Summary
The lifespan of Caenorhabditis elegans is controlled by signaling between the germline and the soma. Germ cell removal extends lifespan by triggering the activation of the DAF-16/FOXO transcription factor in the intestine. Here we analyze microRNA function in C. elegans aging and show that the microRNA mir-71 functions to mediate the effects of germ cell loss on lifespan. mir-71 is required for the lifespan extension caused by germline removal, and overexpression of mir-71 further extends the lifespan of animals lacking germ cells. mir-71 functions in the nervous system to facilitate the localization and transcriptional activity of DAF-16 in the intestine. Our findings reveal a novel microRNA-dependent mechanism of lifespan regulation by the germline and indicate that signaling among the gonad, the nervous system and the intestine coordinates the lifespan of the entire organism.
Introduction
Genetic studies of C. elegans have identified numerous genes that function in highly conserved pathways to control aging (Kenyon, 2010). For example, in an insulin-like signaling pathway the DAF-2 Insulin/IGF-1 receptor homolog activates a conserved phosphatidylinositol 3-kinase pathway to shorten lifespan by inhibiting the activity of DAF-16, a FOXO family transcription factor (Tatar et al., 2003). DAF-16 promotes longevity by regulating the expression of a number of targets, including antioxidant, antimicrobial and metabolic enzymes (Lee et al., 2003a; Murphy et al., 2003; Oh et al., 2006).
The reproductive systems of worms and possibly also those of flies and mammals regulate lifespan (Kenyon, 2010). For example, when the germline of C. elegans is removed either by laser microsurgery or by mutations that block germ-cell proliferation, animals live up to 60% longer than control animals (Arantes-Oliveira et al., 2002; Hsin and Kenyon, 1999). This lifespan extension requires the activities of DAF-16 and of the steroid hormone receptor DAF-12 (Hsin and Kenyon, 1999). In animals lacking germ cells, DAF-16 accumulates specifically in the intestinal nuclei and activates the transcription of stress-related and metabolic genes (Lin et al., 2001; Wang et al., 2008; Yamawaki et al., 2008). Upon germline removal, the somatic gonad promotes longevity by triggering a pathway involved in the biosynthesis of the endogenous ligands for DAF-12 (Gerisch et al., 2007; Gerisch et al., 2001; Yamawaki et al., 2010).
microRNAs, a class of small non-coding RNAs, have emerged as critical posttranscriptional regulators of gene expression in diverse biological processes (Ambros, 2004; Stefani and Slack, 2008). The first microRNAs discovered, the products of the C. elegans genes lin-4 and let-7, control the timing of developmental events (Ambros and Horvitz, 1984; Chalfie et al., 1981; Reinhart et al., 2000). C. elegans microRNAs also control cell-fate specification, embryonic development, physiology, behavior, neural synaptic activity and longevity (Alvarez-Saavedra and Horvitz, 2010; Boehm and Slack, 2005; Chang et al., 2004; Simon et al., 2008; Yoo and Greenwald, 2005). In this study we performed a comprehensive search for microRNA genes that regulate C. elegans lifespan by determining the lifespans of mutants for most known microRNA genes. We show that the microRNA mir-71 acts in the nervous system to mediate the effects of the germline on longevity.
Results
mir-71 functions to promote longevity and stress resistance and delay aging
We have previously described the isolation and initial characterization of a large collection of strains that carry deletions in most of the 115 known C. elegans microRNA genes (Miska et al., 2007). To identify microRNAs that function in the aging process, we tested these microRNA mutants for defects in aging and longevity. Specifically, we determined the lifespans of 81 mutant strains that carried deletions in 90 microRNA genes. We found that most microRNAs are dispensable for normal lifespan (Supplemental Table 1). Strikingly, two independently isolated deletion mutants of mir-71 each displayed a severe decrease in lifespan of about 40% (Fig. 1A). Since longevity and resistance to stresses are frequently coupled, we subjected mir-71 mutant adults to various stresses. We found that mir-71 mutants showed increased sensitivity to both heat shock and oxidative stress (Fig. S1A,B,C). A transgene that contained a wild-type copy of the mir-71 genomic locus rescued both the short lifespan (Fig. 1B) and the heat-stress sensitivity of mir-71 mutants (Fig. S1D). Removing the 22 bp sequence of the mature mir-71 microRNA from this transgene abolished its rescuing activity (Fig. 1C). Taken together, these results indicate that mir-71 is required for normal lifespan and normal responses to heat and oxidative stress. In agreement with our findings, a recent study of the temporal patterns of microRNA expression during aging reported that mir-71 is upregulated in aging adults and promotes longevity and stress resistance (de Lencastre et al., 2010).
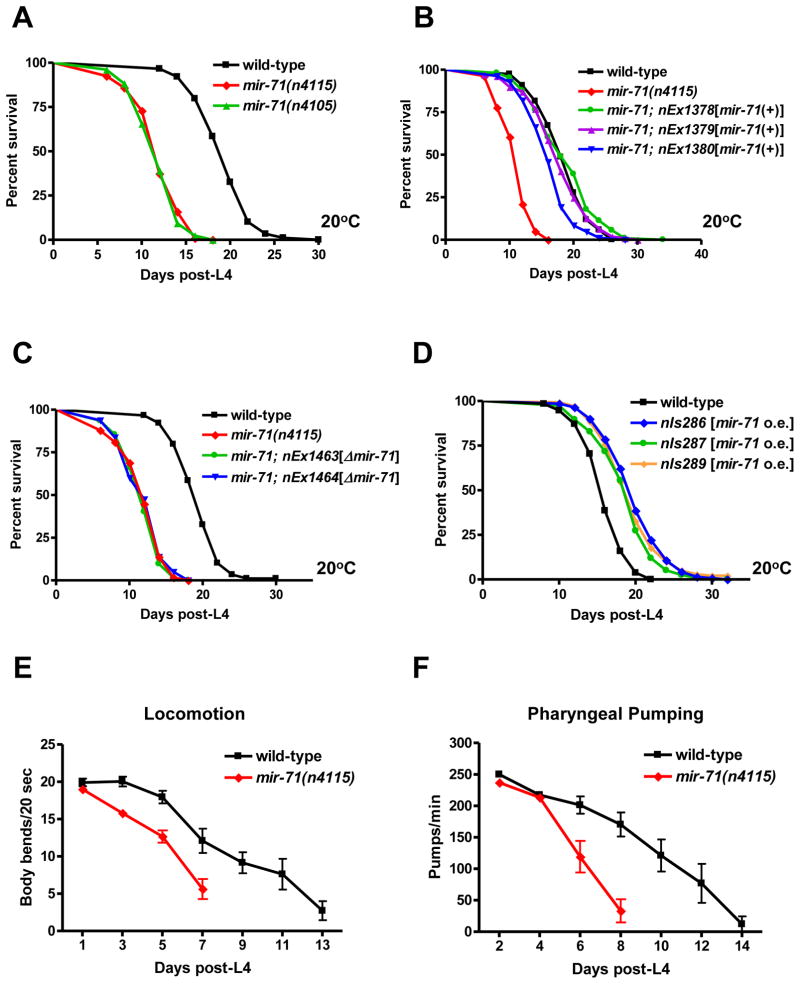
Fig. 1.
The microRNA mir-71 functions to promote longevity and delay aging (A) mir-71 mutants have a short lifespan (p<0.0001; 40% reduction in mean lifespan). (B) A genomic fragment containing the mir-71 locus rescued the lifespan defect of mir-71(n4115) mutants. (p<0.0001; 45–70% mean lifespan extension compared to mir-71(n4115). (C) A genomic fragment lacking the mature mir-71 sequence failed to rescue the lifespan defect of mir-71(n4115) mutants. (p>0.1 compared to mir-71(n4115)). (D) Extra copies of mir-71 extended lifespan. (p<0.0001; 15–25% mean lifespan extension). (E) Young day-1 mir-71 adults showed normal levels of locomotion (p>0.1; mean 19.0 body bends/20 sec) compared to wild-type day-1 adults (mean velocity=19.9 body bends/20 sec). At day 7, mir-71 adults showed a 50% decrease in locomotion (p<0.01; mean 5.6 body bends/20 sec) compared to wild-type adults of the same age (mean 12.1 body bends/20 sec) (wild-type, n=11; mir-71(n4115), n=12). (F) Young mir-71(n4115) adults showed normal levels of pharyngeal pumping compared to the wild-type. The pumping rate of day-2 mir-71 adults was only slightly reduced (p<0.01; mean pumping rate 237.1 pumps/min) compared to the wild-type (mean pumping rate 250.0 pumps/min), and day-4 mir-71 adults had a pumping rate (p>0.1; mean pumping rate 213.3 pumps/min) indistinguishable from that of the wild type (mean pumping rate 217.3 pumps/min). At day 8 mir-71 adults showed a large decrease in pumping rate (p<0.0001; mean pumping rate 33.2 pumps/min) compared to wild-type adults of the same age (mean pumping rate 170.5 pumps/min) (wild-type, n=11; mir-71(n4115), n=12). All experiments were repeated at least once with similar effects. Mean lifespan values and statistical analyses of lifespan assays are shown in Supplementary Table 2. p values refer to the experimental strain and corresponding wild-type control animals unless otherwise noted.
To determine whether overexpression of mir-71 is sufficient to extend C. elegans lifespan, we integrated extrachromosomal arrays carrying the mir-71 locus into the genome and determined the lifespans of multiple resulting transgenic lines. We found that extra copies of mir-71 modestly extended lifespan by 20% (Fig. 1D), suggesting that mir-71 functions to promote longevity and that the short lifespan of mir-71 mutants is not the result of non-specific pathology. Consistent with this hypothesis, aging mir-71 mutant adults showed a premature reduction in the rates of both locomotion and pharyngeal pumping (Fig. 1E,F), two behaviors that normally decline with age (Huang et al., 2004). These results indicate that loss of mir-71 causes an accelerated aging phenotype and suggest that mir-71 acts to delay aging.
mir-71 mediates the effects of germ cell loss on lifespan
To test if mir-71 functions in one of the pathways known to control C. elegans aging, we assayed genetic interactions between mir-71 and various longevity genes. When temperature-sensitive glp-1 mutants grow at the restrictive temperature they fail to develop mature germ cells and as a result live 60% longer than wild-type animals (Fig. 2A) (Arantes-Oliveira et al., 2002). Interestingly, we found that mutations in mir-71 strongly suppressed the long lifespan of germline-deficient glp-1 mutants (Fig. 2A). To confirm that mir-71 is required for the lifespan extension caused by germ cell loss, we used laser microsurgery to ablate the germline precursor cells Z2 and Z3 in wild-type and mir-71 mutant animals. Whereas germline ablation resulted in a robust lifespan extension of wild-type animals, it failed to extend the lifespan of mir-71 mutants (Fig. 2B). These results indicate that mir-71 is required for the increased longevity of germline-less animals and suggest that mir-71 mediates the effects of germ cell loss on lifespan.
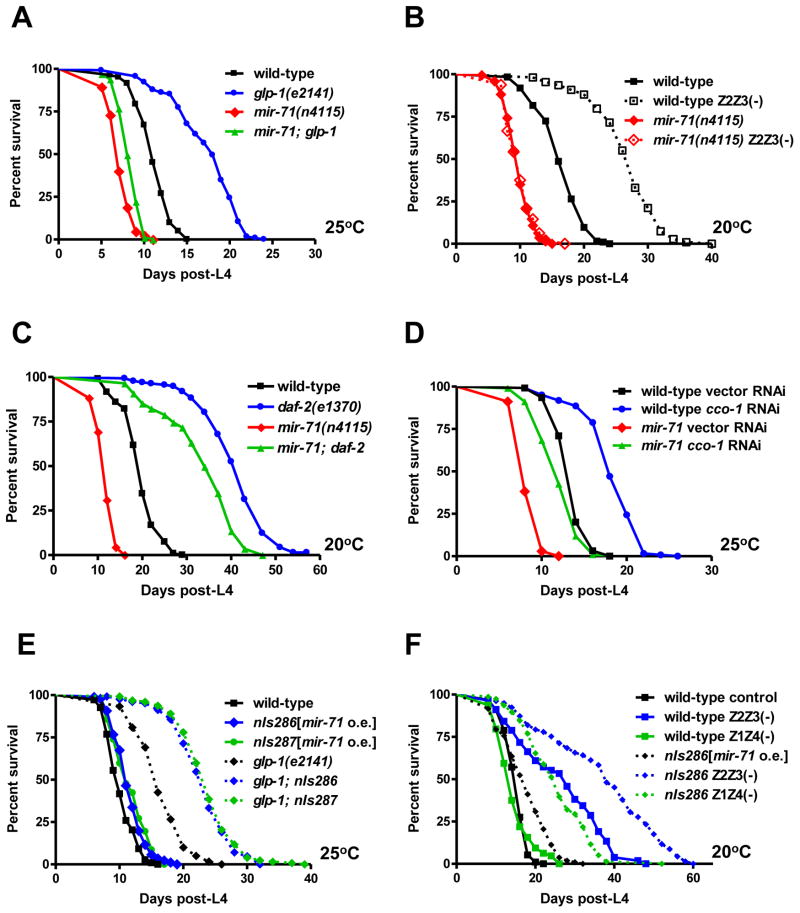
Fig. 2. mir-71 mediates the effects of germ cell loss on longevity.
(A) Loss of mir-71 function suppresses the long lifespan of germline-deficient glp-1(e2141) mutants. Germline removal by glp-1(e2141) resulted in a 55% extension of mean lifespan in otherwise wild-type animals (p<0.0001) compared to a 15% extension in mir-71(n4115) mutants (p<0.0001). (B) Loss of mir-71 function fully suppresses the increased longevity of germline-ablated animals. Ablation of germline precursor cells Z2 and Z3 resulted in a 60% extension of wild-type mean lifespan (p<0.0001), while it had no effect (p>0.5) on mir-71(n4115) mutant lifespan. (C) Loss of daf-2 function extended both wild-type lifespan (p<0.0001; 100% mean lifespan extension) and the lifespan of mir-71(n4115) mutants (p<0.0001; 180% mean lifespan extension) (D) cco-1 RNAi extended both wild-type lifespan (p<0.0001; 35% mean lifespan extension) and the lifespan of mir-71(n4115) mutants (p<0.0001; 45% mean lifespan extension). (E) Extra copies of mir-71 modestly extended the lifespan of intact animals at 25 °C (p<0.0003, 14–15 % mean lifespan extension), whereas it caused a robust extension on the lifespan of germline-deficient glp-1(e2141) animals at 25 °C (p<0.0001, 40–45% mean lifespan extension). (F) Extra copies of mir-71 caused a robust lifespan extension on the lifespan of both germline-ablated (Z2 and Z3) (p<0.0001, 40% mean lifespan extension compared to wild type Z2Z3(−) animals) and somatic gonad-ablated (Z1 and Z4) animals (p<0.0001, 75% mean lifespan extension compared to wild type Z1Z4(−) animals). All experiments were repeated at least once with similar effects. Mean lifespan values and statistical analyses of lifespan assays are shown in Supplementary Table 2.
To examine if mir-71 is specifically required for germ cell loss to extend lifespan, we tested whether deletion of mir-71 suppresses the long lifespan of animals with compromised insulin/IGF signaling or defective mitochondrial function. Partial loss-of-function mutations of the daf-2 Insulin/IGF receptor homolog cause animals to live twice as long as the wild type (Fig. 2C) (Kenyon et al., 1993). We observed that loss of daf-2 function similarly extended the lifespan of mir-71 mutants more than two-fold (Fig. 2C). RNAi that reduced the levels of cco-1 or T02H6.11 or a loss-of-function mutation of isp-1 (all three of these genes encode enzymes that function in mitochondrial respiration (Dillin et al., 2002; Feng et al., 2001; Lee et al., 2003b), extended the lifespan of mir-71 mutants to a similar degree as in a wild-type background (Fig. 2D and Fig. S2A,B). In addition, mir-71 mutants could extend lifespan in response to dietary restriction (Fig. S2C). Taken together, these results indicate that mir-71 is specifically required for the lifespan extension caused by germline removal.
Extra copies of mir-71 further extend the lifespan of germline-less animals
To examine the effect of mir-71 overexpression on the lifespan of germline-deficient animals, we introduced the integrated mir-71 transgenes into glp-1 mutants. While extra copies of mir-71 resulted in a modest lifespan extension in germline-intact animals, overexpression of mir-71 extended the lifespan of germline-deficient glp-1 animals by more than 40% (Fig. 2E). In agreement with this observation, ablation of the germline precursor cells Z2 and Z3 further extended the lifespan of mir-71 overxpressors by about 40%, compared to germline-ablated wild-type controls. Interestingly, extra copies of mir-71 also robustly extended the lifespan of somatic gonad-ablated animals, indicating that the somatic gonad is not required for mir-71-mediated lifespan extension in the absence of the germline (Fig 2F). This effect was specific to germline-deficient animals, as extra copies of mir-71 caused little or no effect on the long lifespan of either daf-2 mutants or animals with defective mitochondrial respiration, respectively (Fig. S3). These results indicate that mir-71 overexpression is sufficient to extend further the lifespan of germline-less animals and suggest that the presence of the germline limits the lifespan-promoting activity of mir-71.
mir-71 is broadly expressed during development and adulthood
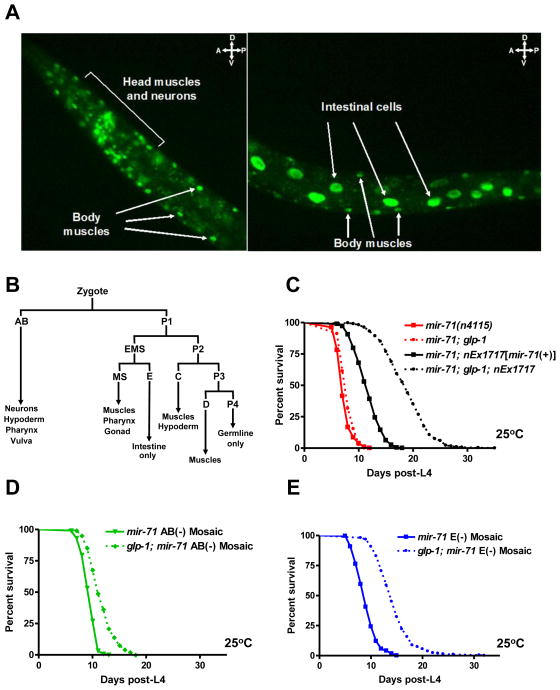
To identify the tissue(s) in which mir-71 functions to regulate longevity, we monitored the spatial pattern of mir-71 expression using a gfp transcriptional reporter. We found that a Pmir-71::gfp reporter was broadly expressed; stronger signal was detected in the intestine, body wall muscles and neurons during larval development and adulthood and weaker expression was observed in the hypoderm of adult animals (Fig. 3A) (Martinez et al., 2008). Germline removal did not affect the expression pattern of this Pmir-71::gfp reporter during development and adulthood (Fig. S4).
Fig. 3.
mir-71 functions in the AB lineage to regulate the lifespan of germline-deficient animals. (A) A Pmir-71::gfp reporter was strongly expressed in the intestine, body wall muscles and neurons during adulthood. (B) A cell-lineage diagram indicating tissues produced by the early blastomeres of C. elegans. (C–E) mir-71 functions primarily in AB-derived tissue(s) to mediate the effects of germline on lifespan. Germline removal had a minor effect on the lifespan of mir-71 mutants (p<0.0001; 7% mean lifespan extension), whereas it robustly extended the lifespan of animals that carried the mir-71-expressing array presumably in all tissues (p<0.0001; 60% mean lifespan extension). Whereas mir-71 E(−) mosaics fully responded to germline removal (p<0.0001; 60% mean lifespan extension), the lifespan of mir-71 AB(−) mosaics was only modestly extended by germ cell loss (p<0.0001; 23% mean lifespan extension). Mean lifespan values and statistical analyses of lifespan assays are shown in Supplementary Table 2.
mir-71 functions in neurons to promote germline-mediated longevity
To test if mir-71 functions in the intestine, muscles, neurons or hypoderm to mediate the effects of germ cell loss on lifespan, we generated genetic mosaics that lacked mir-71 gene function in a subset of cell lineages. Genetic mosaics were produced by the spontaneous loss of an extrachromosomal array that carries the only wild-type gene copy of mir-71 as well as gfp reporters that serve as cell lineage markers (Yochem and Herman, 2003). We identified two classes of genetic mosaics: mir-71 AB(−) mosaics that lacked mir-71 function in the AB lineage, which generates almost the entire nervous system and most of the hypoderm, but retained mir-71 function in the P1 lineage, which gives rise to the intestine, muscles, gonad and part of the hypoderm; and mir-71 E(−) mosaics that lacked mir-71 function in the E lineage (a subset of the P1 lineage), which generates the entire intestine, but retained mir-71 function in the AB lineage (Fig. 3B and Experimental Procedures). As expected, germline removal in animals that carried the mir-71-expressing array in presumably all tissues caused extension of lifespan by 60% (Fig. 3C). We observed that the lifespan of mir-71 AB(−) mosaics, was extended by only 23% by germline removal, indicating that the activity of mir-71 in the AB lineage was largely required for the effect of germ cell loss on lifespan (Fig. 3D). On the other hand, we found that the lifespan of mir-71 E(−) mosaics, was extended by 60% by germline removal, indicating that mir-71 function in the AB lineage is sufficient to fully restore the effect of germ cell loss on lifespan whereas intestinal mir-71 activity is dispensable (Fig. 3E). Therefore, our mosaic analysis shows that mir-71 activity in the AB lineage, which generates almost all neurons and most of the hypoderm, is both necessary and sufficient for germline-mediated longevity.
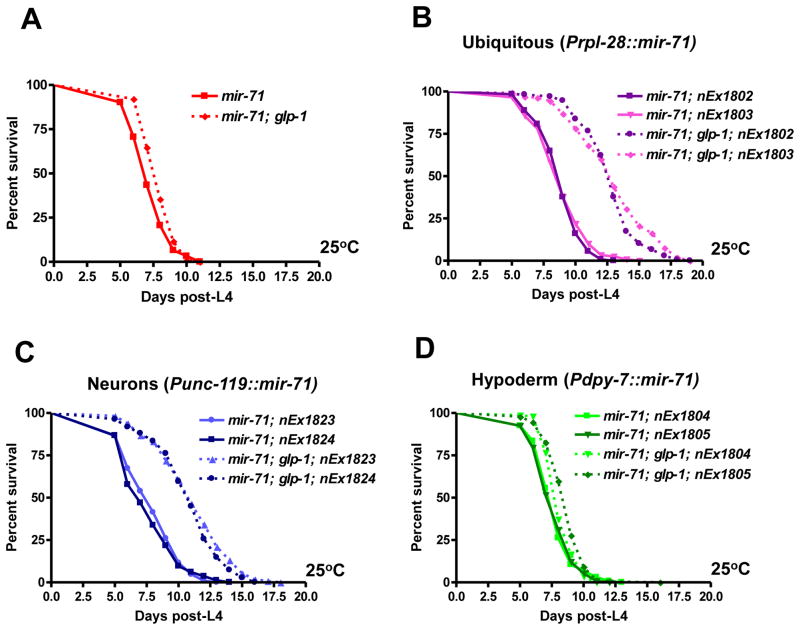
Since Pmir-71::gfp expression was detected in neuronal and hypodermal cells, we hypothesized that mir-71 likely acts in either the nervous system or the hypoderm to mediate the extension of lifespan that occurs in the absence of the germline. To distinguish between those alternatives, we performed tissue-specific rescue experiments. Expression of mir-71 under the control of the ubiquitous rpl-28 promoter strongly rescued the short lifespan of mir-71; glp-1 mutants (Fig. 4A,B). Similarly, driving expression of mir-71 in neurons alone, using the pan-neuronal unc-119 and rab-3 promoters, resulted in strong rescue of the lifespan defect of mir-71 mutants; their lifespan was extended by germline removal by more than 40% or 30%, respectively (Fig. 4C and Fig. S5). By contrast, hypodermal-specific expression of mir-71 failed to restore the effect of germ cell loss on lifespan (Fig. 4D). Taken together, these results indicate that mir-71 functions in the nervous system to promote germline-mediated longevity.
Fig. 4.
Expression of mir-71 in neurons alone was sufficient to promote germline-mediated longevity. (A–D) Driving mir-71 expression either ubiquitously (using the rpl-28 promoter) or in the nervous system of mir-71 mutants (using the pan-neuronal unc-119 promoter) resulted in strong rescue (p<0.0001; 40–45% mean lifespan extension). On the other hand, hypodermal-specific expression of mir-71 (using the dpy-7 promoter) failed to rescue the lifespan defect of mir-71; glp-1 mutants (p<0.0001; 5–10% mean lifespan extension). All experiments were repeated at least once with similar effects. Mean lifespan values and statistical analyses of lifespan assays are shown in Supplementary Table 2.
mir-71-mediated lifespan extension in germline-less animals depends on daf-16
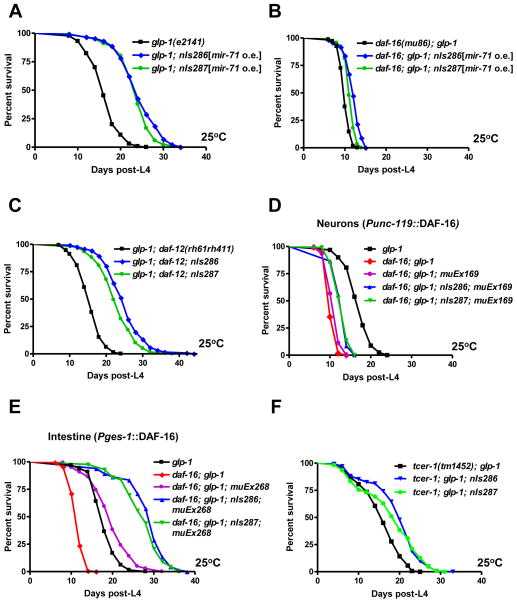
The long lifespan of animals that lack germ cells depends on the activities of DAF-16 and a pathway that signals through the DAF-12 steroid hormone receptor (Gerisch et al., 2007; Hsin and Kenyon, 1999). It is thought that signals from the germline regulate the subcellular localization and transcriptional activity of DAF-16, while signals from the somatic gonad control the biosynthesis of DAF-12 ligands (Lin et al., 2001; Yamawaki et al., 2008; Yamawaki et al., 2010). To test whether mir-71 genetically interacts with either daf-16 or daf-12 to regulate lifespan upon germ cell removal, we examined the effect of mir-71 overexpression in loss-of-function mutants of daf-16 or daf-12. Importantly, we observed that mir-71-mediated lifespan extension was fully suppressed by a null allele of daf-16 but was unaffected by complete loss of daf-12 function (Fig. 5A,B,C and Fig. S6). That daf-12 is dispensable for mir-71 to promote the lifespan of germline-less animals is not surprising, given that the somatic gonad is also not required (Fig. 2F). These results indicate that mir-71 acts upstream of or in parallel to daf-16 to promote germline-mediated longevity.
Fig. 5.
mir-71 functions upstream of or parallel to DAF-16 to promote the longevity of germline-deficient animals. (A) Extra copies of mir-71 caused a robust extension of the lifespan of germline-deficient glp-1(e2141) animals. (p<0.0001, 40–45% mean lifespan extension). (B) Loss of daf-16 function suppressed mir-71-mediated lifespan extension in germline-deficient glp-1(e2141) animals. Extra copies of mir-71 only slightly extended the lifespan of germline-deficient daf-16; glp-1 animals (p<0.0001, 13–20% mean lifespan extension). (C) Loss of daf-12 function did not suppress mir-71-mediated lifespan extension in germline-deficient glp-1(e2141) animals. Extra copies of mir-71 caused a robust extension on the lifespan of germline-deficient glp-1; daf-12 animals (p<0.0001, 45–60% mean lifespan extension). (D) Expression of DAF-16 in neurons was not sufficient for mir-71 overexpression to substantially extend the lifespan of germline-deficient glp-1(e2141) animals. Extra copies of mir-71 caused only a slight extension on the lifespan of germline-deficient glp-1 animals that express daf-16 only in neurons (p<0.0001, 15% mean lifespan extension). (E) Expression of DAF-16 in the intestine was sufficient for mir-71 overexpression to substantially extend the lifespan of germline-deficient glp-1(e2141) animals. Extra copies of mir-71 caused a robust extension on the lifespan of germline-deficient glp-1 animals that express daf-16 only in the intestine (p<0.0001, 40% mean lifespan extension). (F) Deletion of tcer-1 partially suppressed mir-71-mediated lifespan extension in germline-deficient glp-1(e2141) animals. Extra copies of mir-71 only modestly extended the lifespan of germline-deficient tcer-1(tm1452); glp-1 animals (p<0.0001, 15–25% mean lifespan extension). All experiments were repeated at least once with similar effects. Mean lifespan values and statistical analyses of lifespan assays are shown in Supplementary Table 2.
Intestinal daf-16 function is sufficient for mir-71 to promote germline-mediated longevity
To identify the tissue(s) in which daf-16 activity is required for mir-71-mediated lifespan extension, we assayed whether tissue-specific expression of DAF-16 is sufficient for mir-71 overexpression to extend the lifespan of germline-deficient animals. We found that driving daf-16 expression only in neurons had little or no effect on the lifespan of daf-16; glp-1 animals overexpressing mir-71 (Fig. 5D). By contrast, intestinal expression of DAF-16::GFP fully rescued mir-71-mediated lifespan extension, indicating that the activity of DAF-16 in the intestine is sufficient for mir-71 to promote longevity in animals lacking germ cells (Fig. 5E). In short, our results suggest that upon germline removal, mir-71 functions in the nervous system to promote lifespan extension by facilitating the activation of daf-16 in the intestine.
mir-71 promotes the localization and transcriptional activity of DAF-16 in the intestine of germline-less animals
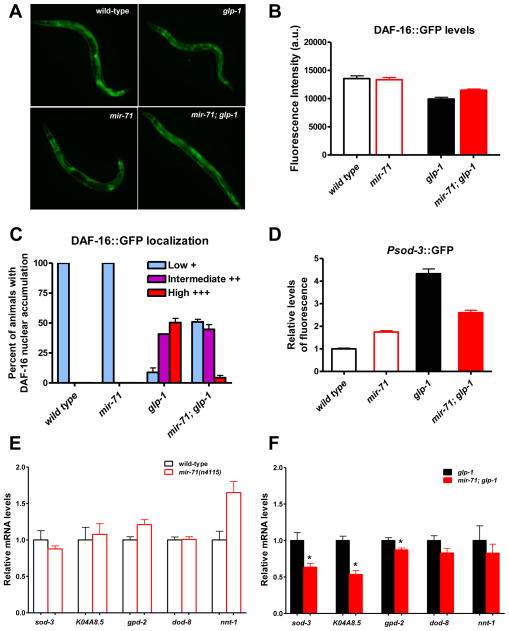
When the germline is removed DAF-16 accumulates in the nuclei of intestinal cells and promotes the expression of several target genes (Lin et al., 2001; Yamawaki et al., 2008). Our findings raise the intriguing possibility that mir-71 regulates the expression, subcellular localization or transcriptional activity of DAF-16 in the intestine of germline-deficient animals. To distinguish among these alternatives, we first examined the expression and subcellular localization of a functional DAF-16::GFP fusion protein. Interestingly, we observed that loss of mir-71 function partially blocked the accumulation of DAF-16::GFP in the intestine of germline-deficient adults without affecting the overall levels of DAF-16 in the presence or absence of germ cells (Fig. 6A,B,C). This effect is specific, since the nuclear translocation of DAF-16 in response to heat shock and in daf-2 mutants was not dependent on mir-71 (Fig. S7). These results suggest that mir-71 specifically facilitates the translocation of DAF-16 to the intestinal nuclei of animals lacking germ cells.
Fig. 6.
mir-71 facilitates the localization and transcriptional activity of DAF-16 in the intestine of animals lacking germ cells. (A–C) Loss of mir-71 function partially affected the nuclear accumulation of DAF-16::GFP in the intestine of germline-deficient glp-1(e2141) animals. (A) Representative images for each genotype. (B) Loss of mir-71 function does not cause major changes in overall levels of DAF-16::GFP expression in day 2 adults (n = 20). Error bar, standard error of the mean (SEM). (C) Intestinal DAF-16::GFP nuclear accumulation was assessed by measuring the number of nuclei from the images of day 2 adults (low accumulation, <10 nuclei; intermediate accumulation, 10–20 nuclei; high accumulation, >20 nuclei) (n = 78 for each genotype). Error bar, standard error of two biological replicates. All strains contain the daf-16(mu86) allele and the functional muIs109[Pdaf-16::DAF-16::GFP] reporter. (D) Loss of mir-71 function blocked the induction of Psod-3::gfp in day-3 germline-deficient glp-1(e2141) animals (p<0.0001; 40% reduction in mean GFP fluorescence intensity) (wild-type, n=32; mir-71(n4115), n=35; glp-1(e2141), n=40; mir-71(n4115); glp-1(e2141), n=40). (E) Loss of mir-71 function did not affect the expression of DAF-16 targets in intact animals. mRNA levels of DAF-16 target genes sod-3, K04A8.5, gpd-2, dod-8 and nnt-1 were measured by qRT-PCR in wild-type and mir-71(n4115) day-2 germline-intact adults. Loss of mir-71 function did not affect the levels of sod-3, K04A8.5, gpd-2 and dod-8, while it resulted in a 50% increase in the levels of nnt-1 (p<0.05). mRNA levels are relative to wild-type levels. Error bars, standard error of four biological replicates. (F) Loss of mir-71 function reduced the expression of a subset of DAF-16 targets in germline-less animals. mRNA levels of DAF-16 target genes sod-3, K04A8.5, gpd-2, dod-8 and nnt-1 were measured by qRT-PCR in germline-deficient glp-1 and mir-71; glp-1 day-2 adults. Loss of mir-71 function in gelmline-defective glp-1 animals resulted in 50% decrease in the levels of sod-3 (p<0.05) and K04A8.5 (p<0.01) and a small reduction in the levels of gpd-2 (p<0.05). mRNA levels are shown relative to glp-1(e2141) levels. Error bars, standard error of four biological replicates.
To examine whether the absence of mir-71 affects the ability of DAF-16 to activate its targets, we examined the expression of Psod-3::gfp, a well-characterized and widely used sensor of DAF-16 activity (Libina et al., 2003; Yamawaki et al., 2008). As previously shown, we found that Psod-3::gfp expression was upregulated primarily in the intestine of germline-deficient glp-1 adults (Fig. 6D) (Yamawaki et al., 2008). Importantly, we found that loss of mir-71 function partially blocked the induction of Psod-3::gfp, suggesting that mir-71 is required for the transcriptional activation of DAF-16-dependent gene targets in the intestine (Fig. 6D). To directly test this, we measured by qRT-PCR the transcript levels of a number of genes known to be upregulated in the intestine of germline-deficient animals in a daf-16-dependent manner. The induction of superoxide dismutase sod-3, the triglyceride lipase K04A8.5 and the glyceraldehyde 3-phospate dehydrogenase gpd-2 is totally dependent on DAF-16, while the upregulation of the putative steroid dehydrogenase dod-8 and the nicotinamide nucleotide transhydrogenase nnt-1 is partially daf-16-dependent (Wang et al., 2008; Yamawaki et al., 2008). We found that loss of mir-71 function in animals lacking germ cells resulted in 50% reduction in the levels of sod-3 and K04A8.5; the levels of gpd-2, dod-8 and nnt-1 were not significantly affected (Fig. 6F). By contrast, the levels of sod-3 and K04A8.5 were not affected by the absence of mir-71 in germline-intact animals (Fig. 6E). Taken together, our results suggest that mir-71 promotes germline-mediated longevity by regulating the localization and transcriptional activity of DAF-16.
Discussion
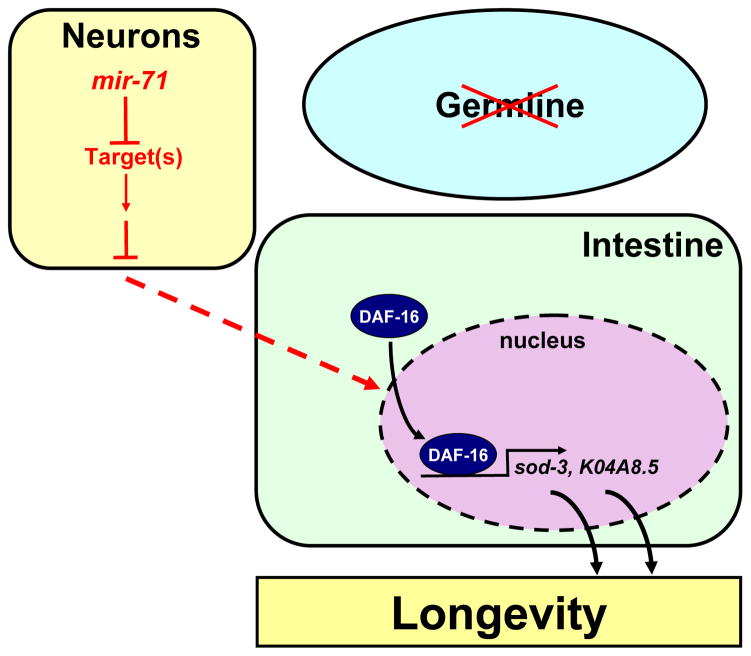
In this study we report a systematic analysis of microRNA genes in the regulation of aging of an entire organism. We have established that the microRNA gene mir-71 is a critical factor in mediating the effect of germ cell loss on lifespan: mir-71 is necessary for the lifespan extension caused by germline removal and promotes the longevity of animals lacking germ cells by regulating the localization and transcriptional activity of DAF-16. We propose a model in which mir-71 functions in the nervous system to mediate the production or modification of a lifespan-extending signal that promotes the intestinal expression of key DAF-16-dependent target genes (Fig. 7). According to this model, mir-71 could inhibit posttranscriptionally the expression of neuronal factor(s) that antagonize cell non-autonomously the activity of DAF-16 in the intestine (Fig. 7).
Figure 7.
A model for the regulation of germline-mediated longevity by the mir-71 microRNA. We propose that mir-71 functions in neurons to inhibit posttranscriptionally the expression of factor(s) involved in the production or modification of a signal that controls the localization and activity of DAF-16 in the intestine.
Several approaches using bioinformatics have been developed to help identify direct targets of microRNAs. Although these algorithms have identified a large number of predicted microRNA targets, experimental validation of most of these putative targets is lacking (Hammell et al., 2008; Lall et al., 2006; Lewis et al., 2005). Recently, de Lencastre et al. (2010) implicated cdc-25.1 as a potential target of mir-71 function. We have tested if cdc-25.1 is a biologically significant target of mir-71 in controlling germline-mediated longevity in genetic epistasis experiments; our preliminary results do not support this hypothesis (K. Boulias and H.R. Horvitz, unpublished observations). Given our finding that mir-71 activity in neurons is important for germline-mediated longevity, it will be interesting to test experimentally whether predicted mir-71 targets known to be highly enriched in the nervous system function to mediate the effects of germline on lifespan.
Our results indicate that the germline strongly suppresses the ability of mir-71 to promote longevity, since extra copies of mir-71 have only a modest effect on the lifespan of intact animals. When the germline is removed, mir-71-mediated lifespan extension requires the intestinal activity of daf-16, whereas lifespan extension does not depend on the presence of the somatic gonad or the daf-12 pathway. Since mir-71 expression is not regulated by the germline, we postulate that mir-71-mediated lifespan extension requires factors that are triggered by germline removal and act on DAF-16 function. Previous studies have shown that germline removal triggers the up-regulation of TCER-1, a transcription elongation factor that acts to promote the transcriptional activity of DAF-16 in the intestine (Ghazi et al., 2009). We found that mir-71-mediated lifespan extension is partially dependent on tcer-1 gene function (Fig. 5F). Thus, mir-71 might act with TCER-1 and possibly other factors to promote intestinal DAF-16 activity in germline-deficient animals.
Current evidence suggests that the rate of aging at least in C. elegans and Drosophila is coordinated through communication and signaling among different tissues. For example, tissue-specific manipulations of insulin/IGF-1 signaling in the intestine or the nervous system have been shown to regulate the lifespan of the whole organism, while genetic ablation of a set of neurons can affect the ability of worms to extend lifespan in response to dietary restriction (Bishop and Guarente, 2007; Broughton et al., 2005; Hwangbo et al., 2004; Libina et al., 2003; Wolkow et al., 2000). In addition, a recent study showed that perturbation of mitochondrial function can modulate C. elegans aging in a cell non-autonomous fashion (Durieux et al., 2011). Furthermore, the germline of C. elegans is thought to send signals that inhibit the lifespan of the entire animal (Kenyon, 2010), while the somatic gonad is thought to be involved in the production of a steroid hormone that promotes the lifespan of animals lacking germ cells (Yamawaki et al., 2010). Tissue-specific rescue experiments suggested that the somatic gonad might act through the hypoderm, the endocrine XXX cells or sensory neurons to promote longevity (Yamawaki et al., 2010). Our finding that mir-71 activity in neurons is sufficient to promote longevity underscores the importance of the nervous system and neuroendocrine signaling for the control of germline-mediated longevity. Based on our results, we suggest that mir-71 functions cell non-autonomously in neurons to promote DAF-16 activity in the intestine. In short, our results implicate the nervous system in lifespan control upon germ cell removal and support a model in which signaling among the germline, the somatic gonad, the intestine and the nervous system coordinates the rate of aging of the whole organism.
Experimental Procedures
Strains
Strains were cultured as described (Brenner, 1974) and maintained at 20°C unless specified otherwise. Strains that contained the glp-1(e2141ts) allele were maintained at 15 °C. mir-71(n4115) was outcrossed 8x, mir-71(n4105) 6x and nIs286, nIs287 and nIs289 4x to the wild type. A list of the strains used in this study is provided in Supplementary Material.
Rescue Experiments and Transgenic Animals
For rescue experiments and mosaic analysis, we amplified a 3 kb fragment surrounding the mir-71 locus (2 kb upstream of and 1 kb downstream of the mir-71 locus) from wild-type genomic DNA using PCR and cloned this fragment into the PCRII-TOPO (Invitrogen) vector. We used site-directed ligase-independent mutagenesis to generate a control plasmid in which the mature microRNA sequence was deleted (Chiu et al., 2004). To generate the Pmir-71::gfp reporter, we amplified by PCR the 2 kb upstream region of the mir-71 locus present in the rescuing construct and cloned this fragment into the pPD96.62 (Adgene) vector.
For tissue-specific rescue experiments we substituted the gfp coding sequence of pPD95.75 with the mir-71 precursor sequence (pPD95.75-mir-71pr). Subsequently, we cloned either the rpl-28 promoter fragment (ubiquitous expression) from pPD129.57 or the unc-119 promoter fragment (pan-neuronal expression) from Punc-119::gfp (Nakano et al., 2010) or the dpy-7 promoter fragment (hypodermal expression) from Pdpy-7::2Xnls::yfp (Myers and Greenwald, 2005) into pPD95.75-mir-71pr.
Germline transformation experiments were performed as described (Mello et al., 1991). For rescue experiments, injection mixes contained plasmids at 5 ng/μl (for mir-71 and for a mir-71 control plasmid with the mir-71 mature sequence deleted), 20 ng/μl of pTG96 (Psur-5::gfp) as a co-transformation marker and 80 ng/μl of 1 kb DNA ladder (Invitrogen) as carrier DNA. For mosaic analyses, mir-71(n4115) hermaphrodites were injected with a mix that contained 5 ng/μl of mir-71 rescuing construct, 50 ng/μl of Posm-6::gfp construct (Collet et al., 1998), 50 ng/μl of Pges-1::gfp construct (Bishop and Guarente, 2007) and 80 ng/μl of 1 kb DNA ladder. For tissue-specific rescue experiments, mir-71(n4115) hermaphrodites were injected with a mix that contained 20 ng/μl of Prpl-28::mir-71 construct or 100 ng/ul Punc-119::mir-71 construct or 100 ng/ul Prab-3::mir-71 construct or 20 ng/ul Pdpy-7::mir-71 construct along with 100 ng/ul pRF4 and 80 ng/μl of 1 kb DNA ladder. We generated the Pmir-71::gfp transgenic strain by injecting lin-15AB(n765) hermaphrodites with 30 ng/μl of Pmir-71::gfp construct, 33 ng/μl plin-15(EK) and 80 ng/μl of 1 kb DNA ladder.
nIs286, nIs287 and nI289, integrants of the Ex[mir-71(+) + pTG96] transgene, and nIs298, an integrant of the Ex[Pmir-71::gfp] transgene, were isolated after a standard γ-ray integration screen and were backcrossed twice to the wild type before analysis (Mello et al., 1991).
Lifespan Analyses
Unless stated otherwise, lifespan assays were performed by standard methods at 20 °C using NGM plates seeded with OP50 bacteria containing 25 μM FUdR. In lifespan experiments assaying strains that carried the glp-1(e2141ts) mutation, animals (glp-1 and control germline-intact controls) were raised at 25 °C during embryogenesis and were either kept at 25 °C on plates without FUdR throughout the analysis or shifted to 20 °C after the L4 stage and for the rest of the lifespan analysis. Statistical analysis was performed with GraphPad Prism 4 software, which uses the log-rank (Mantel-Cox) method to calculate p values. RNAi lifespan assays were performed according to the standard feeding protocol (Kamath et al., 2003).
Behavioral Assays
Single animals were maintained on individual NGM plates throughout adulthood and were transferred to fresh plates seeded with OP50 bacteria before locomotion or pumping rates were counted. Locomotion rates were determined by counting body bends per 20 sec of animals moving on a fresh bacterial lawn using a dissecting microscope. Pumping rates were assayed by counting the number of movements per min of the rear bulb of the pharynx of animals within the bacterial lawn using a dissecting microscope.
Laser Ablation Experiments
Laser ablations of germline precursor cells (Z2 and Z3) and somatic gonad precursor cells (Z1 and Z4) of newly hatched L1 larvae were performed as described previously (Avery and Horvitz, 1987). At adulthood the absence of a germline was determined using a dissecting microscope. Intact controls were anesthetized and recovered from the same sodium azide agarose pads as experimental animals.
Mosaic analysis
We used mir-71(n4115); nEx1717 and mir-71(n4115); glp-1(e2141); nEx1717 animals to generate mir-71 genetic mosaics. nEx1717 is an extrachromosomal array containing a genomic copy of mir-71 as well as lineage specific markers (see Rescue Experiments and Transgenic Animals). Approximately 200,000 progeny of each of mir-71(n4115); nEx1717 and mir-71(n4115); glp-1(e2141); nEx1717 animals were raised at 25 °C until the L4 stage and then were screened using a fluorescence dissecting microscope (Olympus) for mosaic animals in which either the AB-specific marker Posm-6::gfp (expressed in ciliated neurons) or the E-specific marker Pges-1::gfp (expressed in the intestine generated by the P1 lineage) was absent. AB(−) mosaics (Posm-6::gfp negative, Pges-1::gfp positive) lost the mir-71 locus in the AB lineage and presumably retained mir-71 locus in the P1 lineage. E(−) mosaics (Posm-6::gfp positive, Pges-1::gfp negative) lost the mir-71 locus in the E lineage, presumably retained mir-71 locus in the AB lineage and might or might not have carried the mir-71 locus in the MS and P2 lineages. Thus E(−) mosaics probably included a mix of E(−), EMS(−) and P1(−) mosaic animals. Mosaics were selected as L4 larvae and were transferred to 20 °C for lifespan analysis. mir-71(n4115); nEx1717 and mir-71(n4115); glp-1(e2141); nEx1717 controls (array present in all cells), and mir-71(n4115) and mir-71(n4115); glp-1(e2141) controls (array lost in all cells) underwent the same procedure. They were selected using the fluorescence dissecting microscope in parallel with the mosaic animals and were exposed to UV radiation for approximately the same time.
GFP Fluorescence Microscopy and Quantification
Animals were anaesthetized on agarose pads containing 20–50 mM NaN3. Images were taken with a CCD digital camera using a 5X objective on a Zeiss Axioskop microscope. For each trial, exposure time was calibrated to minimize the number of saturated pixels and was kept constant though the experiment. The ImageJ software was used to quantify mean fluorescence intensity per worm as measured by intensity of each pixel in the selected area. No expression of the transgene was visible in embryos prior to egg laying.
Quantitative RT-PCR Analysis
Germline-deficient glp-1(e2141ts) and wild-type N2 animals were raised at 25 °C until the L4 stage and then shifted to 20 °C. On day 2 of adulthood, animals were collected for RNA extraction. RNA extraction, purification, and reverse transcription and qPCR were carried as described (Andersen et al., 2008). Data were generated from four biological replicates. mRNA levels of snb-1 and rpl-26 were used for normalization (Curran et al., 2009). Primer sequences are available upon request.
Statistical Analysis
Error bars represent the standard error of the mean (S.E.M). p values were calculated using the unpaired Student’s t test.
Supplementary Material
Highlights.
C. elegans lifespan extension caused by germ cell loss depends on the microRNA mir-71
mir-71 functions in neurons to promote germline-mediated longevity
mir-71-mediated lifespan extension depends on intestinal daf-16 function
mir-71 facilitates the localization and activity of DAF-16 in the intestine
Acknowledgments
We thank S. Nakano, D.P. Denning, A. Saffer, E. Alvarez-Saavedra and A. Chalkiadaki for critically reading the manuscript; N.A. Bishop and L. Guarente for plasmids and for the dietary restriction analyses; A. Antebi, R.K. Herman and the Caenorhabditis Genetics Center for strains and plasmids and members of the Horvitz laboratory for discussions. This work was supported by a European Molecular Biology Organization fellowship, a grant from the Ellison Medical Foundation and by the Howard Hughes Medical Institute. H.R.H is an Investigator of the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alvarez-Saavedra E, Horvitz HR. Many families of C. elegans microRNAs are not essential for development or viability. Curr Biol. 2010;20:367–373. doi: 10.1016/j.cub.2009.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- Ambros V, Horvitz HR. Heterochronic mutants of the nematode Caenorhabditis elegans. Science. 1984;226:409–416. doi: 10.1126/science.6494891. [DOI] [PubMed] [Google Scholar]
- Andersen EC, Saffer AM, Horvitz HR. Multiple levels of redundant processes inhibit Caenorhabditis elegans vulval cell fates. Genetics. 2008;179:2001–2012. doi: 10.1534/genetics.108.092197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arantes-Oliveira N, Apfeld J, Dillin A, Kenyon C. Regulation of life-span by germ-line stem cells in Caenorhabditis elegans. Science. 2002;295:502–505. doi: 10.1126/science.1065768. [DOI] [PubMed] [Google Scholar]
- Avery L, Horvitz HR. A cell that dies during wild-type C. elegans development can function as a neuron in a ced-3 mutant. Cell. 1987;51:1071–1078. doi: 10.1016/0092-8674(87)90593-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop NA, Guarente L. Two neurons mediate diet-restriction-induced longevity in C. elegans. Nature. 2007;447:545–549. doi: 10.1038/nature05904. [DOI] [PubMed] [Google Scholar]
- Boehm M, Slack F. A developmental timing microRNA and its target regulate life span in C. elegans. Science. 2005;310:1954–1957. doi: 10.1126/science.1115596. [DOI] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broughton SJ, Piper MD, Ikeya T, Bass TM, Jacobson J, Driege Y, Martinez P, Hafen E, Withers DJ, Leevers SJ, et al. Longer lifespan, altered metabolism, and stress resistance in Drosophila from ablation of cells making insulin-like ligands. Proc Natl Acad Sci U S A. 2005;102:3105–3110. doi: 10.1073/pnas.0405775102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalfie M, Horvitz HR, Sulston JE. Mutations that lead to reiterations in the cell lineages of C. elegans. Cell. 1981;24:59–69. doi: 10.1016/0092-8674(81)90501-8. [DOI] [PubMed] [Google Scholar]
- Chang S, Johnston RJ, Jr, Frokjaer-Jensen C, Lockery S, Hobert O. MicroRNAs act sequentially and asymmetrically to control chemosensory laterality in the nematode. Nature. 2004;430:785–789. doi: 10.1038/nature02752. [DOI] [PubMed] [Google Scholar]
- Chiu J, March PE, Lee R, Tillett D. Site-directed, Ligase-Independent Mutagenesis (SLIM): a single-tube methodology approaching 100% efficiency in 4 h. Nucleic Acids Res. 2004;32:e174. doi: 10.1093/nar/gnh172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collet J, Spike CA, Lundquist EA, Shaw JE, Herman RK. Analysis of osm-6, a gene that affects sensory cilium structure and sensory neuron function in Caenorhabditis elegans. Genetics. 1998;148:187–200. doi: 10.1093/genetics/148.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran SP, Wu X, Riedel CG, Ruvkun G. A soma-to-germline transformation in long-lived Caenorhabditis elegans mutants. Nature. 2009;459:1079–1084. doi: 10.1038/nature08106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lencastre A, Pincus Z, Zhou K, Kato M, Lee SS, Slack FJ. MicroRNAs Both Promote and Antagonize Longevity in C. elegans. Curr Biol. 2010;20:2159–2168. doi: 10.1016/j.cub.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillin A, Hsu AL, Arantes-Oliveira N, Lehrer-Graiwer J, Hsin H, Fraser AG, Kamath RS, Ahringer J, Kenyon C. Rates of behavior and aging specified by mitochondrial function during development. Science. 2002;298:2398–2401. doi: 10.1126/science.1077780. [DOI] [PubMed] [Google Scholar]
- Durieux J, Wolff S, Dillin A. The cell-non-autonomous nature of electron transport chain-mediated longevity. Cell. 2011;144:79–91. doi: 10.1016/j.cell.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, Bussiere F, Hekimi S. Mitochondrial electron transport is a key determinant of life span in Caenorhabditis elegans. Dev Cell. 2001;1:633–644. doi: 10.1016/s1534-5807(01)00071-5. [DOI] [PubMed] [Google Scholar]
- Gerisch B, Rottiers V, Li D, Motola DL, Cummins CL, Lehrach H, Mangelsdorf DJ, Antebi A. A bile acid-like steroid modulates Caenorhabditis elegans lifespan through nuclear receptor signaling. Proc Natl Acad Sci U S A. 2007;104:5014–5019. doi: 10.1073/pnas.0700847104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerisch B, Weitzel C, Kober-Eisermann C, Rottiers V, Antebi A. A hormonal signaling pathway influencing C. elegans metabolism, reproductive development, and life span. Dev Cell. 2001;1:841–851. doi: 10.1016/s1534-5807(01)00085-5. [DOI] [PubMed] [Google Scholar]
- Ghazi A, Henis-Korenblit S, Kenyon C. A transcription elongation factor that links signals from the reproductive system to lifespan extension in Caenorhabditis elegans. PLoS Genet. 2009;5:e1000639. doi: 10.1371/journal.pgen.1000639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammell M, Long D, Zhang L, Lee A, Carmack CS, Han M, Ding Y, Ambros V. mirWIP: microRNA target prediction based on microRNA-containing ribonucleoprotein-enriched transcripts. Nat Methods. 2008;5:813–819. doi: 10.1038/nmeth.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsin H, Kenyon C. Signals from the reproductive system regulate the lifespan of C. elegans. Nature. 1999;399:362–366. doi: 10.1038/20694. [DOI] [PubMed] [Google Scholar]
- Huang C, Xiong C, Kornfeld K. Measurements of age-related changes of physiological processes that predict lifespan of Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2004;101:8084–8089. doi: 10.1073/pnas.0400848101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwangbo DS, Gershman B, Tu MP, Palmer M, Tatar M. Drosophila dFOXO controls lifespan and regulates insulin signalling in brain and fat body. Nature. 2004;429:562–566. doi: 10.1038/nature02549. [DOI] [PubMed] [Google Scholar]
- Kamath RS, Fraser AG, Dong Y, Poulin G, Durbin R, Gotta M, Kanapin A, Le Bot N, Moreno S, Sohrmann M, et al. Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature. 2003;421:231–237. doi: 10.1038/nature01278. [DOI] [PubMed] [Google Scholar]
- Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature. 1993;366:461–464. doi: 10.1038/366461a0. [DOI] [PubMed] [Google Scholar]
- Kenyon CJ. The genetics of ageing. Nature. 2010;464:504–512. doi: 10.1038/nature08980. [DOI] [PubMed] [Google Scholar]
- Lall S, Grun D, Krek A, Chen K, Wang YL, Dewey CN, Sood P, Colombo T, Bray N, Macmenamin P, et al. A Genome-Wide Map of Conserved MicroRNA Targets in C. elegans. Curr Biol. 2006;16:461–470. doi: 10.1016/j.cub.2006.01.050. [DOI] [PubMed] [Google Scholar]
- Lee SS, Kennedy S, Tolonen AC, Ruvkun G. DAF-16 target genes that control C. elegans life-span and metabolism. Science. 2003a;300:644–647. doi: 10.1126/science.1083614. [DOI] [PubMed] [Google Scholar]
- Lee SS, Lee RY, Fraser AG, Kamath RS, Ahringer J, Ruvkun G. A systematic RNAi screen identifies a critical role for mitochondria in C. elegans longevity. Nat Genet. 2003b;33:40–48. doi: 10.1038/ng1056. [DOI] [PubMed] [Google Scholar]
- Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- Libina N, Berman JR, Kenyon C. Tissue-specific activities of C. elegans DAF-16 in the regulation of lifespan. Cell. 2003;115:489–502. doi: 10.1016/s0092-8674(03)00889-4. [DOI] [PubMed] [Google Scholar]
- Lin K, Hsin H, Libina N, Kenyon C. Regulation of the Caenorhabditis elegans longevity protein DAF-16 by insulin/IGF-1 and germline signaling. Nat Genet. 2001;28:139–145. doi: 10.1038/88850. [DOI] [PubMed] [Google Scholar]
- Martinez NJ, Ow MC, Reece-Hoyes JS, Barrasa MI, Ambros VR, Walhout AJ. Genome-scale spatiotemporal analysis of Caenorhabditis elegans microRNA promoter activity. Genome Res. 2008;18:2005–2015. doi: 10.1101/gr.083055.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello CC, Kramer JM, Stinchcomb D, Ambros V. Efficient gene transfer in C. elegans: extrachromosomal maintenance and integration of transforming sequences. Embo J. 1991;10:3959–3970. doi: 10.1002/j.1460-2075.1991.tb04966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miska EA, Alvarez-Saavedra E, Abbott AL, Lau NC, Hellman AB, McGonagle SM, Bartel DP, Ambros VR, Horvitz HR. Most Caenorhabditis elegans microRNAs are individually not essential for development or viability. PLoS Genet. 2007;3:e215. doi: 10.1371/journal.pgen.0030215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy CT, McCarroll SA, Bargmann CI, Fraser A, Kamath RS, Ahringer J, Li H, Kenyon C. Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature. 2003;424:277–283. doi: 10.1038/nature01789. [DOI] [PubMed] [Google Scholar]
- Myers TR, Greenwald I. lin-35 Rb acts in the major hypodermis to oppose ras-mediated vulval induction in C. elegans. Dev Cell. 2005;8:117–123. doi: 10.1016/j.devcel.2004.11.015. [DOI] [PubMed] [Google Scholar]
- Nakano S, Ellis RE, Horvitz HR. Otx-dependent expression of proneural bHLH genes establishes a neuronal bilateral asymmetry in C. elegans. Development. 2010;137:4017–4027. doi: 10.1242/dev.058834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh SW, Mukhopadhyay A, Dixit BL, Raha T, Green MR, Tissenbaum HA. Identification of direct DAF-16 targets controlling longevity, metabolism and diapause by chromatin immunoprecipitation. Nat Genet. 2006;38:251–257. doi: 10.1038/ng1723. [DOI] [PubMed] [Google Scholar]
- Reinhart BJ, Slack FJ, Basson M, Pasquinelli AE, Bettinger JC, Rougvie AE, Horvitz HR, Ruvkun G. The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans. Nature. 2000;403:901–906. doi: 10.1038/35002607. [DOI] [PubMed] [Google Scholar]
- Simon DJ, Madison JM, Conery AL, Thompson-Peer KL, Soskis M, Ruvkun GB, Kaplan JM, Kim JK. The microRNA miR-1 regulates a MEF-2-dependent retrograde signal at neuromuscular junctions. Cell. 2008;133:903–915. doi: 10.1016/j.cell.2008.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefani G, Slack FJ. Small non-coding RNAs in animal development. Nat Rev Mol Cell Biol. 2008;9:219–230. doi: 10.1038/nrm2347. [DOI] [PubMed] [Google Scholar]
- Tatar M, Bartke A, Antebi A. The endocrine regulation of aging by insulin-like signals. Science. 2003;299:1346–1351. doi: 10.1126/science.1081447. [DOI] [PubMed] [Google Scholar]
- Wang MC, O’Rourke EJ, Ruvkun G. Fat metabolism links germline stem cells and longevity in C. elegans. Science. 2008;322:957–960. doi: 10.1126/science.1162011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolkow CA, Kimura KD, Lee MS, Ruvkun G. Regulation of C. elegans lifespan by insulinlike signaling in the nervous system. Science. 2000;290:147–150. doi: 10.1126/science.290.5489.147. [DOI] [PubMed] [Google Scholar]
- Yamawaki TM, Arantes-Oliveira N, Berman JR, Zhang P, Kenyon C. Distinct activities of the germline and somatic reproductive tissues in the regulation of Caenorhabditis elegans’ longevity. Genetics. 2008;178:513–526. doi: 10.1534/genetics.107.083253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamawaki TM, Berman JR, Suchanek-Kavipurapu M, McCormick M, Maria Gaglia M, Lee SJ, Kenyon C. The somatic reproductive tissues of C. elegans promote longevity through steroid hormone signaling. PLoS Biol. 2010;8:e1000468. doi: 10.1371/journal.pbio.1000468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yochem J, Herman RK. Investigating C. elegans development through mosaic analysis. Development. 2003;130:4761–4768. doi: 10.1242/dev.00701. [DOI] [PubMed] [Google Scholar]
- Yoo AS, Greenwald I. LIN-12/Notch activation leads to microRNA-mediated down-regulation of Vav in C. elegans. Science. 2005;310:1330–1333. doi: 10.1126/science.1119481. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.