Abstract
In the title compound, C13H12N3O3 +·Br−, the benzene and pyridinium rings form a dihedral angle of 82.0 (1)°. In the crystal, N—H⋯Br and N—H⋯O hydrogen bonds link the components into chains along [001]. In addition, weak C—H⋯O and C—H⋯Br hydrogen bonds are observed.
Related literature
The title compound was prepared as an NAD+ (nicotinamide adenine dinucleotide) model. For effective regeneration systems for co-enzymes (e.g. NADH), see: Hollmann et al. (2001 ▶); Lee et al. (2011 ▶); Maenaka et al. (2012 ▶); Park et al. (2008 ▶); Ruppert et al. (1988 ▶); Zhu et al. (2006 ▶). For the mechanisms of redox interconversions (NADH/NAD+), see: Zhu et al. (2003 ▶); Song et al. (2008 ▶).
Experimental
Crystal data
C13H12N3O3 +·Br−
M r = 338.17
Monoclinic,

a = 17.576 (4) Å
b = 7.9990 (16) Å
c = 10.152 (2) Å
β = 105.88 (3)°
V = 1372.8 (5) Å3
Z = 4
Mo Kα radiation
μ = 3.01 mm−1
T = 293 K
0.15 × 0.15 × 0.10 mm
Data collection
Bruker SMART CCD diffractometer
Absorption correction: multi-scan (SADABS; Bruker, 1997 ▶) T min = 0.661, T max = 0.753
7399 measured reflections
2684 independent reflections
2081 reflections with I > 2σ(I)
R int = 0.034
Refinement
R[F 2 > 2σ(F 2)] = 0.036
wR(F 2) = 0.086
S = 1.04
2684 reflections
187 parameters
2 restraints
H atoms treated by a mixture of independent and constrained refinement
Δρmax = 0.36 e Å−3
Δρmin = −0.38 e Å−3
Data collection: SMART (Bruker, 1997 ▶); cell refinement: SAINT (Bruker, 1997 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: SHELXTL (Sheldrick, 2008 ▶); software used to prepare material for publication: SHELXTL.
Supplementary Material
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S1600536812015917/lh5450sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536812015917/lh5450Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536812015917/lh5450Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N1—H1B⋯O1i | 0.86 (1) | 2.30 (1) | 3.143 (4) | 168 (4) |
| N1—H1A⋯Br1ii | 0.86 (1) | 2.61 (1) | 3.454 (3) | 166 (3) |
| C4—H4⋯Br1 | 0.93 | 2.82 | 3.743 (3) | 173 |
| C7—H7B⋯Br1iii | 0.97 | 2.82 | 3.595 (3) | 137 |
| C5—H5⋯O2iv | 0.93 | 2.36 | 3.271 (4) | 167 |
| C3—H3⋯O1i | 0.93 | 2.27 | 3.150 (4) | 157 |
Symmetry codes: (i)  ; (ii)
; (ii)  ; (iii)
; (iii)  ; (iv)
; (iv)  .
.
Acknowledgments
Financial support from the Converging Research Center Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2011 K000675 and 2011 K000660), and Seoul National University of Science & Technology is gratefully acknowledged.
supplementary crystallographic information
Comment
One of the most important challenges in applying mono-oxygenases reactions in vitro is to find an effective regeneration system for the necessary co-enzyme (mostly NAD(P)H) (Hollmann et al., 2001; Lee et al.,2011; Maenaka et al., 2012; Park et al., 2008; Ruppert et al., 1988; Zhu et al., 2006). The well established methods for the regeneration of the nicotinamide co-enzyme mainly consist of an enzyme-coupled approach utilizing formate dehydrogenase or glucose-6-phosphate dehydrogenase. Because the redox coenzyme couple NADH/NAD+ is ubiquitous and controls so much of our oxidation/reduction nature, there has been a long-standing interest in the mechanisms of the redox interconversions (Zhu et al., 2003). The high cost of these co-factors, however, is prohibitive of industrialization of many promising enzymatic processes. An efficient method of their in situ regeneration is the only means for making the processes economically and industrially feasible (Song et al., 2008). Therefore, many researchers have given considerable attention to the chemistry of NADH and its models (Hollmann et al., 2001). In this work, we have synthesized the title compound as a NAD+ model and report herein its crystal structure.
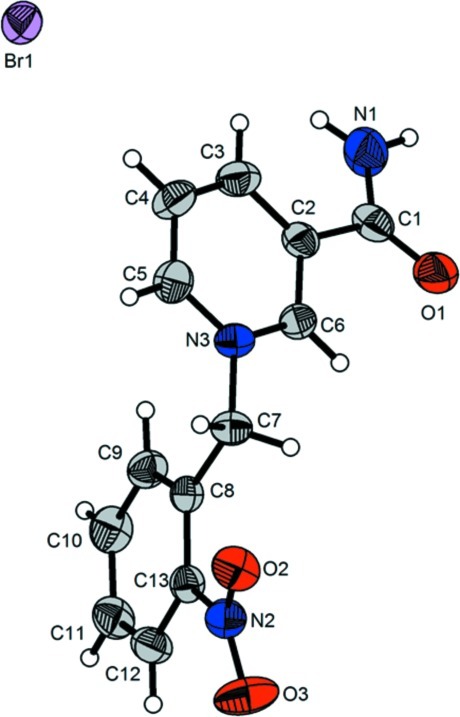
The molecular structure of the title compound is shown in Fig. 1. The benzene ring (C8-C13) and pyridine ring (N3/C2-C6) form a dihedral angle of 82.0 (1)°. In the crystal, intermolecular N—H···Br and N—H···O hydrogen bonds link the components to form chains along [001]. In addition, weak C—H···O and C—H···Br hydrogen bonds are observed.
Experimental
Nicotinamide (123.4 mg, 1 mmol) was dissolved in 10 ml acetonitrile. After stirring for a few minutes, 2-nitrobenzyl bromide (220.4 mg, 1 mmol) was carefully added to the reaction mixture. The solution was stirred for 3 h at 353K. The precipitate was filtered, washed three times with methylene chloride, and dried under vacuum. Crystals suitable for X-ray analysis were obtained from a methanol soution of the title compound in a few days.
Refinement
H atoms bonded to C atoms were placed in calculated positions with C—H distances of 0.93 Å for aromatic C atoms and 0.97 Å for a methylene C atoms. They were included in the refinement in riding-motion approximation with Uiso(H) = 1.2Ueq(C). The positions of N—H atoms of the amine were refined with N—H = 0.860 (2) Å and Uiso(H) =1.2Ueq(N).
Figures
Fig. 1.
The molecular structure with displacement ellipsoids shown at the 50% probability level.
Crystal data
| C13H12N3O3+·Br− | F(000) = 680 |
| Mr = 338.17 | Dx = 1.636 Mg m−3 |
| Monoclinic, P21/c | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: -P 2ybc | Cell parameters from 11909 reflections |
| a = 17.576 (4) Å | θ = 2.7–27.6° |
| b = 7.9990 (16) Å | µ = 3.01 mm−1 |
| c = 10.152 (2) Å | T = 293 K |
| β = 105.88 (3)° | Block, colorless |
| V = 1372.8 (5) Å3 | 0.15 × 0.15 × 0.10 mm |
| Z = 4 |
Data collection
| Bruker SMART CCD diffractometer | 2684 independent reflections |
| Radiation source: fine-focus sealed tube | 2081 reflections with I > 2σ(I) |
| Graphite monochromator | Rint = 0.034 |
| φ and ω scans | θmax = 26.0°, θmin = 2.4° |
| Absorption correction: multi-scan (SADABS; Bruker, 1997) | h = −21→21 |
| Tmin = 0.661, Tmax = 0.753 | k = −9→9 |
| 7399 measured reflections | l = −10→12 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.036 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.086 | H atoms treated by a mixture of independent and constrained refinement |
| S = 1.04 | w = 1/[σ2(Fo2) + (0.0287P)2 + 0.3626P] where P = (Fo2 + 2Fc2)/3 |
| 2684 reflections | (Δ/σ)max = 0.001 |
| 187 parameters | Δρmax = 0.36 e Å−3 |
| 2 restraints | Δρmin = −0.38 e Å−3 |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Br1 | 0.299001 (18) | 0.53711 (4) | 0.36253 (3) | 0.04487 (13) | |
| N1 | 0.51901 (17) | 0.2174 (4) | 0.9902 (3) | 0.0556 (7) | |
| H1A | 0.5634 (10) | 0.181 (4) | 1.041 (3) | 0.067* | |
| H1B | 0.506 (2) | 0.193 (5) | 0.9045 (10) | 0.067* | |
| N2 | 0.09788 (14) | 0.6339 (3) | 1.1536 (2) | 0.0374 (6) | |
| N3 | 0.27770 (14) | 0.5158 (3) | 0.9346 (2) | 0.0333 (5) | |
| O1 | 0.48818 (12) | 0.3289 (3) | 1.1724 (2) | 0.0515 (6) | |
| O2 | 0.11894 (12) | 0.7569 (3) | 1.1007 (2) | 0.0468 (5) | |
| O3 | 0.06762 (15) | 0.6449 (3) | 1.2487 (3) | 0.0648 (7) | |
| C1 | 0.47318 (17) | 0.3051 (4) | 1.0485 (3) | 0.0396 (7) | |
| C2 | 0.40031 (16) | 0.3825 (3) | 0.9549 (3) | 0.0330 (6) | |
| C3 | 0.38718 (18) | 0.3982 (4) | 0.8142 (3) | 0.0411 (7) | |
| H3 | 0.4249 | 0.3592 | 0.7728 | 0.049* | |
| C4 | 0.31891 (19) | 0.4710 (4) | 0.7355 (3) | 0.0444 (8) | |
| H4 | 0.3101 | 0.4811 | 0.6412 | 0.053* | |
| C5 | 0.26429 (18) | 0.5282 (4) | 0.7981 (3) | 0.0397 (7) | |
| H5 | 0.2175 | 0.5760 | 0.7459 | 0.048* | |
| C6 | 0.34414 (16) | 0.4456 (3) | 1.0136 (3) | 0.0329 (6) | |
| H6 | 0.3523 | 0.4396 | 1.1080 | 0.040* | |
| C7 | 0.21733 (16) | 0.5811 (3) | 0.9988 (3) | 0.0340 (7) | |
| H7A | 0.1859 | 0.6657 | 0.9398 | 0.041* | |
| H7B | 0.2436 | 0.6335 | 1.0854 | 0.041* | |
| C8 | 0.16318 (15) | 0.4428 (3) | 1.0230 (3) | 0.0298 (6) | |
| C9 | 0.16802 (17) | 0.2812 (4) | 0.9765 (3) | 0.0372 (7) | |
| H9 | 0.2030 | 0.2585 | 0.9246 | 0.045* | |
| C10 | 0.12235 (18) | 0.1532 (4) | 1.0052 (3) | 0.0443 (8) | |
| H10 | 0.1267 | 0.0464 | 0.9717 | 0.053* | |
| C11 | 0.07044 (18) | 0.1813 (4) | 1.0828 (3) | 0.0429 (7) | |
| H11 | 0.0404 | 0.0941 | 1.1028 | 0.051* | |
| C12 | 0.06342 (17) | 0.3402 (4) | 1.1305 (3) | 0.0394 (7) | |
| H12 | 0.0284 | 0.3615 | 1.1825 | 0.047* | |
| C13 | 0.10931 (15) | 0.4683 (3) | 1.1001 (3) | 0.0307 (6) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Br1 | 0.0467 (2) | 0.0500 (2) | 0.0367 (2) | −0.00517 (15) | 0.00936 (14) | 0.00137 (15) |
| N1 | 0.0406 (17) | 0.066 (2) | 0.0561 (19) | 0.0114 (15) | 0.0063 (15) | −0.0072 (16) |
| N2 | 0.0361 (14) | 0.0353 (15) | 0.0415 (15) | 0.0064 (11) | 0.0120 (11) | −0.0022 (12) |
| N3 | 0.0364 (14) | 0.0322 (13) | 0.0346 (13) | −0.0001 (10) | 0.0152 (11) | 0.0025 (10) |
| O1 | 0.0402 (12) | 0.0710 (16) | 0.0435 (14) | 0.0001 (11) | 0.0117 (10) | 0.0118 (12) |
| O2 | 0.0491 (13) | 0.0332 (12) | 0.0613 (15) | −0.0007 (10) | 0.0202 (11) | −0.0023 (11) |
| O3 | 0.0894 (19) | 0.0559 (16) | 0.0689 (16) | 0.0129 (13) | 0.0549 (15) | −0.0026 (13) |
| C1 | 0.0320 (16) | 0.0412 (18) | 0.047 (2) | −0.0050 (13) | 0.0134 (14) | 0.0044 (15) |
| C2 | 0.0314 (15) | 0.0317 (16) | 0.0369 (16) | −0.0060 (12) | 0.0111 (12) | 0.0002 (13) |
| C3 | 0.0445 (18) | 0.0419 (17) | 0.0431 (18) | −0.0009 (14) | 0.0224 (15) | −0.0049 (15) |
| C4 | 0.053 (2) | 0.0526 (19) | 0.0301 (16) | 0.0042 (16) | 0.0158 (14) | 0.0022 (15) |
| C5 | 0.0418 (17) | 0.0398 (17) | 0.0348 (17) | 0.0044 (14) | 0.0056 (14) | 0.0070 (14) |
| C6 | 0.0343 (15) | 0.0359 (17) | 0.0289 (15) | −0.0028 (13) | 0.0092 (12) | 0.0045 (12) |
| C7 | 0.0352 (16) | 0.0331 (16) | 0.0373 (16) | 0.0037 (12) | 0.0160 (13) | 0.0013 (12) |
| C8 | 0.0278 (14) | 0.0323 (16) | 0.0274 (14) | 0.0010 (11) | 0.0044 (11) | 0.0011 (12) |
| C9 | 0.0395 (16) | 0.0374 (17) | 0.0363 (17) | 0.0032 (13) | 0.0131 (13) | −0.0050 (13) |
| C10 | 0.0530 (19) | 0.0298 (17) | 0.0490 (19) | −0.0039 (14) | 0.0121 (15) | −0.0054 (14) |
| C11 | 0.0438 (18) | 0.0368 (18) | 0.0486 (19) | −0.0129 (14) | 0.0134 (15) | 0.0015 (15) |
| C12 | 0.0330 (16) | 0.0449 (19) | 0.0425 (18) | −0.0027 (13) | 0.0141 (13) | 0.0028 (14) |
| C13 | 0.0307 (14) | 0.0283 (14) | 0.0320 (15) | −0.0001 (12) | 0.0067 (12) | −0.0019 (12) |
Geometric parameters (Å, º)
| N1—C1 | 1.324 (4) | C4—H4 | 0.9300 |
| N1—H1A | 0.860 (2) | C5—H5 | 0.9300 |
| N1—H1B | 0.860 (2) | C6—H6 | 0.9300 |
| N2—O2 | 1.226 (3) | C7—C8 | 1.523 (4) |
| N2—O3 | 1.227 (3) | C7—H7A | 0.9700 |
| N2—C13 | 1.466 (4) | C7—H7B | 0.9700 |
| N3—C5 | 1.344 (4) | C8—C9 | 1.387 (4) |
| N3—C6 | 1.345 (4) | C8—C13 | 1.398 (4) |
| N3—C7 | 1.484 (3) | C9—C10 | 1.381 (4) |
| O1—C1 | 1.227 (4) | C9—H9 | 0.9300 |
| C1—C2 | 1.503 (4) | C10—C11 | 1.377 (4) |
| C2—C6 | 1.381 (4) | C10—H10 | 0.9300 |
| C2—C3 | 1.389 (4) | C11—C12 | 1.377 (4) |
| C3—C4 | 1.376 (4) | C11—H11 | 0.9300 |
| C3—H3 | 0.9300 | C12—C13 | 1.390 (4) |
| C4—C5 | 1.367 (4) | C12—H12 | 0.9300 |
| C1—N1—H1A | 119 (2) | C2—C6—H6 | 120.0 |
| C1—N1—H1B | 124 (3) | N3—C7—C8 | 111.6 (2) |
| H1A—N1—H1B | 118 (4) | N3—C7—H7A | 109.3 |
| O2—N2—O3 | 122.4 (3) | C8—C7—H7A | 109.3 |
| O2—N2—C13 | 118.3 (2) | N3—C7—H7B | 109.3 |
| O3—N2—C13 | 119.3 (3) | C8—C7—H7B | 109.3 |
| C5—N3—C6 | 121.6 (2) | H7A—C7—H7B | 108.0 |
| C5—N3—C7 | 118.8 (2) | C9—C8—C13 | 116.1 (2) |
| C6—N3—C7 | 119.6 (2) | C9—C8—C7 | 121.5 (2) |
| O1—C1—N1 | 123.7 (3) | C13—C8—C7 | 122.2 (2) |
| O1—C1—C2 | 119.4 (3) | C10—C9—C8 | 121.8 (3) |
| N1—C1—C2 | 116.9 (3) | C10—C9—H9 | 119.1 |
| C6—C2—C3 | 118.3 (3) | C8—C9—H9 | 119.1 |
| C6—C2—C1 | 117.6 (3) | C11—C10—C9 | 120.9 (3) |
| C3—C2—C1 | 124.1 (3) | C11—C10—H10 | 119.5 |
| C4—C3—C2 | 120.6 (3) | C9—C10—H10 | 119.5 |
| C4—C3—H3 | 119.7 | C10—C11—C12 | 119.3 (3) |
| C2—C3—H3 | 119.7 | C10—C11—H11 | 120.4 |
| C5—C4—C3 | 118.9 (3) | C12—C11—H11 | 120.4 |
| C5—C4—H4 | 120.6 | C11—C12—C13 | 119.3 (3) |
| C3—C4—H4 | 120.6 | C11—C12—H12 | 120.4 |
| N3—C5—C4 | 120.5 (3) | C13—C12—H12 | 120.4 |
| N3—C5—H5 | 119.8 | C12—C13—C8 | 122.6 (3) |
| C4—C5—H5 | 119.8 | C12—C13—N2 | 115.9 (2) |
| N3—C6—C2 | 120.1 (3) | C8—C13—N2 | 121.4 (2) |
| N3—C6—H6 | 120.0 | ||
| O1—C1—C2—C6 | −14.2 (4) | N3—C7—C8—C13 | 170.4 (2) |
| N1—C1—C2—C6 | 167.5 (3) | C13—C8—C9—C10 | −0.2 (4) |
| O1—C1—C2—C3 | 164.0 (3) | C7—C8—C9—C10 | 176.1 (3) |
| N1—C1—C2—C3 | −14.3 (4) | C8—C9—C10—C11 | −0.6 (5) |
| C6—C2—C3—C4 | −1.7 (4) | C9—C10—C11—C12 | 0.9 (5) |
| C1—C2—C3—C4 | −179.9 (3) | C10—C11—C12—C13 | −0.5 (5) |
| C2—C3—C4—C5 | 0.2 (5) | C11—C12—C13—C8 | −0.3 (4) |
| C6—N3—C5—C4 | −0.8 (4) | C11—C12—C13—N2 | 179.5 (3) |
| C7—N3—C5—C4 | 179.3 (3) | C9—C8—C13—C12 | 0.7 (4) |
| C3—C4—C5—N3 | 1.0 (5) | C7—C8—C13—C12 | −175.6 (3) |
| C5—N3—C6—C2 | −0.7 (4) | C9—C8—C13—N2 | −179.2 (2) |
| C7—N3—C6—C2 | 179.2 (2) | C7—C8—C13—N2 | 4.6 (4) |
| C3—C2—C6—N3 | 1.9 (4) | O2—N2—C13—C12 | −160.2 (3) |
| C1—C2—C6—N3 | −179.8 (2) | O3—N2—C13—C12 | 19.6 (4) |
| C5—N3—C7—C8 | 97.2 (3) | O2—N2—C13—C8 | 19.7 (4) |
| C6—N3—C7—C8 | −82.7 (3) | O3—N2—C13—C8 | −160.5 (3) |
| N3—C7—C8—C9 | −5.6 (4) |
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N1—H1B···O1i | 0.86 (1) | 2.30 (1) | 3.143 (4) | 168 (4) |
| N1—H1A···Br1ii | 0.86 (1) | 2.61 (1) | 3.454 (3) | 166 (3) |
| C4—H4···Br1 | 0.93 | 2.82 | 3.743 (3) | 173 |
| C7—H7B···Br1iii | 0.97 | 2.82 | 3.595 (3) | 137 |
| C5—H5···O2iv | 0.93 | 2.36 | 3.271 (4) | 167 |
| C3—H3···O1i | 0.93 | 2.27 | 3.150 (4) | 157 |
Symmetry codes: (i) x, −y+1/2, z−1/2; (ii) −x+1, y−1/2, −z+3/2; (iii) x, y, z+1; (iv) x, −y+3/2, z−1/2.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: LH5450).
References
- Bruker (1997). SMART and SAINT Bruker AXS Inc., Madison, Wisconsin, USA.
- Hollmann, F., Schmid, A. & Steckhan, E. (2001). Angew. Chem. Int. Ed. 40, 169–171. [DOI] [PubMed]
- Lee, H. J., Lee, S. H., Park, C. B. & Won, K. (2011). Chem. Commun. 47, 12538–12540. [DOI] [PubMed]
- Maenaka, Y., Suenobu, T. & Fukuzumi, S. (2012). J. Am. Chem. Soc. 134, 367–374. [DOI] [PubMed]
- Park, C. B., Lee, S. H., Subramanian, E., Kale, B. B., Lee, S. M. & Baeg, J.-O. (2008). Chem. Commun. pp. 5423–5425. [DOI] [PubMed]
- Ruppert, R., Herrmann, S. & Steckhan, E. (1988). J. Chem. Soc. Chem. Commun. pp. 1150–1151.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Song, H.-K., Lee, S. H., Won, K., Park, J. H., Kim, J. K., Lee, H., Moon, S.-J., Kim, D. K. & Park, C. B. (2008). Angew. Chem. Int. Ed. 47, 1749–1752. [DOI] [PubMed]
- Zhu, X.-Q., Yang, Y., Zhang, M. & Cheng, J.-P. (2003). J. Am. Chem. Soc. 125, 15298–15299. [DOI] [PubMed]
- Zhu, X.-Q., Zhang, J.-Y. & Cheng, J.-P. (2006). J. Org. Chem. 71, 7007–7015. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S1600536812015917/lh5450sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536812015917/lh5450Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536812015917/lh5450Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report