Abstract
In the study, we have examined the antitumor and antimicrobial activities of the methanol extract, the fractions, a fraction of total alkaloids and two alkaloids isolated from the stem of Erythroxylum caatingae Plowman. All test fractions, except the hexane fractions, showed antimicrobial activity on gram-positive bacteria and fungi. The acetate: methanol (95:5), acetate, chloroform and hexane fractions show the highest cytotoxicity activity against the NCI-H292, HEp-2 and K562 cell lines using MTT. The absence of hemolysis in the erythrocytes of mice was observed in these fractions and 6β-Benzoyloxy-3α-(3,4,5- trimethoxybenzoyloxy) tropane (catuabine B). Staining with Annexin V-FITC and JC-1 was used to verify the mechanism of action of the compounds of E. caatingae that showed cytotoxicity less than 30 μg/mL in leukemic cells. After 48 h of incubation, we observed that the acetate: methanol (95:5), acetate, and chloroform fractions, as well as the catuabine B, increased in the number of cells in early apoptosis, from 53.0 to 74.8%. An analysis of the potential of the mitochondrial membrane by incorporation of JC-1 showed that most cells during incubation of the acetate: methanol (95:5) and acetate fractions (63.85 and 59.2%) were stained, suggesting the involvement of an intrinsic pathway of apoptosis.
Keywords: Erythroxylum caatingae, antimicrobial activity, cytotoxic activity, hemolytic activity, apoptosis
1. Introduction
The research with medicinal plants aiming at the development of phytotherapeutic medicines and the promotion of the rational use of these products by the populations of developing countries such as Brazil, have a great importance not only in socio-economic aspects but also because they enable a greater knowledge of the culture of such people and a better utilization of the biodiversity of the respective countries [1]. Approximately 25% of the medicines prescribed in the industrialized countries originate from plants and about 120 compounds of natural origin, obtained from approximately 90 species of plants, are used in modern therapy. In Brazil, approximately 80,000 species of plants are described, offering a wide range of raw material for the discovery of new drugs [2,3].
It is well established that plants have always been useful sources of antitumor or cancer prevention compounds [4–6]. Approximately more than 60% of currently-used anticancer chemotherapeutic drugs are derived in one way or another from natural sources, including plants [7,8]. The search for anticancer agents from plant sources began in the 1950s with the discovery and development of the vinca alkaloids, vinblastine and vincristine, isolated from Catharanthus roseus, and etoposide and teniposide, the semisynthetic derivatives of epipodophyllotoxin, isolated from Podophyllum peltatum and P. endoii [7].
The indiscriminate use of antibiotics has led to antibiotic resistance in many pathogenic microorganisms and has led researchers to seek new antibiotics that are effective. Several alternatives have been suggested to solve this problem, such as the search for new antimicrobials in plant species [9]. Since the early 1980s, the number of drugs in development has decreased considerably, while the resistance of microorganisms, due to the constant use of antibiotics, has grown immensely. This is because series of new mechanisms of resistance are constantly being developed. The study of bacterial resistance is usually based on microorganisms of epidemiological significance, such as Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa and fungi, which are responsible for different etiological processes in both immunocompetent and immunosuppressed patients [10].
The Erythroxylaceae family comprises approximately 250 species distributed across four genera, Aneulophus Benth, Nectaropetalum Engl., Pinacopodium Exell and Mendonça and Erythroxylum P. Browne [11,12]. The genus Erythroxylum consists of approximately 200 species, diversely distributed over South America, Africa and the island of Madagascar [13,14]. It has been used in many ethnomedical practices as an anti-inflammatory agent, an antibacterial agent and a tonic for its stimulant properties and as a powerful diuretic for liver, renal and vesicular afflictions. Erythroxylum has been used to treat venereal diseases, rheumatism, arthrosis, respiratory affections, amenorrhea and hemorrhages [15,16]. In Brazil, the family is represented by the genus Erythroxylum, with approximately 114 species, and there are 13 species registered in state of Paraíba [12].
The genus is characterized by the presence of tropane alkaloids, tannins, terpenes, flavonoids and phenylpropanoids [14,17]. Tropane alkaloids are an important class of natural products because of their analgesic, anesthetic, anticholinergic, antiemetic, antihypertensive, parasympatholytic, and other pharmacological properties [18].
The aim of the present study was to evaluate the antimicrobial and cytotoxic activities of the methanol extract fractions and two alkaloids isolated from the stem of Erythroxylum caatingae Plowman. For the evaluation of antimicrobial activity, the following fractions were tested against gram-positive and gram-negative bacteria and against fungi: the acetate phase (AcOEt), various ratios of acetate: methanol [AcOEt:MeOH (60:40); AcOEt:MeOH (80:20); AcOEt:MeOH (90:10) and AcOEt:MeOH (95:5)], chloroform (CHCl3) and hexane (C6H6). Antiproliferative activity was tested against tumor cell lines (NCI-H292, human lung mucoepidermoid carcinoma cells, K562, chronic myelocytic leukemia, and HEp-2, human larynx epidermoid carcinoma cells) and hemolytic activity was measured for the methanol extracts, its fractions and alkaloids. Another set of experiments was performed with K562, which was used to investigate the mechanisms involved in the antitumor activity of the fractions and the alkaloids of E. caatingae.
2. Results and Discussion
2.1. Antimicrobial Activity
The antimicrobial activities of methanol extract, fractions from the stem of E. caatingae Plowman were tested using the disc diffusion method at a concentration of 2000 μg/disc. The results are presented in Tables 1–3. All test fractions, except the hexane fractions, showed antimicrobial activity on gram-positive bacteria and fungi. The DMSO (control) did not produce inhibition haloes against the microorganisms studied, indicating that this solvent does not interfere with the antimicrobial activity results for the extract or fractions. The bacteria that were the most sensitive to the fractions tested were Micrococcus luteus and Mycobacterium smegmatis, with each showing inhibition zones of 13.5 ± 0.7 to 29.5 ± 0.7 mm and 14.0 ± 1.4 to 31.5 ± 1.4 mm, respectively. The AcOEt:MeOH (80:20), AcOEt:MeOH (90:10), AcOEt:MeOH (95:5) and the CHCl3 phases showed significant inhibition zones of 18.0 ± 0.0 to 19.0 ± 1.4 mm for Candida albicans (Table 1). Evaluation of the minimum inhibitory concentration of the extract and fractions of E. caatingae (zone of inhibition > 12 mm) showed significant inhibitory concentrations for S. aureus, M. luteus, B. subtilis, M. smegmatis, E. faecalis and C. albicans. However, the lowest values of the minimum inhibitory concentration (MIC) and minimum bacteriostatic concentration (MBC) against M. luteus went to the AcOEt:MeOH (95:5), AcOEt:MeOH (90:10) and CHCl3 fractions (Tables 1–3).
Table 1.
Antimicrobial activity of the methanol extract of the stem of Erythroxylum caatingae and of its fractions (2000 μg/disc).
| Zone of Inhibition (mm) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Microorganisms | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | Kanamicin (30 μg/disc) | ketoconazole (300 μg/disc) |
| S. aureus | 10.5 ± 0.7 | 10.0 ± 0.0 | 11.0 ± 0.7 | 12.0 ± 0.0 | 12.0 ± 0.0 | 0.0 | 10.5 ± 0.7 | 0.0 | 28 | - |
| M. luteus | 21.5 ± 2.1 | 26.0 ± 1.4 | 28.5 ± 0.7 | 28.5 ± 0.7 | 29.5 ± 0.7 | 13.5 ± 0.7 | 26.0 ± 0.0 | 0.0 | 34 | - |
| B. subitilis | 10.0 ± 0.0 | 10.0 ± 0.0 | 13.5 ± 0.7 | 13.5 ± 0.7 | 13.0 ± 0.7 | 0.0 | 12.0 ± 0.0 | 0.0 | 29 | - |
| M. smegmatis | 14.0 ± 1.4 | 22.5 ± 0.7 | 30.0 ± 0.0 | 31.5 ± 1.4 | 30.0 ± 0.0 | 15.0 ± 0.0 | 15.0 ± 0.0 | 0.0 | 40 | - |
| E. faecalis | 0.0 | 0.0 | 11.5 ± 0.7 | 11.5 ± 0.7 | 12.5 ± 0.7 | 0.0 | 17.0 ± 0.0 | 0.0 | 13 | - |
| E. coli | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 15 | - |
| S. marcescens | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 15 | - |
| P. aeruginosa | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 20 | - |
| C. albicans | 0.0 | 0.0 | 19.0 ± 1.4 | 19.0 ± 1.4 | 18.0 ± 1.4 | 0.0 | 18.0 ± 0.0 | 0.0 | - | 24 |
1: Methanol extract of stem of E. caatingae (MEEC); 2: AcOEt:MeOH (60:40); 3: AcOEt:MeOH (80:20); 4: AcOEt:MeOH (90:10); 5: AcOEt:MeOH (95:5); 6: AcOEt; 7: CHCl3; 8: Hexane fraction. Data are presented as the mean ± SD of two independent experiments. Each experiment was done in triplicate.
Table 3.
Evaluates the minimum bacteriostatic concentration of the methanol extract of the stem of Erythroxylum caatingae and of its fractions.
| Microorganisms | Minimum Bacteriostatic Concentration (μg/mL) | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| S. aureus | n.t. | n.t. | n.t. | 1000 | 500 | n.t. | n.t. |
| M. luteus | 250 | 500 | n.t. | <31.25 | <31.25 | >2000 | <31.25 |
| B. subtilis | n.t. | n.t. | n.t. | 1000 | 500 | n.t. | 250 |
| M. smegmatis | 1000 | 1000 | n.t. | 500 | 250 | 1,000 | 250 |
| E. faecalis | n.t. | n.t. | n.t. | n.t. | 1000 | n.t. | 1000 |
| C. albicans | n.t. | n.t. | n.t. | 500 | 500 | n.t. | 250 |
1: Methanol extract of stem of E. caatingae (MEEC); 2: AcOEt:MeOH (60:40); 3: AcOEt:MeOH (80:20); 4: AcOEt:MeOH (90:10); 5: AcOEt:MeOH (95:5); 6: AcOEt; 7: CHCl3; n.t.: not tested. Each experiment was done in triplicate.
Plants are an important source of potentially useful structures for the development of new chemotherapeutic agents. The first step to identification of new chemotherapeutic agents is through trials of antibacterial activity in vitro. Many reports are available on the antiviral, antibacterial, antifungal, anthelmintic, antimolluskal and anti-inflammatory properties of plants. Some of these observations have proven helpful in the identification of the active principle compounds responsible for such activities and in the development of drugs for therapeutic use in humans [19].
There are a few reports of the antimicrobial activity of the genus Erythroxylum. Among them, we highlight the alkaline extract of the bark of Erythroxylum catuaba that has been shown to have a protective action against lethal infections of E. coli and S. aureus [20]. However, Rahman et al. (1998) [21] reported the absence of antibacterial activity against S. aureus, E. coli, B. subtilis and P. aeruginosa at the concentrations of 100 and 200 μg/mL and the presence of antifungal activity of the alcoholic extract of Erythroxylum moonii against C. albicans. In our study, the absence of any antimicrobial activity of E. caatingae against S. aureus, P. aeruginosa and E. coli was also verified, while the AcOEt:MeOH (80:20), AcOEt:MeOH (90:10), AcOEt:MeOH (95:5) and the CHCl3 fractions inhibited the growth of C. albicans. However, the lowest values of MIC and MBC went to the AcOEt:MeOH (90:10), AcOEt:MeOH (95:5) and chloroform fractions against M. luteus. It was observed from the data that the most active fractions were more polar, probably due to the presence of phenolic compounds such as flavonoids or tannins. According Haslan (1996) [22], the antimicrobial activity exhibited by tannins is explained by the ability of these compounds present in complexing with macromolecules, such as polysaccharides and proteins present in bacteria.
2.2. Cytotoxicity Activity
The cytotoxicity of the 11 samples, including the extract, fractions and two isolated compounds on the human tumor cell lines were evaluated after 72 h using MTT, and the results are presented in Table 4. The AcOEt:MeOH (95:5) (5), AcOEt (6), CHCl3 (7) and C6H6 (8) fractions were the most cytotoxic against the NCI-H292, HEp-2 and K562 cell lines. The most sensitive strains tested were the HEp-2 cell line, with an IC50 value equal to 8.25 ± 0.36 μg/mL for the CHCl3 fraction, and the K562 cell line, with an IC50 value equal to 13.10 ± 0.63 μg/mL, 9.86 ± 0.56 μg/mL and 11.21 ± 0.46 μg/mL for the AcOEt:MeOH (95:5), AcOEt and CHCl3 fractions, respectively. To verify whether the cytotoxicity observed was related to the injury of the cell plasma membrane, fractions 3–8 (Table 4) and the Catuabine B of E. caatingae were tested in the erythrocytes of mice (Mus musculus). The absence of hemolysis was observed in all substances.
Table 4.
Cytotoxic and hemolytic activity de Erytroxylum caatingae.
| Products/Extracts | Cell Line, IC50 (μg/mL) | EC50 (μg/mL) | ||
|---|---|---|---|---|
| HEp-2 | NCI-H292 | K562 | ||
| 1 | >50 | >50 | >50 | n.t. |
| 2 | >50 | >50 | >50 | n.t. |
| 3 | >50 | 25.76 ± 1.71 | >50 | >2000 |
| 4 | >50 | 25.50 ± 1.08 | >50 | >2000 |
| 5 | 40.59 ± 0.72 | 28.19 ± 2.09 | 13.10 ± 0.63 | >2000 |
| 6 | 34.12 ± 1.01 | 20.37 ± 0.75 | 9.86 ± 0.56 | 403.24 ± 9.64 |
| 7 | 8.25 ± 0.36 | 34.39 ± 1.71 | 11.21 ± 0.46 | 1036.52 ± 35.17 |
| 8 | 31.42 ± 1.01 | 15.79 ± 1.01 | 33.58 ± 1.33 | 510.00 ± 8.60 |
| 9 | >50 | >50 | >50 | n.t. |
| 10 | >50 | >50 | 9.36 ± 0.77 | >250 |
| 11 | >50 | >50 | >50 | n.t. |
| Etoposide | 6.10 ± 0.19 | 2.75 ± 0.10 | 4.48 ± 0.23 | n.t. |
1: Methanol extract of stem of E. caatingae (MEEC); 2: AcOEt:MeOH (40:60); 3: AcOEt:MeOH (80:20); 4: AcOEt:MeOH (90:10); 5: AcOEt:MeOH (95:5); 6: acetate fraction (AcOEt); 7: chloroform fraction (CHCl3); 8: hexane fraction (C6H6); 9: Fraction total of alkaloids; 10: catuabine B; 11: 3α,6β-dibenzoyloxytropane; n.t.: not tested. The IC50 and EC50 and its 95% confidence interval (CI 95%) were obtained by non-linear regression. Data are presented as mean ± SD of two independent experiments. Each experiment was done in triplicate.
The cytotoxic activity of Erytroxylum in carcinoma and adenocarcinoma cells was first described by Silva et al. (2001) [15]. This study showed that the methanolic extract and alkaloids of tropanes of Erytroxylum pervillei inhibited the growth of KB-V1 cells (human cervix carcinoma multidrug-resistant cells) in the presence of vinblastine and were much less cytotoxic to KB-V1 cells in the absence of vinblastine or KB cells (oral squamous cell carcinoma). The alkaloids of the species pervillene B and C showed cytotoxic activity in adenocarcinomas of ovary (SKOV3) cells and in adenocarcinomas of multidrug-resistant ovary (SKVLB) cells incubated with adriamycin. The following year, alkaloids isolated from the stem of Erytroxylum rotundifolium were also found to be cytotoxic in KB-V1 cells [23].
Extracts of Erytroxylum minutifolium and Erytroxylum confusum produced a significant cytotoxic effect in rat hepatocytes and displayed hepatoprotective, anti-inflammatory and antimicrobial activities [24,25].
Among the few reports of cytotoxicity of the genus Erythroxulum, our group found an absence of cytotoxicity in the methanol extract of E. caatingae stems and in the catuabine B against human cancer cell lines (HEp-2, NCI-H292 and KB) of cytotoxicity within in the tested concentration range of 50.0–1.56 μg/mL [26]. However, this cytotoxicity test showed that the AcOEt: MeOH (95:5), AcOEt, CHCl3 and hexane fraction were the most active against the HEp-2, NCI-H292 and K562 cell lines. The extracts showed promising activity, with IC50 values ranging between 8.2–40.5 μg/mL. Most of these values were within the cutoff point of the National Cancer Institute criteria for cytotoxicity (IC50 < 30 μg/mL) in the screening of crude plant extracts, which indicates that these extracts are promising for further purification and study [27].
Although we observed cytotoxic activity, the E. caatingae extracts and fraction did not possess any activity against the mouse erythrocytes. These data suggest that the cytotoxic activity was not related to the lytic properties or the membrane instability induced by the extracts.
2.3. Study of the Mechanisms of Action of the Phases of Erythroxylum caatingae
2.3.1. Annexin/PI Cell Death Assay
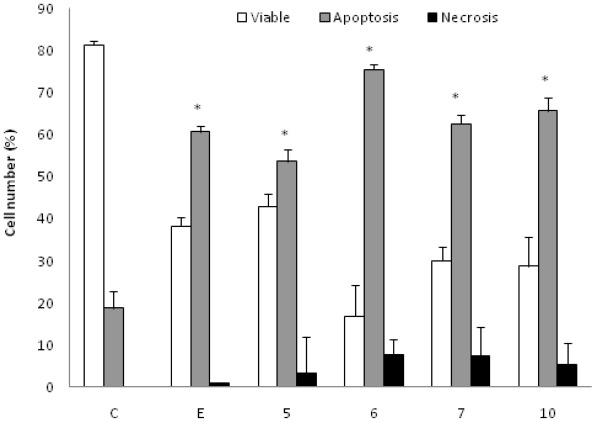
K562 cells incubated with the AcOEt:MeOH (95:5) (5), acetate (6) and CHCl3 fractions (7) and the catuabine B (10) were evaluated with the Annexin V-FITC kit by fluorescent microscopy to verify that cell death was induced by apoptosis through the externalization of phosphatidylserine. The doses chosen for the test were equal to the IC50 values. After 48 h of incubation, we observed that the AcOEt:MeOH (95:5) (13 μg/mL), AcOEt (9.8 μg/mL) and CHCl3 (11.2 μg/mL) fractions, as well as the catuabine B alkaloid (10 μg/mL), all increased the number of cells in early apoptosis (Ann Vpos/PIneg) by 53.0%, 74.8%, 61.7% and 65.0%, respectively, when compared to the control. These substances exhibited values less than 1% of late apoptotic cells (AnnVpos/PIpos) and values less than 8% of cells in necrosis (AnnVneg/PIpos) (Figures 1 and 2).
Figure 1.
Effect of AcOEt:MeOH (95:5) (5), AcOEt (6), CHCl3 (7) and Catuabine B (10) in K562 cell population determined by fluorescence microscopy using Annexin V—FITC Kit, after 48 h incubation. The negative control (C) was the vehicle used for diluting the tested substances. Etoposide (E) was used as positive control. * p < 0.01 compared to control by ANOVA, followed by Newman-Keuls multiple comparison test. Data are presented as mean ± SD from three independent experiments.
Figure 2.
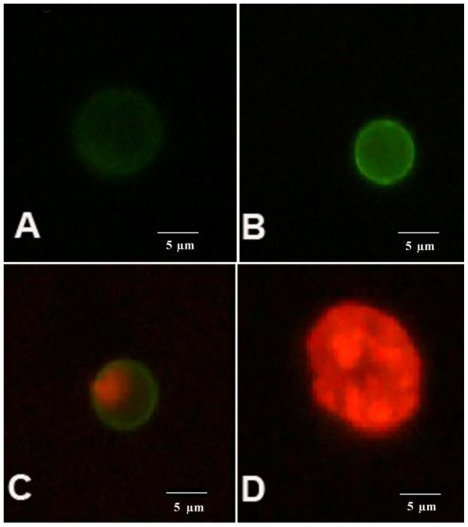
AnnV/PI staining of the cells. An image of K562 showing: (A) viable (AnnVneg/PIneg); (B) early apoptotic (AnnVpos/PIneg); (C) late apoptotic (AnnVpos/PIpos) and (D) necrotic (AnnVneg/PIpos) cell.
Apoptosis is currently a subject of research interest, in part because tumor cells are susceptible to death by apoptosis in response to various drugs used in chemotherapy [28].
Apoptosis is regulated by two major pathways: the extrinsic (receptor mediated) and intrinsic (mitochondrial mediated) pathways. Due to the sensitivity of the intrinsic pathway, tumors arise more often through this pathway than through the extrinsic pathway [29]. The extrinsic pathway involves execution through cell surface death receptors, recruiting the Fas-associated death domain, which leads to the activation of caspase-8 [30]. The intrinsic pathway requires a loss/disruption of the mitochondrial membrane potential in the cells, which eventually may cause the initiation and activation of apoptotic cascades. The cascade triggers the release of cytochrome c and other apoptogenic molecules, such as Smac/DIABLO, from the mitochondria to the cytosol. Once in the cytosol, cytochrome c binds to Apaf-1 and recruits and activates caspase-9 in the apoptosome. Active caspase-9 cleaves and activates caspase-3, the caspase required to complete the induction of apoptosis [31]. Therefore, we sought to determine whether the phases that induced apoptosis were associated with the disruption of the mitochondrial membrane potential in K562 cells. As shown in Figure 2A, the treatment of K562 cells with the AcOEt:MeOH (95:5) (13 μg/mL), AcOEt (9.8 μg/mL) and CHCl3 fraction (11.2 μg/mL), as well as the catuabine B for 48 h resulted in an increase in the number of cells in early apoptosis by 53.0, 74.8, 61.6 and 65.0%, respectively. These data show that the main mechanism of cell death caused by the extracts is apoptosis, which suggests the involvement of the intrinsic pathway.
2.3.2. Measurement of the Mitochondrial Membrane Potential
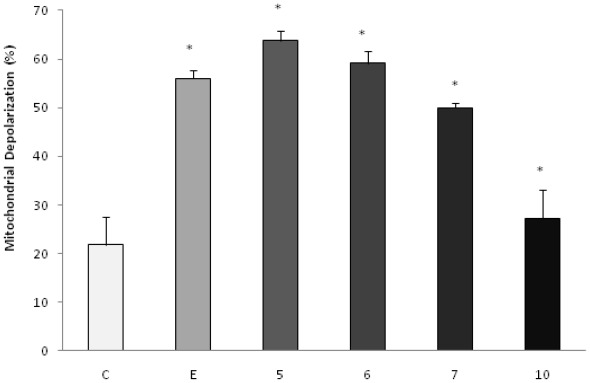
To investigate the possible involvement of the mitochondrial pathway in apoptosis induced by the products under study, the analysis of the mitochondrial membrane potential by the incorporation of JC-1 was evaluated by fluorescence microscopy. The doses chosen for the test were equal to the IC50 values. After 48 h of incubation, we observed that the AcOEt:MeOH (95:5), AcOEt and CHCl3 fractions, as well as the catuabine B (10), all showed 63.8%, 59.2%, 50.0% and 27.2% apoptotic cells, respectively (Figures 3 and 4).
Figure 3.
Effect of AcOEt:MeOH (95:5) (5), AcOEt (6), CHCl3 (7) and catuabine B (10) in K562 cell population determined by fluorescence microscopy using JC-1, after 48 h incubation. The negative control (C) was the vehicle used for diluting the tested substances. Etoposide (E) was used as positive control. * p < 0.01 compared to control by ANOVA, followed by Newman-Keuls multiple comparison test. Data are presented as mean ± SD from three independent experiments.
Figure 4.
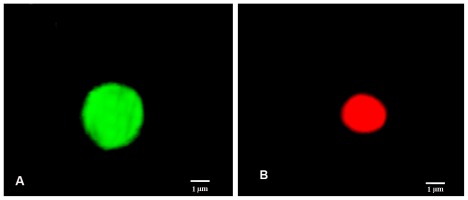
JC-1 staining of the cells. An image of K562 showing: (A) Green fluorescence emitted by cells is attributed to depolarized mitochondrial membrane; (B) No depolarized mitochondrial membrane emits red fluorescence.
To confirm the involvement of the intrinsic pathway in cell death induced by treatment with the fractions and the alkaloids isolated from E. caatingae, an analysis of the mitochondrial membrane potential was made by the incorporation of JC-1. Cells treated with the AcOEt:MeOH (95:5), AcOEt, and CHCl3 fractions, as well as the catuabine B for 48 h, resulted in an increase in the number of green fluorescence-positive cells of 63.8, 59.2, 50.0, and 27%, respectively, thus, confirming the disruption of the mitochondrial membrane potential on treatment.
The intrinsic pathway requires the disruption of the mitochondrial membrane and the release of mitochondrial proteins, such as cytochrome c, which work together with the other two cytosolic protein factors, Apaf-1 (apoptotic protease activating factor-1) and procaspase-9, to promote the assembly of the caspase-activating complex (termed the apoptosome), which in return induces the activation of caspase-9 and initiates the apoptotic caspase cascade [32,33]. In our study, the AcOEt:MeOH (95:5), AcOEt, and CHCl3 fractions showed death by apoptosis, which mainly involved the mitochondrial pathway, especially with the acetate fraction.
3. Experimental Section
3.1. Chemicals
Phosphate buffered saline (PBS), penicillin-streptomycin liquid and DMEM (Dulbecco’s Modified Eagle’s Medium) were purchased from Gibco. MTT (3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide) was purchased from Invitrogen. Eposide (Etoposide) was purchased from Blausiegel. Fetal bovine serum (FBS), glutamine, Triton X-100 and JC-1 (5,5′,6,6′-Tetrachloro-1,1′, 3,3′-tetraethylbenzimidazolcarbocyanine iodide) were purchased from Sigma-Aldrich Brazil. The Annexin V-FITC Apoptosis Detection Kit was purchased from Calbiochem. DMSO (Dimethyl sulfoxide), CaCl2, NaCl, and paraformaldehyde were purchased from Vetec. Mueller Hinton Agar and Sabouraud were purchased from Difco, and kanamycin and ketoconazole were purchased from Cecon.
3.2. Plant Material
The stems of Erythroxylum caatingae Plowman were collected in Picuí, Paraíba, Brazil. The botanical material was identified by Maria de Fátima Agra of the Laboratory of Pharmaceutical Technology, Federal University of Paraíba. A dried specimen was deposited in the herbarium of Lauro Pires Xavier (JPB) of the Federal University of Paraíba under the identification label AGRA 5666.
3.3. Extraction and Isolation
The crude methanol extract (500 g) was dissolved in water and defatted with C6H6. The defatted aqueous extract was acidified with 3% HCl with mechanical mixing and filtered through Celite, which yielded a residue, which was discarded, and an acidic solution. The acidic solution was extracted with CHCl3 (3 × 500 mL), which resulted in an acid chloroform phase and an aqueous phase that was neutralized to pH 7.0 with ammonium hydroxide. The aqueous phase, at pH 7.0, was extracted with CHCl3 to yield an aqueous phase and the basic chloroform phase (4.0 g), which was submitted to column chromatography (CC) using a silica gel as the stationary phase and CHCl3 and MeOH as the eluents, which were used either independently or in binary mixtures with increasing polarity. The result was 55 fractions of 100 mL. The 55 fractions were monitored by thin layer chromatography (TLC) and eluted with various solvent systems (CHCl3 and/or CHCl3-MeOH in order of increasing polarity) in chambers that were pre-saturated with NH3 vapor. They were then revealed with Dragendorff’s reagent and placed in 21 groups based on their Rf. Fraction 25, 45 and 46 were submitted to repeated recrystallization with acetone and ether to obtain alkaloids. The elucidation of the chemical structures was described by Oliveira et al. (2011) [26]. 6β-Benzoyloxy-3α-(3,4,5-trimethoxybenzoyloxy) tropane (Catuabine B) and 3α,6β-dibenzoyloxytropane were also isolated. The extract and fractions obtained were evaporated until dry. The following extracts were all tested: the methanol extract of the stem of E. caatingae (MEEC) and the AcOEt:MeOH (60:40), AcOEt:MeOH (80:20), AcOEt:MeOH (90:10), AcOEt:MeOH (95:5), AcOEt, CHCl3, and the hexane fractions, as well as the total alkaloids fraction.
3.4. Microorganisms
The microorganisms, which were from the Antibiotics Department collection of the Federal University of Pernambuco were as follows: S. aureus, UFPEDA 01; Bacillus subtilis, UFPEDA 16; Enterococcus faecalis, UFPEDA 138; Micrococcus luteus, UFPEDA 06; Escherichia coli, UFPEDA 224; P. aeruginosa, UFPEDA 39; Serratia marcescens, UFPEDA 398; Mycobacterium smegmatis, UFPEDA 71; and Candida albicans, UFPEDA 1007, and these were used in the antimicrobial activity assays.
3.5. Cell Lines and Cell Culture
The cell lines used for the in vitro cytotoxicity tests were K562 (human chronic myelocytic leukemia), NCI-H292 (human lung mucoepidermoid carcinoma cells) and HEp-2 (human larynx epidermoid carcinoma cells), and these were obtained from the Adolph Lutz Institute (São Paulo, Brazil). The cells were maintained in DMEM supplemented with 10% fetal bovine serum, 2 mM glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin at 37 °C with 5% CO2.
3.6. Animals
Three Swiss mice (female, 25–30 g), obtained from the central animal house of the Universidade Federal de Pernambuco, Brazil, were used. The animals were housed in cages with free access to food and water. All animals were kept under a 12 h light:12 h dark cycle (lights on at 6:00 a.m.). The animals were treated according to the ethical principles of animal experimentation of the Brazilian College of Animal Experimentation (COBEA), Brazil. The Animal Studies Committee of the Universidade Federal do Pernambuco approved the experimental protocols (Number 23076.012173/2007-77).
3.7. Antimicrobial Activity
Antimicrobial activity was verified in vitro by the method of diffusion in paper record [34]. The microorganisms were standardized with an optical density of McFarland 0.5 in physiological solution [35], which corresponds to a concentration of approximately 107 UFC/mL for yeast and filamentous fungi and 108 UFC/mL for bacteria. On the inoculated medium, Mueller Hinton Agar (S. aureus, B. subtilis, M. luteus, E. coli, P. aeruginosa and S. marcescens), glucose extracts of yeast (E. faecalis, M. smegmatis) and Sabouraud (C. albicans), and discs of barren paper (6 mm) were absorbed with 10 μL of the solution 200,000 μg/mL of the methanol extract from the stem of E. caatingae and its fractions and were placed on the agar. After placing the discs, the plates were incubated for 24 h and 48 h at 30 °C and 35 °C, respectively. The antibiotics kanamycin and ketoconazole were used as standards at 30 μg/disc and 300 μg/disc, respectively.
The Minimum Inhibitory Concentration (MIC) and Minimum Bacteriostatic Concentration (MBC) were applied to the substances that showed halos larger than 12 mm by serial dilutions in half-solids [36]. Aliquots of different volumes (0.03–1.0 mL) of the solutions at 20.000 μg/mL were placed in Petri dishes and homogenized with 10 mL of the appropriate culture medium. The microorganisms were streaked on the surface of the medium, and the plates were incubated at 35 °C and 30 °C for 24 h and 48 h, respectively. For values over 1000 μg/mL, the extract was considered inactive [37].
3.8. Cell Viability Assay (MTT Assay)
The cytotoxicity of the methanol extract of the stems of E. caatingae and its fractions were tested against the K562, NCI-H292 and HEp-2 tumor cell lines. For these experiments, the cells were plated in 96-well plates (105 cells/mL for adherent cells or 0.3 × 106 cells/mL for suspended cells). After 24 h, the extracts, fractions (6.25–50 μg/mL) and alkaloids (1.25–10 μg/mL) dissolved in DMSO were added to each well and incubated for 72 h. Control groups received DMSO (0.2%) in DMEN. Etoposide (1.25–20 μg/mL) was used as a positive control. The growth of the tumor cells was quantified by the ability of the living cells to reduce yellow tetrazolium MTT (3-(4,5-dimethylthiazolyl-2)-2,5- diphenyltetrazolium bromide) to a blue formazan product [38,39]. At the end of the 72 h incubation period, MTT (5.0 mg/mL) was added to the plate. After three hours for the suspended cells or two hours for the adherent cells, the formazan product from the reduction of MTT was dissolved in DMSO, and the absorbance was measured using a multi-plate reader. The effect of the drug was quantified as the percentage of control absorbance the reduced dye at 450 nm (Multi-plate Reader Thermoplate).
3.9. Hemolytic Assay
The hemolytic assay was performed in 96-well plates, following the method previously described by Costa-Lotufo et al. (2005) [40]. Each well received 100 μL of a 0.85% NaCl solution containing 10 mM CaCl2. The first well served as the negative control and only contained the vehicle (10% DMSO). The second well contained 100 μL of the test substance that was diluted 1:2. The extracts were tested at concentrations ranging from 15.62 to 2000 μg/mL. The serial dilution continued until the 11th well. The last well received 20 μL of 0.1% Triton X-100 (in 0.85% saline) to obtain 100% hemolysis (positive control). Each well received 100 μL of a 2% suspension of mouse erythrocytes in 0.85% saline containing 10 mM CaCl2. After incubation at room temperature for 30 min and centrifugation, the supernatant was removed, and the released hemoglobin was measured by spectroscopic absorbance at 450 nm. Extracts with an EC50 value lower than 200 μg/mL were considered to be active.
3.10. Analysis of the Mechanisms Involved in the Cytotoxic Activity
The following experiments were performed to elucidate the mechanisms involved in the cytotoxic activity of the fractions and alkaloids on K562 cells after 48 h of incubation.
3.10.1. Annexin/PI Cell Death Assay
Cellular apoptosis was stained with Annexin V and propidium iodide (PI) using an Annexin V—FITC kit (Calbiochem) following the protocol provided by the manufacturer. The suspension of K562 cells (0.3 × 106 cells/mL) was seeded into 96-well plates. The plates were incubated at 37 °C with 5% CO2 for 24 h. After this period, the fractions and alkaloids that presented cytotoxicity were added at the concentration that was equal to the IC50 value. After 48 h of treatment, the cells were stained with Annexin V and PI following the manufacturer’s recommended protocol and were analyzed by an epifluorescence microscope (Carl Zeiss, Göttingen, Germany) at 1000× magnification under an oil immersion with an emission filter of LP 515 nm and excitation filter of BP 450–490 nm. A minimum of 200 cells were counted in each sample.
3.10.2. Measurement of the Mitochondrial
Membrane Potential Mitochondrial depolarization was evaluated by the incorporation of JC-1. This assay was performed as previously described, with some modifications [41,42]. The JC-1 probe is freely permeable to cells and undergoes reversible transformation from a monomer to an aggregate form (Jagg). The suspension of K562 cells (0.3 × 106 cells/mL) was seeded into 96-well plates. The plates were incubated at 37 °C and 5% CO2 for 24 h. The fractions that presented cytotoxicity were added at a final concentration equal to the IC50 value. After 48 h of treatment, 50 μL was collected, incubated with JC-1 (0.15 mM in DMSO) for 10 min in the dark and then washed twice with PBS. The cells were fixed with 4% paraformaldehyde, mounted on a glass slide and observed under an epifluorescence microscope (Carl Zeiss, Göttingen, Germany) with 1000× magnification under an oil immersion with an emission filter of LP 515 nm and an excitation filter of BP 450–490 nm. A minimum of 200 cells were counted in each sample. The cells stained in red were classified as having a high potential mitochondrial membrane, while those stained in green were classified as having a low potential membrane.
3.11. Statistical Analysis
Data are presented as the mean ± S.D. The IC50 and EC50 values and their 95% confidence intervals were obtained by nonlinear regression using SigmaPlot 11.0 (version 11.0.1; Systat Software Inc.: Chicago, IL, USA, 2011). The differences between the experimental groups were compared by one-way analysis of variance (ANOVA), followed by Newman-Keuls test; the significance level was set at 1%.
4. Conclusions
According to the results of investigations, methanol extract of the stem of Erythroxylum caatingae and all tested fractions, except the hexane fraction, showed antimicrobial activity on gram-positive bacteria and fungi. The absence of hemolysis in the erythrocytes of mice was observed in these fractions and 6β-Benzoyloxy-3α-(3,4,5-trimethoxybenzoyloxy) tropane (catuabine B). We observed that the acetate:methanol (95:5), acetate, and chloroform fractions, as well as the catuabine B induced apoptosis in K562 cells. An analysis of the potential of the mitochondrial membrane by incorporation of JC-1 showed that most cells during incubation of the acetate:methanol (95:5) and acetate phases were stained, suggesting the involvement of an intrinsic pathway of apoptosis.
Table 2.
Evaluates the minimum inhibitory concentration of the methanol extract of the stem of Erythroxylum caatingae and of its fractions.
| Microorganisms | Minimum Inhibitory Concentration (μg/mL) | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| S. aureus | n.t. | n.t. | n.t. | 250 | 250 | n.t. | n.t. |
| M. luteus | 250 | 250 | n.t. | <31.25 | <31.25 | 250 | <31.25 |
| B. subtilis | n.t. | n.t. | n.t. | 250 | 250 | n.t. | 125 |
| M. smegmatis | 500 | 500 | n.t. | 125 | 125 | 1000 | 125 |
| E. faecalis | n.t. | n.t. | n.t. | n.t. | 500 | n.t. | 500 |
| C. albicans | n.t. | n.t. | n.t. | 250 | 125 | n.t. | 125 |
1: Methanol extract of stem of E. caatingae (MEEC); 2: AcOEt:MeOH (60:40); 3: AcOEt:MeOH (80:20); 4: AcOEt:MeOH (90:10); 5: AcOEt:MeOH (95:5); 6: AcOEt; 7: CHCl3; n.t.: not tested. Each experiment was done in triplicate.
Acknowledgments
The authors thank Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for their financial support.
References
- 1.Brandão M.G.L., Cosenza G.P., Moreira R.A., Monte-Mor R.L.M. Medicinal plants and other botanical products from the Brazilian Official Pharmacopoeia. Rev. Bras. Farmacogn. 2006;16:408–420. [Google Scholar]
- 2.Politi F.A., de Mello J.C., Migliato K.F., Nepomuceno A.L., Moreira R.R., Pietro R.C. Antimicrobial, cytotoxic and antioxidant activities and determination of the total tannin content of bark extracts endopleura uchi. Int. J. Mol. Sci. 2011;12:2757–2768. doi: 10.3390/ijms12042757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yunes R.A., Calixto J.B. Plantas Medicinais sob a ótica da Química Medicinal Moderna. Chapecó (SC); Argos, Brazil: [1st ed]. 2001. pp. 17–44. [Google Scholar]
- 4.Stankovic M.S., Curcic M.G., Zizic J.B., Topuzovic M.D., Solujic S.R., Markovic S.D. Teucrium plant species as natural sources of novel anticancer compounds: Antiproliferative, proapoptotic and antioxidant properties. Int. J. Mol. Sci. 2011;12:4190–4205. doi: 10.3390/ijms12074190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reddy L., Odhav B., Bhoola K. Natural products for cancer prevention: Global perspective. Pharmacol. Ther. 2003;99:1–13. doi: 10.1016/s0163-7258(03)00042-1. [DOI] [PubMed] [Google Scholar]
- 6.Guo X., Zhu K., Zhang H., Yao H. Anti-tumor activity of a novel protein obtained from Tartary Buckwheat. Int. J. Mol. Sci. 2010;11:5201–5211. doi: 10.3390/ijms11125201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cragg G.M., Newman D.J. Plants as a source of anticancer agents. J. Ethopharmacol. 2005;100:72–79. doi: 10.1016/j.jep.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 8.Tan G., Gyllenhaal C., Sorjarto D.D. Biodiversity as a source of anticancer drugs. Curr. Drug Targets. 2006;7:265–277. doi: 10.2174/138945006776054942. [DOI] [PubMed] [Google Scholar]
- 9.Cechinel Filho V. Principais avanços e perspectivas na área de produtos naturais ativos: Estudos desenvolvidos no NIQFAR/Univali. Quim. Nova. 2000;23:680–685. [Google Scholar]
- 10.Antunes R.M.P., Lima E.O., Pereira M.S.V., Camara C.A., Arruda T.A., Catão R.M.R., Barbosa T.P., Nunes X.P., Dias C.S., Silva T.M.S. Atividade antimicrobiana “in vitro” e determinação da concentração inibitória mínina (CIM) de fitoconstituintes e produtos sintéticos sobre bactérias e fungos leveduriformes. Braz. J. Pharmacogn. 2006;16:517–524. [Google Scholar]
- 11.Barreiros M.L., David J.M., Queiroz L.P., David J.P. Flavonoids and triterpenes from leaves of Erythroxylum nummularia. Biochem. Syst. Ecol. 2005;33:537–540. [Google Scholar]
- 12.Loiola M.I.B., Agra M.F., Baracho G.S., Queiroz R.T. Flora da Paraíba, Brasil: Erythroxylaceae Kunth. Acta Bot. Bras. 2007;21:473–487. [Google Scholar]
- 13.Santos C.C., Lima M.A.S., Silveira E.R. Micromolecular secondary metabolites of Erythroxylum barbatum. Biochem. Syst. Ecol. 2003;31:661–664. [Google Scholar]
- 14.Chin Y.W., Jones W.P., Waybright T.J., McCloud T.G., Rasoanaivo P., Cragg G.M., Cassady J.M., Kinghorn A.D. Tropane aromatic ester alkaloids from a large-scale re-collection of Erythroxylum pervillei stem bark obtained in Madagascar. J. Nat. Prod. 2006;69:414–417. doi: 10.1021/np050366v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silva G.L., Cui B., Chávez D., You M., Chai H.B., Rasoanaivo P., Lynn S.M., O’Neill M.J., Lewis J.A., Besterman J.M., et al. Modulation of the multidrug-resistance phenotype by new tropane alkaloid aromatic esters from Erythroxylum pervillei. J. Nat. Prod. 2001;64:1514–1520. doi: 10.1021/np010295+. [DOI] [PubMed] [Google Scholar]
- 16.González-Guevara J.L., Veélez-Castro H., González-García K.L., Payo-Hill A.L., González-Lavaut J.A., Molina-Torres J., Prieto-Gonzaález S. Flavonoid glycosides from Cuban Erythroxylum species. Biochem. Syst. Ecol. 2006;34:539–542. [Google Scholar]
- 17.Zuanazzi J.A.S., Tremea V., Limberger R.P., Sobral M., Henriques A.T. Alkaloids of Erythroxylum (Erythroxylaceae) species from Southern Brazil. Biochem. Syst. Ecol. 2001;29:819–825. doi: 10.1016/s0305-1978(01)00022-9. [DOI] [PubMed] [Google Scholar]
- 18.Khattak K.F., Atta-ur-Rahman Choudhary M.I., Hemalal K.D., Tillekeratne L.M. New tropane alkaloids from Erythroxylum moonii. J. Nat. Prod. 2002;65:929–931. doi: 10.1021/np010221y. [DOI] [PubMed] [Google Scholar]
- 19.Mahesh B., Satish S. Antimicrobial activity of some important medicinal plant against plant and human pathogens. J. Agric. Sci. 2008;4:839–843. [Google Scholar]
- 20.Manabe H. Effects of Catuaba extracts on microbial and HIV infection. In Vivo. 1992;6:161–165. [PubMed] [Google Scholar]
- 21.Atta-ur-Rahman Khattak K.F., Nighat F., Shabbir M., Hemalal K.D., Tillekeratne L.M. Dimeric tropane alkaloids from Erythroxylum moonii. Phytochemistry. 1998;48:377–383. [Google Scholar]
- 22.Haslan E. Natural polyphenols (vegetable tannins) as drugs: Possible modes of action. J. Nat. Prod. 1996;59:205–215. doi: 10.1021/np960040+. [DOI] [PubMed] [Google Scholar]
- 23.Chavez D., Cui B., Chai H.B., Garcia R., Meija M., Farnsworth N.R., Cordell G.A., Pezzuto J.M., Kinghorn A.D. Reversal of multidrug resistance by tropane alkaloids from the stems of Erythroxylum rotundifolium. J. Nat. Prod. 2002;65:606–610. doi: 10.1021/np0104774. [DOI] [PubMed] [Google Scholar]
- 24.Rodeiro I., Donato M.T., Lahoz A., González-Lavaut J.A., Laguna A., Castell J.V., Delgado R., Gómez-Lechón M.J. Modulation of P450 enzymes by Cuban natural products rich in polyphenolic compounds in rat hepatocytes. Chem. Biol. Interact. 2008a;172:1–10. doi: 10.1016/j.cbi.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 25.Rodeiro I., Donato M.T., Martínez I., Hermández I., Garrido G., González-Lavaut J.A., Menéndez R., Laguna A., Castell J.V., Gómez-Lechón M.J. Potential hepatoprotective effects of new Cuban natural products in rat hepatocytes culture. Toxicol. In Vitro. 2008;22:1242–1249. doi: 10.1016/j.tiv.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 26.Oliveira S.L., Silva M.S., Tavares J.F., Branco M.V.S.C., Lucena H.F.S., Barbosa-Filho J.M., Agra M.F., Silva T.G., Nascimento S.C., Aguiar J.S., et al. Tropane alkaloids from Erythroxylum caatingae plowman. Chem. Biodivers. 2011;8:155–165. doi: 10.1002/cbdv.200900400. [DOI] [PubMed] [Google Scholar]
- 27.Mesquita M.L., Paula J.E., Pessoa C., Moraes M.O., Costa-Lotufo L.V., Grougnet R., Michel S., Tillequin F., Espindola L.S. Cytotoxic activity of Brazilian Cerrado plants used in traditional medicine against cancer cell lines. J. Ethopharmacol. 2009;123:439–445. doi: 10.1016/j.jep.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 28.Lima A.P., Pereira F.C., Vilanova-Costa C.A.S.T., Mello F.M.S., Ribeiro A.S.B.B., Benfica P.L., Valadares M.C., Pavanin L.A., Santos W.B., Lacerda E.P.S. The compound cis-(dichloro)tetrammineruthenium(III) chloride induces caspase-mediated apoptosis in K562 cells. Toxicol. In Vitro. 2010;24:1562–1568. doi: 10.1016/j.tiv.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 29.Johnstone R.W., Ruefli A.A., Lowe S.W. Apoptosis: A link between cancer genetics and chemotherapy. Cell. 2002;108:153–164. doi: 10.1016/s0092-8674(02)00625-6. [DOI] [PubMed] [Google Scholar]
- 30.Fesik S.W. Promoting apoptosis as a strategy for cancer drug discovery. Nat. Rev. Cancer. 2005;5:876–885. doi: 10.1038/nrc1736. [DOI] [PubMed] [Google Scholar]
- 31.Meeran S.M., Katiyar S., Katiyar S.K. Berberine-induced apoptosis in human prostate cancer cells is initiated by reactive oxygen species generation. Toxicol. Appl. Pharmacol. 2008;229:33–43. doi: 10.1016/j.taap.2007.12.027. [DOI] [PubMed] [Google Scholar]
- 32.Sun S.Y., Hail N., Jr, Lotan R. Apoptosis as a novel target for cancer chemoprevention. J. Natl. Cancer Inst. 2004;96:662–672. doi: 10.1093/jnci/djh123. [DOI] [PubMed] [Google Scholar]
- 33.Konopleva M., Zhao S., Xie Z., Segall H., Younes A., Claxton D.F., Estrov Z., Kornblau S.M., Andreeff M. Apoptosis: Molecules and mechanisms. Adv. Exp. Med. Biol. 1998;457:217–236. [PubMed] [Google Scholar]
- 34.Bauer A.W., Kirby W.M.M., Sherris J.C., Turk M. Antibiotic susceptibility testing by the standardized single disk method. Am. J. Clin. Pathol. 1966;45:493–496. [PubMed] [Google Scholar]
- 35.Barry A.L. Lorian, Antibiotics in Laboratory Medicine. 5th ed. Williams & Wilkins; Baltimore, MD, USA: 1986. Procedure for Testing Antimicrobial Agents in Agar Media: Theoretical Considerations; p. 13. [Google Scholar]
- 36.Carvalho A.A., Sampaio M.C.C., Sampaio F.C., Melo A.F.M., Sena K.X.F.R., Chiappeta A.A., Higino J.S. Atividade antimicrobiana in vitro de extratos hidroalcoólico de Psidium guajava L. sobre bactérias Gram-negativas. Lat. Am. J. Pharm. 2002;21:255–258. [Google Scholar]
- 37.Holetz F.B., Pessini G.L., Sanches N.R., Cortez D.A.G., Nakamura C.V., Dias Filho B.P. Screening of some plants used in the Brazilian folk medicine for the treatment of infectious diseases. Mem. Inst. Oswaldo Cruz. 2002;97:1027–1031. doi: 10.1590/s0074-02762002000700017. [DOI] [PubMed] [Google Scholar]
- 38.Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;16:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 39.Alley M.C., Scudiere D.A., Monks A., Hursey M.L., Czerwinski M.J., Fine D.L., Abbott B.J., Mayo J.G., Shoemaker R.H., Boyd M.R. Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Res. 1988;48:589–601. [PubMed] [Google Scholar]
- 40.Costa-Lotufo L.V., Cunha G.M.A., Farias P.A.M., Viana G.S.B., Cunha K.M.A., Pessoa C., Moraes M.O., Silveira E.R., Gramosa N.V., Rao V.S.N. The cytotoxic and embryotoxic effects of kaurenoic acid, a diterpene isolated from Copaifera langsdorffii oleo-resin. Toxicon. 2002;40:1231–1234. doi: 10.1016/s0041-0101(02)00128-9. [DOI] [PubMed] [Google Scholar]
- 41.Guthrie H.D., Welch G.R. Determination of intracellular reactive oxygen species and high mitochondrial membrane potential in Percoll-treated viable boar sperm using fluorescence-activated flow cytometry. J. Anim. Sci. 2006;84:2089–2100. doi: 10.2527/jas.2005-766. [DOI] [PubMed] [Google Scholar]
- 42.Kang K.S., Yun J.W., Lee Y.S. Protective effect of l-carnosine against 12-Otetradecanoylphorbol-13-acetate- or hydrogen peroxide-induced apoptosis on v-myc transformed rat liver epithelial cells. Cancer Lett. 2002;178:53–62. doi: 10.1016/s0304-3835(01)00821-7. [DOI] [PubMed] [Google Scholar]