically stressed above 10 cm H2O (although no correlation was seen with magnitude of stress). This may at first seem discordant with in vivo observations showing low (<1) MMP-9/TIMP-1 ratios in BAL fluid from asthmatic patients (16, 18). However, inflammatory cells are a significant source of protease and protease inhibitors in the clinical situation and are deliberately excluded in our system. It also is possible that a higher MMP-9 release precedes an increase in TIMP-1 as the airway wall is first degraded and then remodeled. Furthermore, epithelial expression of MMP-9 has been correlated with subepithelial deposition of collagen III in asthmatic airways (14). This corroborates our data in an isolated system, which demonstrate that mechanical stress leads to high levels of collagen III production (Fig. 4B). The fact that insignificant amounts of either MMP-9 or TIMP-1 were seen in fibroblast-only wells indicates that epithelial cells, alone or under the influence of cocultured fibroblasts, were primarily responsible for the regulation of this protease–anti-protease balance.
We observed that FN is up-regulated as a result of mechanotransduction and may play a role in the epithelial–fibroblast signaling. The consistent and dose-dependent up-regulation of FN protein production by airway epithelial cells undergoing normal stresses highlights the relevance of mechanical stress in asthma as contributing to the associated changes in the airway wall. Our data also support other studies demonstrating that Egr-1 is stimulated by mechanical stress (11, 19, 20).
Although a three-dimensional coculture system might provide a more realistic model of an airway wall, the isolation of the two cell types from each other (such as our model provides) is necessary to study the communication of mechanical stress from one cell type to the other because the ECM is a deformable material and thus mechanically couples the cells that reside in it. Furthermore, neither cell type was grown on or within its own isolated three-dimensional matrix (except for whatever matrix proteins the cells had produced while in culture) because of hindrance to the transport and distribution of cell–cell signaling molecules and the added difficulty in evaluating collagen neosynthesis.
The issue of mechanotransduction mechanism has not been addressed in this paper. In a previous paper that investigated the epithelial response in isolation (11), we demonstrated by placing a rigid but porous support beneath the membrane that substrate stretch did not produce the stimulus. We also have ruled out hydrostatic pressure. Potential mechanisms may include deformation of the basal membrane into the porous substrate, cell volume regulation, or intercellular shear stress because of the flow of intercellular fluid or apical mucus. Any of these effects could produce the internal stresses or deformations necessary to elicit a biologic response, and studies to elucidate this mechanism are currently underway.
When considered as a whole, the integrated response of the coculture system to transepithelial pressure on the epithelial cell layer demonstrates remarkable similarities to the asthmatic airway, including increased fibrous protein synthesis, the most prevalent of those examined being collagen type III [the isoform most frequently associated with airway wall thickening (5, 13–15)], increased HBEC production of FN and its transcriptional regulator Egr-1, and an elevated MMP-9/TIMP-1 ratio. Because these in vitro responses to mechanical force occurred in the absence of inflammatory cells, our data provide strong evidence that many of the phenotypic characteristics of asthma can be induced by mechanical events without the need for inflammatory cells per se.
We observed striking differences in fibroblast proliferation between the conditions of (i) coculture of epithelial cells and fibroblasts and (ii) fibroblasts grown in epithelial cell-conditioned medium (Fig. 5), which illustrates the complex nature of soluble communication between cells. Our observation that medium from epithelial cells exposed to mechanical stress failed to induce in fibroblasts the same phenotype achieved when fibroblasts were cocultured with epithelial cells is prima-facie evidence of two-way communication between epithelial cells and fibroblasts. The nature of the factors mediating these phenotypic changes in both epithelial cells and fibroblasts is unknown. Indeed, the number of factors that serve to facilitate epithelial–fibroblast communication, along with the dynamics of their expression, synthesis, secretion, binding, and signaling, evidence the tremendous complexity in the regulation of proliferation in this simple model. Although the molecular systems studied here are far from exhaustive, they represent a subset with great relevance to remodeling of the asthmatic airway. With respect to asthma pathogenesis, our data indicate that airway wall remodeling may occur in the absence of inflammatory mediators and may in fact be instigated by the sustained mechanical stresses associated with bronchial constriction and buckling of the epithelial layer lining the airways. This result would suggest that therapy aimed at both the inflammatory response, which initiated the release of bronchoactive mediators, as well as its consequence, i.e., airway narrowing, might be superior to either one alone.
Acknowledgments
We thank Drs. Kathy Haley and Eric Silverman for assistance with protein detection methods and stimulating discussions. This work was supported by the National Institutes of Health (HLP50–56383 and HL-64075).
Abbreviations
- ECM
extracellular matrix, Egr-1, early growth response-1
- FN
fibronectin
- HBEC
human bronchial epithelial cell
- HLF
human lung fibroblast
- PMA
phorbol 12-myristate 13-acetate
- MMP-9
matrix metalloproteinase type 9
- TCA
trichloroacetic acid
- PDGF
platelet-derived growth factor
References
- 1.Chetta A, Foresi A, del Donno M, Bertorelli G, Pesci A, Olivieri D. Chest. 1997;111:852–857. doi: 10.1378/chest.111.4.852. [DOI] [PubMed] [Google Scholar]
- 2.Laitinen A, Laitinen L A. Am J Respir Crit Care Med. 1994;150:S14–S17. doi: 10.1164/ajrccm/150.5_Pt_2.S14. [DOI] [PubMed] [Google Scholar]
- 3.Kuwano K, Bosken C H, Pare P D, Bai T R, Wiggs B R, Hogg J C. Am Rev Respir Dis. 1993;148:1220–1225. doi: 10.1164/ajrccm/148.5.1220. [DOI] [PubMed] [Google Scholar]
- 4.Jeffery P K. Respiration. 1992;59, Suppl. 1:13–16. doi: 10.1159/000196096. [DOI] [PubMed] [Google Scholar]
- 5.Brewster C E, Howarth P H, Djukanovic R, Wilson J, Holgate S T, Roche W R. Am J Respir Cell Mol Biol. 1990;3:507–511. doi: 10.1165/ajrcmb/3.5.507. [DOI] [PubMed] [Google Scholar]
- 6.Kamm R D. Annu Rev Biomed Eng. 1999;1:47–72. doi: 10.1146/annurev.bioeng.1.1.47. [DOI] [PubMed] [Google Scholar]
- 7.Burger E H, Klein-Nulend J. Bone. 1998;22:127S–130S. doi: 10.1016/s8756-3282(98)00010-6. [DOI] [PubMed] [Google Scholar]
- 8.Sadoshima J, Izumo S. Annu Rev Physiol. 1997;59:551–557. doi: 10.1146/annurev.physiol.59.1.551. [DOI] [PubMed] [Google Scholar]
- 9.Mio T, Liu X, Adachi Y, Striz I, Skold C M, Romberger D J, Spurzem J R, Illig M G, Ertl R, Rennard S I. Am J Physiol. 1998;274:L119–L126. doi: 10.1152/ajplung.1998.274.1.L119. [DOI] [PubMed] [Google Scholar]
- 10.Infeld M D, Brennan J A, Davis P B. Am J Resp Cell Mol Biol. 1993;8:69–76. doi: 10.1165/ajrcmb/8.1.69. [DOI] [PubMed] [Google Scholar]
- 11.Ressler B H, Lee R, Randell S H, Drazen J M, Kamm R D. Am J Physiol. 2000;278:L1264–L1272. doi: 10.1152/ajplung.2000.278.6.L1264. [DOI] [PubMed] [Google Scholar]
- 12.Wiggs B R, Hrousis C A, Drazen J M, Kamm R D. J Appl Physiol. 1997;83:1814–1821. doi: 10.1152/jappl.1997.83.6.1814. [DOI] [PubMed] [Google Scholar]
- 13.Arm J P, Lee T H. Adv Immunol. 1992;51:323–382. doi: 10.1016/s0065-2776(08)60491-5. [DOI] [PubMed] [Google Scholar]
- 14.Hoshino M, Nakamura Y, Sim J J, Shimojo J, Isogai S. J Allergy Clin Immunol. 1998;102:783–788. doi: 10.1016/s0091-6749(98)70018-1. [DOI] [PubMed] [Google Scholar]
- 15.Kawamoto M, Romberger D J, Nakamura Y, Adachi Y, Tatte L, Ertl R, Spurzem J R, Rennard S I. Am J Respir Cell Mol Biol. 1995;12:425–433. doi: 10.1165/ajrcmb.12.4.7695922. [DOI] [PubMed] [Google Scholar]
- 16.Mautino G, Henriquet C, Jaffuel D, Bousquet J, Capony F. Am J Resp Crit Care Med. 1999;160:324–330. doi: 10.1164/ajrccm.160.1.9808087. [DOI] [PubMed] [Google Scholar]
- 17.Hoshino M, Takahashi M, Takai Y, Sim J. J Allergy Clin Immunol. 1999;104:356–363. doi: 10.1016/s0091-6749(99)70379-9. [DOI] [PubMed] [Google Scholar]
- 18.Bosse M, Chakir J, Rouabhia M, Boulet L, Audette M, Laviolette M. Am J Respir Crit Care Med. 1999;159:596–602. doi: 10.1164/ajrccm.159.2.9802045. [DOI] [PubMed] [Google Scholar]
- 19.Schwachtgen J L, Houston P, Campbell C, Sukhatme V, Braddock M. J Clin Invest. 1998;101:2540–2549. doi: 10.1172/JCI1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ogata T. J Cell Physiol. 1997;170:27–34. doi: 10.1002/(SICI)1097-4652(199701)170:1<27::AID-JCP4>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 21.Liu C, Yao J, de Belle I, Huang R-P, Adamson E, Mercola D. J Biol Chem. 1999;274:4400–4411. doi: 10.1074/jbc.274.7.4400. [DOI] [PubMed] [Google Scholar]
- 22.Khachigian L M, Linder V, Williams A J, Collins T. Science. 1996;271:1427–1431. doi: 10.1126/science.271.5254.1427. [DOI] [PubMed] [Google Scholar]
- 23.Meerschaert J, Kelly E A, Mosher D F, Busse W W, Jarjour N N. Am J Respir Crit Care Med. 1999;159:619–625. doi: 10.1164/ajrccm.159.2.9806053. [DOI] [PubMed] [Google Scholar]
- 24.Vignola A M, Chanez P, Campbell A M, Bousquet J, Michel F B, Godard P. Allergy (Copenhagen) 1993;48, Suppl. 17:32–38. doi: 10.1111/j.1398-9995.1993.tb04696.x. [DOI] [PubMed] [Google Scholar]
- 25.Mattoli S, Mattoso V L, Soloperto M, Allegra L, Fasoli A. J Allergy Clin Immunol. 1991;87:794–802. doi: 10.1016/0091-6749(91)90125-8. [DOI] [PubMed] [Google Scholar]
- 26.Lobb R, Pepinsky B, Leone D R, Abraham W M. Eur Respir J Suppl. 1996;22:104s–108s. [PubMed] [Google Scholar]
- 27.Shoji S, Rickard K A, Ertl R F, Robbins R A, Linder J, Rennard S I. Am J Resp Cell Mol Biol. 1989;1:13–20. doi: 10.1165/ajrcmb/1.1.13. [DOI] [PubMed] [Google Scholar]
- 28.Yoon J-H, Gray T, Guzman K, Koo J S, Nettesheim P. Am J Respir Cell Mol Biol. 1997;16:724–731. doi: 10.1165/ajrcmb.16.6.9191474. [DOI] [PubMed] [Google Scholar]
- 29.Gray T E, Guzman K, Davis C W, Abdullah L H, Nettesheim P. Am J Respir Cell Mol Biol. 1996;14:104–112. doi: 10.1165/ajrcmb.14.1.8534481. [DOI] [PubMed] [Google Scholar]
- 30.Sah R L Y, Kim Y-J, Doong J-Y, Grodzinsky A J, Plaas A H K, Sandy J D. J Orthop Res. 1989;7:619–636. doi: 10.1002/jor.1100070502. [DOI] [PubMed] [Google Scholar]
- 31.Abrami L, Tacnet F, Ripoche P. Pflügers Arch. 1995;430:447–458. doi: 10.1007/BF00373921. [DOI] [PubMed] [Google Scholar]
- 32.Cambrey A D, Kwon O J, Gray A J, Harrison N K, Yacoub M, Barnes P J, Laurent G J, Chung K F. Clin Sci. 1995;89:611–617. doi: 10.1042/cs0890611. [DOI] [PubMed] [Google Scholar]
- 33.Nakamura Y, Tate L, Ertl R F, Kawamoto M, Mio T, Adachi Y, Romberger D J, Koizumi S, Gossman G, Robbins R A. Am J Physiol. 1995;269:L377–L387. doi: 10.1152/ajplung.1995.269.3.L377. [DOI] [PubMed] [Google Scholar]
- 34.Zhang S, Smartt H, Holgate S T, Roche W R. Lab Invest. 1999;79:395–405. [PubMed] [Google Scholar]
- 35.Adamson I Y, Young L, King G M. Exp Lung Res. 1991;17:821–835. doi: 10.3109/01902149109062880. [DOI] [PubMed] [Google Scholar]
- 36.Pasternack M, Liu X, Goodman R A, Rannels E. Am J Physiol. 1997;272:L619–L630. doi: 10.1152/ajplung.1997.272.4.L619. [DOI] [PubMed] [Google Scholar]
- 37.Liu M, J, X, Tanswell A K, Post M. Exp Lung Res. 1993;19:505–517. doi: 10.3109/01902149309064360. [DOI] [PubMed] [Google Scholar]
- 38.Shoji S, Rickard K A, Takizawa H, Ertl R F, Linder J, Rennard S I. Am Rev Resp Dis. 1990;141:433–439. doi: 10.1164/ajrccm/141.2.433. [DOI] [PubMed] [Google Scholar]
- 39.Mautino G, Oliver N, Chanez P, Bousquet J, Capony F. Am J Respir Cell Mol Biol. 1997;17:583–592. doi: 10.1165/ajrcmb.17.5.2562. [DOI] [PubMed] [Google Scholar]
- 40.Ohno I, Ohtani H, Nitta Y, Suzuki J, Hoshi H, Honma M, Isoyama S, Tanno Y, Tamura G, Yamuchi K, Nagura H, Shirato K. Am J Respir Cell Mol Biol. 1997;16:212–219. doi: 10.1165/ajrcmb.16.3.9070604. [DOI] [PubMed] [Google Scholar]
- 41.Nagase H. In: Zinc Metalloproteinases in Health and Disease. Hopper N M, editor. London: Taylor & Francis; 1996. pp. 153–204. [Google Scholar]