Abstract
In the title moleclue, C16H13ClN2O2, the cyclohexene ring is in a sofa conformation. The pyran ring is essentialy planar [maximum deviation = 0.038 (2) Å] and forms a dihedral angle of 89.68 (10)° with the benzene ring. In the crystal, molecules are linked by pairs of N—H⋯N hydrogen bonds, forming inversion dimers with R 2 2(12) ring motifs. These dimers are further linked by N—H⋯O hydrogen bonds into chains along [110]. Weak C—H⋯O hydrogen bonds are also present.
Related literature
For pharmaceutical background to 2-amino-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile derivatives, see: Gao et al. (2001 ▶); Xu et al. (2011 ▶); Luan et al. (2011 ▶); Wang & Zhu, (2007 ▶); O’Callaghan et al. (1995 ▶). For similar structures, see: Tu et al. (2001 ▶); Qiao et al. (2011 ▶); Kong et al. (2011 ▶); Hu et al. (2012 ▶). For standard bond lengths, see: Allen et al. (1987 ▶). For geometric analysis, see: Cremer & Pople (1975 ▶). For hydrogen-bond motifs, see: Bernstein et al. (1995 ▶); Etter et al. (1990 ▶).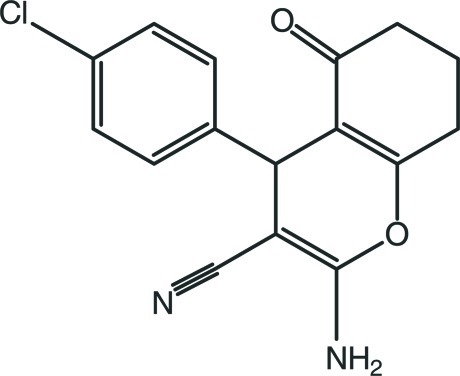
Experimental
Crystal data
C16H13ClN2O2
M r = 300.73
Monoclinic,
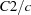
a = 13.753 (4) Å
b = 11.077 (3) Å
c = 19.370 (6) Å
β = 107.856 (5)°
V = 2808.7 (14) Å3
Z = 8
Mo Kα radiation
μ = 0.28 mm−1
T = 150 K
0.43 × 0.27 × 0.07 mm
Data collection
Bruker APEX 2000 CCD area-detector diffractometer
Absorption correction: multi-scan (SADABS; Sheldrick, 1996 ▶) T min = 0.914, T max = 0.981
10643 measured reflections
2755 independent reflections
2124 reflections with I > 2σ(I)
R int = 0.063
Refinement
R[F 2 > 2σ(F 2)] = 0.049
wR(F 2) = 0.122
S = 1.02
2755 reflections
190 parameters
H-atom parameters constrained
Δρmax = 0.35 e Å−3
Δρmin = −0.23 e Å−3
Data collection: APEX2 (Bruker, 2005 ▶); cell refinement: SAINT (Bruker, 2005 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: ORTEP-3 for Windows (Farrugia, 1997 ▶) and PLATON (Spek, 2009 ▶); software used to prepare material for publication: WinGX (Farrugia, 1999 ▶) and PLATON.
Supplementary Material
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S1600536812015838/lh5452sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536812015838/lh5452Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536812015838/lh5452Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N1—H1A⋯N2i | 0.88 | 2.25 | 3.132 (3) | 177 |
| N1—H1B⋯O1ii | 0.88 | 2.15 | 2.955 (2) | 151 |
| C3—H3⋯N2iii | 0.95 | 2.48 | 3.226 (3) | 135 |
Symmetry codes: (i) 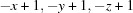 ; (ii)
; (ii) 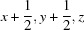 ; (iii)
; (iii) 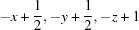 .
.
Acknowledgments
The authors gratefully thank the Higher Education Ministries in both the Arab Republic of Egypt and the Republic of Azerbaijan for their financial support to conduct this project. They also extend their thanks to Manchester Metropolitan University for facilitating this study.
supplementary crystallographic information
Comment
The existence of an amino group and cyano group in tetrahydrochromenone compounds make them great substrates for building up multi organic transformations (Luan et al., 2011), preparing poly-functionalized substituted pyran derivatives (Wang & Zhu, 2007) and designing of poly-heterocyclic compounds (O'Callaghan et al.,1995). Moreover, such derivatives of tetrahydrochromenones have attracted strong interests of pharmacists and biologists because of their potential application in the treatment of psoriatic arthritis and rheumatoid arthritis (Xu et al., 2011). They also have wide biological applications such as anti-anaphylaxis, anti-achondroplasty and anti-cancer activity (Gao et al., 2001). To continue to our interest in the synthesis of biologically active compounds we report herein the crystal structure of the title compound.
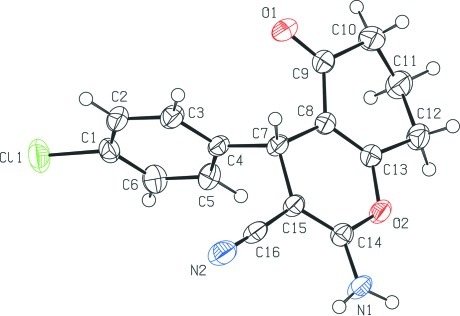
The molecular structure of the title compound is shown in Fig. 1. The (C8–C13) cyclohexene ring is in a sofa conformation with puckering parameters (Cremer & Pople, 1975) of QT = 0.466 (3) Å, θ = 58.3 (2) ° and φ = 173.5 (3) °. The pyran ring (O2/C7/C8/C13—C15) is essentialy planar with a maximum deviation of 0.038 (2) Å for C7, and forms a dihedral angle of 89.68 (10)° with the benzene ring (C1-C6). The bond lengths (Allen et al., 1987) and angles are similar to those for reported structures (Tu et al., 2001; Qiao et al., 2011; Kong et al., 2011; Hu et al., 2012).
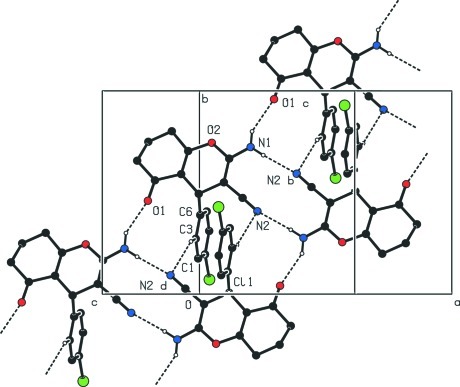
In the crystal, molecules are linked by pairs of intermolecular N—H···N hydrogen bonds, forming inversion dimers with R22(12) ring motifs (Bernstein et al., 1995; Etter et al., 1990), and these dimers are connected by weak C—H···N and N—H···O hydrogen bonds, generating one-dimensional chains along [110] (Table 1, Fig. 2).
Experimental
The title compound (I) was formed during a three component reaction of an equimolar ratios of (4-chlorobenzylidene)propanedinitrile (1 mmol), (4-aminophenyl)methanol (1 mmol) and cyclohexane-1,3-dione (1 mmol). The reaction mixture was heated in ethanol at 351 K. The reaction was monitored with TLC until completed after 5 h, then left in fume cupboard at room temperature until solvent evaporated. The resulting solid mass was recrystallized from ethanol to afford good quality crystals suitable for X-ray diffraction. [Yield: 83%, m.p.: 513 K].
Refinement
All H atoms were positioned geometrically and refined using as riding model with N—H = 0.88 Å for NH2, C—H = 0.95 Å for aromatic, C—H = 0.99 Å for methylene and C—H = 1.00 Å for methine, and with Uiso(H) = 1.2Ueq(C,N)
Figures
Fig. 1.
A view of the molecule of (I), with displacement ellipsoids for non-H atoms drawn at the 50% probability level.
Fig. 2.
Part of the crystal structure of (I) with hydrogen bonds shown as dashed lines. H atoms not involved in H-bonding are omitted for clarity. Symmetry codes: (b) -x+1, -y+1, -z+1; (c) x+1/2, y+1/2, z; (d) -x+1/2, -y+1/2, -z+1.
Crystal data
| C16H13ClN2O2 | F(000) = 1248 |
| Mr = 300.73 | Dx = 1.422 Mg m−3 |
| Monoclinic, C2/c | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: -C 2yc | Cell parameters from 833 reflections |
| a = 13.753 (4) Å | θ = 2.2–28.2° |
| b = 11.077 (3) Å | µ = 0.28 mm−1 |
| c = 19.370 (6) Å | T = 150 K |
| β = 107.856 (5)° | Plate, colourless |
| V = 2808.7 (14) Å3 | 0.43 × 0.27 × 0.07 mm |
| Z = 8 |
Data collection
| Bruker APEX 2000 CCD area-detector diffractometer | 2755 independent reflections |
| Radiation source: fine-focus sealed tube | 2124 reflections with I > 2σ(I) |
| Graphite monochromator | Rint = 0.063 |
| phi and ω scans | θmax = 26.0°, θmin = 2.2° |
| Absorption correction: multi-scan (SADABS; Sheldrick, 1996) | h = −16→16 |
| Tmin = 0.914, Tmax = 0.981 | k = −13→13 |
| 10643 measured reflections | l = −23→23 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.049 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.122 | H-atom parameters constrained |
| S = 1.02 | w = 1/[σ2(Fo2) + (0.0649P)2] where P = (Fo2 + 2Fc2)/3 |
| 2755 reflections | (Δ/σ)max < 0.001 |
| 190 parameters | Δρmax = 0.35 e Å−3 |
| 0 restraints | Δρmin = −0.23 e Å−3 |
Special details
| Geometry. Bond distances, angles etc. have been calculated using the rounded fractional coordinates. All su's are estimated from the variances of the (full) variance-covariance matrix. The cell e.s.d.'s are taken into account in the estimation of distances, angles and torsion angles |
| Refinement. Refinement on F2 for ALL reflections except those flagged by the user for potential systematic errors. Weighted R-factors wR and all goodnesses of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The observed criterion of F2 > σ(F2) is used only for calculating -R-factor-obs etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R-factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Cl1 | 0.12556 (5) | 0.07141 (5) | 0.22343 (4) | 0.0510 (2) | |
| O1 | −0.04515 (11) | 0.45923 (13) | 0.41373 (8) | 0.0395 (5) | |
| O2 | 0.20240 (10) | 0.74455 (12) | 0.39806 (8) | 0.0320 (4) | |
| N1 | 0.37218 (13) | 0.72339 (15) | 0.44001 (9) | 0.0347 (6) | |
| N2 | 0.41870 (14) | 0.40760 (17) | 0.49094 (11) | 0.0440 (7) | |
| C1 | 0.13012 (15) | 0.19541 (19) | 0.28039 (12) | 0.0337 (7) | |
| C2 | 0.12955 (16) | 0.17534 (19) | 0.35014 (13) | 0.0374 (7) | |
| C3 | 0.13788 (15) | 0.27229 (18) | 0.39614 (12) | 0.0332 (7) | |
| C4 | 0.14684 (14) | 0.38895 (17) | 0.37333 (11) | 0.0274 (6) | |
| C5 | 0.14494 (16) | 0.40667 (18) | 0.30168 (11) | 0.0348 (7) | |
| C6 | 0.13673 (16) | 0.3107 (2) | 0.25501 (12) | 0.0378 (7) | |
| C7 | 0.16120 (14) | 0.49363 (17) | 0.42613 (11) | 0.0270 (6) | |
| C8 | 0.08175 (14) | 0.59034 (17) | 0.40049 (10) | 0.0274 (6) | |
| C9 | −0.02395 (15) | 0.55980 (18) | 0.39594 (11) | 0.0311 (7) | |
| C10 | −0.10332 (17) | 0.6565 (2) | 0.37309 (14) | 0.0470 (8) | |
| C11 | −0.07795 (17) | 0.7496 (2) | 0.32396 (14) | 0.0465 (8) | |
| C12 | 0.02871 (15) | 0.80147 (18) | 0.35920 (12) | 0.0360 (7) | |
| C13 | 0.10411 (15) | 0.70407 (18) | 0.38660 (11) | 0.0286 (6) | |
| C14 | 0.28249 (15) | 0.66677 (18) | 0.42763 (10) | 0.0285 (6) | |
| C15 | 0.26669 (14) | 0.54985 (17) | 0.44117 (11) | 0.0271 (6) | |
| C16 | 0.35154 (15) | 0.47326 (19) | 0.46903 (11) | 0.0304 (7) | |
| H1A | 0.42960 | 0.68370 | 0.45910 | 0.0420* | |
| H1B | 0.37370 | 0.80030 | 0.42910 | 0.0420* | |
| H2 | 0.12350 | 0.09570 | 0.36650 | 0.0450* | |
| H3 | 0.13750 | 0.25880 | 0.44450 | 0.0400* | |
| H5 | 0.14940 | 0.48630 | 0.28470 | 0.0420* | |
| H6 | 0.13560 | 0.32360 | 0.20630 | 0.0450* | |
| H7 | 0.15570 | 0.46120 | 0.47300 | 0.0320* | |
| H10A | −0.11020 | 0.69730 | 0.41680 | 0.0560* | |
| H10B | −0.16990 | 0.61890 | 0.34740 | 0.0560* | |
| H11A | −0.12890 | 0.81560 | 0.31430 | 0.0560* | |
| H11B | −0.08110 | 0.71180 | 0.27700 | 0.0560* | |
| H12A | 0.04920 | 0.85040 | 0.32320 | 0.0430* | |
| H12B | 0.02750 | 0.85500 | 0.39980 | 0.0430* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Cl1 | 0.0542 (4) | 0.0358 (4) | 0.0625 (4) | 0.0042 (3) | 0.0171 (3) | −0.0181 (3) |
| O1 | 0.0357 (8) | 0.0312 (9) | 0.0545 (10) | −0.0086 (7) | 0.0182 (7) | 0.0011 (7) |
| O2 | 0.0301 (8) | 0.0212 (7) | 0.0432 (8) | −0.0032 (6) | 0.0090 (6) | 0.0040 (6) |
| N1 | 0.0288 (9) | 0.0249 (9) | 0.0471 (11) | −0.0069 (7) | 0.0070 (8) | 0.0050 (8) |
| N2 | 0.0314 (10) | 0.0353 (11) | 0.0633 (13) | −0.0011 (8) | 0.0116 (9) | 0.0139 (10) |
| C1 | 0.0238 (10) | 0.0288 (12) | 0.0460 (13) | 0.0027 (9) | 0.0071 (9) | −0.0091 (10) |
| C2 | 0.0363 (12) | 0.0224 (11) | 0.0545 (14) | −0.0011 (9) | 0.0155 (10) | 0.0015 (10) |
| C3 | 0.0345 (12) | 0.0275 (11) | 0.0383 (12) | −0.0036 (9) | 0.0120 (9) | 0.0030 (9) |
| C4 | 0.0224 (10) | 0.0224 (10) | 0.0366 (11) | −0.0036 (8) | 0.0078 (8) | 0.0000 (8) |
| C5 | 0.0405 (12) | 0.0240 (11) | 0.0390 (12) | −0.0003 (9) | 0.0111 (10) | 0.0028 (9) |
| C6 | 0.0410 (12) | 0.0350 (13) | 0.0359 (12) | 0.0044 (10) | 0.0095 (10) | −0.0001 (10) |
| C7 | 0.0279 (10) | 0.0222 (10) | 0.0309 (11) | −0.0038 (8) | 0.0091 (8) | 0.0027 (8) |
| C8 | 0.0268 (10) | 0.0234 (11) | 0.0319 (11) | −0.0009 (8) | 0.0089 (8) | −0.0005 (8) |
| C9 | 0.0303 (11) | 0.0285 (12) | 0.0344 (11) | −0.0032 (9) | 0.0099 (9) | −0.0045 (9) |
| C10 | 0.0290 (12) | 0.0374 (13) | 0.0759 (18) | 0.0014 (10) | 0.0182 (11) | 0.0005 (12) |
| C11 | 0.0323 (13) | 0.0334 (13) | 0.0691 (17) | 0.0046 (10) | 0.0085 (12) | 0.0082 (12) |
| C12 | 0.0365 (12) | 0.0261 (11) | 0.0441 (13) | 0.0016 (9) | 0.0104 (10) | 0.0042 (10) |
| C13 | 0.0282 (11) | 0.0261 (11) | 0.0316 (11) | −0.0014 (9) | 0.0095 (8) | −0.0025 (9) |
| C14 | 0.0278 (11) | 0.0277 (11) | 0.0287 (11) | −0.0018 (8) | 0.0066 (8) | −0.0004 (8) |
| C15 | 0.0256 (10) | 0.0230 (10) | 0.0311 (11) | −0.0035 (8) | 0.0064 (8) | 0.0008 (8) |
| C16 | 0.0273 (11) | 0.0262 (11) | 0.0356 (12) | −0.0077 (9) | 0.0067 (9) | 0.0032 (9) |
Geometric parameters (Å, º)
| Cl1—C1 | 1.751 (2) | C8—C13 | 1.343 (3) |
| O1—C9 | 1.227 (3) | C9—C10 | 1.496 (3) |
| O2—C13 | 1.376 (3) | C10—C11 | 1.515 (3) |
| O2—C14 | 1.377 (3) | C11—C12 | 1.528 (3) |
| N1—C14 | 1.338 (3) | C12—C13 | 1.478 (3) |
| N2—C16 | 1.150 (3) | C14—C15 | 1.352 (3) |
| N1—H1B | 0.8800 | C15—C16 | 1.410 (3) |
| N1—H1A | 0.8800 | C2—H2 | 0.9500 |
| C1—C6 | 1.381 (3) | C3—H3 | 0.9500 |
| C1—C2 | 1.372 (3) | C5—H5 | 0.9500 |
| C2—C3 | 1.378 (3) | C6—H6 | 0.9500 |
| C3—C4 | 1.383 (3) | C7—H7 | 1.0000 |
| C4—C7 | 1.518 (3) | C10—H10A | 0.9900 |
| C4—C5 | 1.394 (3) | C10—H10B | 0.9900 |
| C5—C6 | 1.378 (3) | C11—H11A | 0.9900 |
| C7—C8 | 1.502 (3) | C11—H11B | 0.9900 |
| C7—C15 | 1.523 (3) | C12—H12A | 0.9900 |
| C8—C9 | 1.469 (3) | C12—H12B | 0.9900 |
| C13—O2—C14 | 118.95 (15) | O2—C14—C15 | 121.62 (19) |
| H1A—N1—H1B | 120.00 | C7—C15—C14 | 123.71 (18) |
| C14—N1—H1A | 120.00 | C7—C15—C16 | 117.10 (17) |
| C14—N1—H1B | 120.00 | C14—C15—C16 | 119.17 (19) |
| Cl1—C1—C2 | 118.92 (16) | N2—C16—C15 | 177.8 (2) |
| C2—C1—C6 | 121.4 (2) | C1—C2—H2 | 120.00 |
| Cl1—C1—C6 | 119.64 (17) | C3—C2—H2 | 120.00 |
| C1—C2—C3 | 119.1 (2) | C2—C3—H3 | 119.00 |
| C2—C3—C4 | 121.3 (2) | C4—C3—H3 | 119.00 |
| C3—C4—C7 | 120.28 (18) | C4—C5—H5 | 119.00 |
| C3—C4—C5 | 118.24 (18) | C6—C5—H5 | 119.00 |
| C5—C4—C7 | 121.46 (17) | C1—C6—H6 | 121.00 |
| C4—C5—C6 | 121.16 (19) | C5—C6—H6 | 121.00 |
| C1—C6—C5 | 118.8 (2) | C4—C7—H7 | 108.00 |
| C8—C7—C15 | 109.00 (16) | C8—C7—H7 | 108.00 |
| C4—C7—C8 | 113.00 (17) | C15—C7—H7 | 108.00 |
| C4—C7—C15 | 111.14 (16) | C9—C10—H10A | 109.00 |
| C7—C8—C9 | 117.51 (17) | C9—C10—H10B | 109.00 |
| C7—C8—C13 | 123.17 (19) | C11—C10—H10A | 109.00 |
| C9—C8—C13 | 119.15 (18) | C11—C10—H10B | 109.00 |
| O1—C9—C8 | 120.54 (19) | H10A—C10—H10B | 108.00 |
| C8—C9—C10 | 118.15 (18) | C10—C11—H11A | 110.00 |
| O1—C9—C10 | 121.2 (2) | C10—C11—H11B | 110.00 |
| C9—C10—C11 | 112.7 (2) | C12—C11—H11A | 109.00 |
| C10—C11—C12 | 110.5 (2) | C12—C11—H11B | 110.00 |
| C11—C12—C13 | 110.97 (17) | H11A—C11—H11B | 108.00 |
| O2—C13—C8 | 123.17 (18) | C11—C12—H12A | 109.00 |
| O2—C13—C12 | 111.50 (17) | C11—C12—H12B | 109.00 |
| C8—C13—C12 | 125.3 (2) | C13—C12—H12A | 109.00 |
| N1—C14—C15 | 127.46 (19) | C13—C12—H12B | 109.00 |
| O2—C14—N1 | 110.92 (17) | H12A—C12—H12B | 108.00 |
| C13—O2—C14—N1 | −175.33 (16) | C4—C7—C8—C13 | −117.7 (2) |
| C13—O2—C14—C15 | 4.4 (3) | C15—C7—C8—C9 | −168.89 (17) |
| C14—O2—C13—C8 | −2.4 (3) | C15—C7—C8—C13 | 6.4 (3) |
| C14—O2—C13—C12 | 176.27 (17) | C8—C7—C15—C16 | 177.19 (17) |
| Cl1—C1—C2—C3 | 177.03 (18) | C13—C8—C9—C10 | 2.3 (3) |
| Cl1—C1—C6—C5 | −177.07 (18) | C7—C8—C13—C12 | 178.03 (19) |
| C2—C1—C6—C5 | 1.3 (3) | C9—C8—C13—O2 | 171.79 (18) |
| C6—C1—C2—C3 | −1.4 (3) | C7—C8—C13—O2 | −3.5 (3) |
| C1—C2—C3—C4 | −0.1 (3) | C7—C8—C9—O1 | 1.2 (3) |
| C2—C3—C4—C7 | −177.0 (2) | C7—C8—C9—C10 | 177.81 (18) |
| C2—C3—C4—C5 | 1.4 (3) | C13—C8—C9—O1 | −174.36 (19) |
| C3—C4—C7—C15 | 112.8 (2) | C9—C8—C13—C12 | −6.7 (3) |
| C3—C4—C7—C8 | −124.3 (2) | O1—C9—C10—C11 | −154.9 (2) |
| C3—C4—C5—C6 | −1.5 (3) | C8—C9—C10—C11 | 28.4 (3) |
| C5—C4—C7—C8 | 57.4 (3) | C9—C10—C11—C12 | −53.7 (3) |
| C5—C4—C7—C15 | −65.5 (2) | C10—C11—C12—C13 | 48.8 (2) |
| C7—C4—C5—C6 | 176.9 (2) | C11—C12—C13—O2 | 161.65 (18) |
| C4—C5—C6—C1 | 0.1 (3) | C11—C12—C13—C8 | −19.7 (3) |
| C4—C7—C15—C14 | 120.7 (2) | O2—C14—C15—C7 | −0.6 (3) |
| C4—C7—C15—C16 | −57.6 (2) | O2—C14—C15—C16 | 177.71 (18) |
| C8—C7—C15—C14 | −4.5 (3) | N1—C14—C15—C7 | 179.11 (19) |
| C4—C7—C8—C9 | 67.0 (2) | N1—C14—C15—C16 | −2.6 (3) |
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N1—H1A···N2i | 0.88 | 2.25 | 3.132 (3) | 177 |
| N1—H1B···O1ii | 0.88 | 2.15 | 2.955 (2) | 151 |
| C3—H3···N2iii | 0.95 | 2.48 | 3.226 (3) | 135 |
Symmetry codes: (i) −x+1, −y+1, −z+1; (ii) x+1/2, y+1/2, z; (iii) −x+1/2, −y+1/2, −z+1.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: LH5452).
References
- Allen, F. H., Kennard, O., Watson, D. G., Brammer, L., Orpen, A. G. & Taylor, R. (1987). J. Chem. Soc. Perkin Trans. 2, pp. S1–19.
- Bernstein, J., Davis, R. E., Shimoni, L. & Chang, N.-L. (1995). Angew. Chem. Int. Ed. Engl. 34, 1555–1573.
- Bruker (2005). APEX2 and SAINT Bruker AXS Inc., Madison, Wisconsin, USA.
- Cremer, D. & Pople, J. A. (1975). J. Am. Chem. Soc. 97, 1354–1358.
- Etter, M. C., MacDonald, J. C. & Bernstein, J. (1990). Acta Cryst. B46, 256–262. [DOI] [PubMed]
- Farrugia, L. J. (1997). J. Appl. Cryst. 30, 565.
- Farrugia, L. J. (1999). J. Appl. Cryst. 32, 837–838.
- Gao, Y., Tu, S.-J., Zhou, J.-F. & Shi, D.-Q. (2001). J. Org. Chem. 7, 535–537.
- Hu, X.-L., Wang, Z.-X., Wang, F.-M. & Han, G.-F. (2012). Acta Cryst. E68, o823. [DOI] [PMC free article] [PubMed]
- Kong, L., Ju, X., Qiao, Y., Zhang, J. & Gao, Z. (2011). Acta Cryst. E67, o3100. [DOI] [PMC free article] [PubMed]
- Luan, C.-J., Wang, J.-Q., Zhang, G.-H., Wang, W., Tang, S.-G. & Guo, C. (2011). J. Org. Chem. 31, 860–864.
- O’Callaghan, C. N., McMurry, T. B. H. & O’Brien, J. E. (1995). J. Chem. Soc. Perkin Trans. 1, pp. 417–420.
- Qiao, Y., Kong, L., Chen, G., Li, S. & Gao, Z. (2011). Acta Cryst. E67, o3099. [DOI] [PMC free article] [PubMed]
- Sheldrick, G. M. (1996). SADABS University of Göttingen, Germany.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Spek, A. L. (2009). Acta Cryst. D65, 148–155. [DOI] [PMC free article] [PubMed]
- Tu, S.-J., Deng, X., Fang, Y.-Y., Guo, Y.-M., Du, M. & Liu, X.-H. (2001). Acta Cryst. E57, o358–o359.
- Wang, J. & Zhu, S.-L. (2007). Acta Cryst. E63, o4190.
- Xu, J.-C., Li, W.-M., Zheng, H., Lai, Y.-F. & Zhang, P.-F. (2011). Tetrahedron, 67, 9582–9587.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S1600536812015838/lh5452sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536812015838/lh5452Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536812015838/lh5452Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report