Abstract
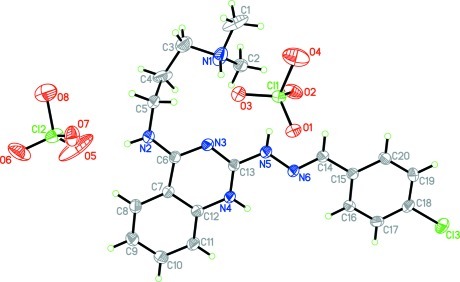
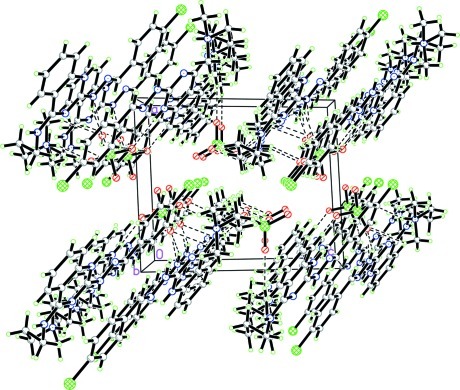
In the title compound, C20H25ClN6 2+·2ClO4 −, the organic cation is roughly planar, as shown by the dihedral angle of 3.78 (3)° between the quinazoline and chlorophenyl rings. The quinazoline ring is itself approximately planar, with an average deviation of 0.018 (4) Å. The organic cation adopts an E configuration with respect to the C= N double bond of the hyrazinyl group. The (dimethylazaniumyl)propylamino side chain is disordered over two sets of sites with occupancies of 0.768 (10) and 0.232 (10). In the crystal, two cations and four anions are linked by strong N—H⋯O hydrogen bonds. Weak C—H⋯O hydrogen bonds exist among these aggregates.
Related literature
For antitumor background to the title compound, see: Abouzid & Shouman (2008 ▶); Zhang et al. (2008 ▶); An et al. (2010 ▶); Horiuchi et al. (2009) ▶. For the structures of closely related compounds, see: Fun et al. (2010 ▶); Ferreira et al. (2009 ▶); de Souza et al. (2010 ▶); Loh et al. (2011 ▶).
Experimental
Crystal data
C20H25ClN6 2+·2ClO4 −
M r = 583.81
Triclinic,
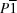
a = 10.4533 (18) Å
b = 10.5018 (18) Å
c = 12.626 (2) Å
α = 104.745 (9)°
β = 91.146 (10)°
γ = 96.21 (1)°
V = 1330.9 (4) Å3
Z = 2
Mo Kα radiation
μ = 0.40 mm−1
T = 293 K
0.25 × 0.23 × 0.18 mm
Data collection
Siemens SMART CCD area-detector diffractometer
Absorption correction: multi-scan (SADABS; Sheldrick, 1996 ▶) T min = 0.907, T max = 0.932
12435 measured reflections
4889 independent reflections
3979 reflections with I > 2σ(I)
R int = 0.023
Refinement
R[F 2 > 2σ(F 2)] = 0.071
wR(F 2) = 0.219
S = 1.09
4889 reflections
371 parameters
62 restraints
H-atom parameters constrained
Δρmax = 0.69 e Å−3
Δρmin = −0.92 e Å−3
Data collection: SMART (Siemens, 1996 ▶); cell refinement: SAINT (Siemens, 1996 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: SHELXTL (Sheldrick, 2008 ▶); software used to prepare material for publication: SHELXTL.
Supplementary Material
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S1600536812018272/im2363sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536812018272/im2363Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536812018272/im2363Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N1—H1A⋯O3 | 0.91 | 2.19 | 2.990 (5) | 147 |
| N5—H5C⋯O2 | 0.86 | 2.11 | 2.922 (4) | 157 |
| N4—H4C⋯O1i | 0.86 | 2.14 | 2.964 (4) | 160 |
| N2—H2D⋯O7 | 0.86 | 2.10 | 2.873 (5) | 149 |
| C19—H19⋯O2ii | 0.93 | 2.48 | 3.316 (5) | 150 |
| C1—H1D⋯O6iii | 0.96 | 2.56 | 3.445 (8) | 153 |
| C1—H1C⋯O6iv | 0.96 | 2.62 | 3.555 (10) | 166 |
Symmetry codes: (i) 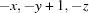 ; (ii)
; (ii) 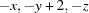 ; (iii)
; (iii) 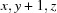 ; (iv)
; (iv) 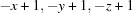 .
.
Acknowledgments
The authors thank Dalian University of Technology for providing research facilities. This work was supported by the National S & T Major Project of China (No. 2009ZX09301–012) and the S & T Project of Liaoning Province (No. LJQ201107).
supplementary crystallographic information
Comment
The target compound was designed and synthesized as part of on-going studies aimed at developing antitumor agents based on 4-aminoquinazoline and 4-aminoquinoline nuclei. These have aroused increasing attentions for excellent antitumor potency in recent years, such as gefitinib, the traditional immunostimulatory agents CQ and their derivatives (Abouzid et al., 2008; Zhang et al., 2008; An et al., 2010). With the aim to improve the electron affinity and better biological interactions, a hydrazone fragment was introduced (Horiuchi et al., 2009).
The crystal structure of the title compound is given in Fig. 1. The quinazoline ring is approximately planar, with an average deviation of 0.018 (4) Å. The dihedral angle between the quinazoline ring and the chlorophenyl ring is 3.78 (3) °. The (dimethylazaniumyl)propylamino side chain of the compound is disordered over two sites with occupancies of 0.768 (10) and 0.232 (10), respectively. The cationic part of the compound establishes strong N–H···O hydrogen bonds (N5—H5C–O2, N2—H2D–O7, N1—H1A–O3, N4—H4C–O1, Table 1) with the perchlorate anions. The resulting aggregates of two cations and four anions are linked by weak C–H···O hydrogen bonds (C1—H1C–O6, C1—H1D–O6, C19—H19–O2, Table 1) in the crystal structure (Fig. 2).
Experimental
Using 2-aminobenzoic acid and urea as the starting materials, (E)-N-(2-(2-(4-chlorobenzylidene)hydrazinyl) quinazolin-4-yl)-N',N'-dimethylpropane-1,3-diamine was prepared according to literature methods (Abouzid et al., 2008; Horiuchi et al., 2009). The compound was purified by silica gel column chromatography (CH2Cl2/Methanol 15:1). 70% Perchloric acid (24 mmol, 1.96 ml) was added to a solution of the compound (20 mmol, 7.7 g) in acetone (50 ml) at room temperature. Then the reaction mixture was stirred at 313 K for 3 h. After cooling to ambient temperature, the resulting precipitate was filtered and washed with acetone. The resulting solids were dissolved in methanol for 15 days to yield the title compound as colorless single crystals (70% yield).
Refinement
All H-atoms were positioned geometrically and refined using a riding model, with C—H = 0.96 Å (methyl), C—H = 0.97 Å (methylene), 0.93 Å (aromatic), N—H = 0.86 Å (amine and aromatic), and Uiso(H) =1.2Ueq(C,N).
Figures
Fig. 1.
Molecular structure of the title compound, showing 30% probability displacement ellipsoids and the atom-numbering scheme.
Fig. 2.
Packing diagram of the title compound.
Crystal data
| C20H25ClN62+·2ClO4− | Z = 2 |
| Mr = 583.81 | F(000) = 604 |
| Triclinic, P1 | Dx = 1.457 Mg m−3 |
| Hall symbol: -P 1 | Mo Kα radiation, λ = 0.71073 Å |
| a = 10.4533 (18) Å | Cell parameters from 4829 reflections |
| b = 10.5018 (18) Å | θ = 2.5–30.1° |
| c = 12.626 (2) Å | µ = 0.40 mm−1 |
| α = 104.745 (9)° | T = 293 K |
| β = 91.146 (10)° | Block, colorless |
| γ = 96.21 (1)° | 0.25 × 0.23 × 0.18 mm |
| V = 1330.9 (4) Å3 |
Data collection
| Siemens SMART CCD area-detector diffractometer | 4889 independent reflections |
| Radiation source: fine-focus sealed tube | 3979 reflections with I > 2σ(I) |
| Graphite monochromator | Rint = 0.023 |
| phi and ω scans | θmax = 25.5°, θmin = 1.7° |
| Absorption correction: multi-scan (SADABS; Sheldrick, 1996) | h = −12→12 |
| Tmin = 0.907, Tmax = 0.932 | k = −12→12 |
| 12435 measured reflections | l = −14→15 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.071 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.219 | H-atom parameters constrained |
| S = 1.09 | w = 1/[σ2(Fo2) + (0.1339P)2 + 1.0689P] where P = (Fo2 + 2Fc2)/3 |
| 4889 reflections | (Δ/σ)max < 0.001 |
| 371 parameters | Δρmax = 0.69 e Å−3 |
| 62 restraints | Δρmin = −0.92 e Å−3 |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | Occ. (<1) | |
| Cl1 | 0.32941 (8) | 0.74253 (8) | 0.10002 (7) | 0.0433 (3) | |
| Cl2 | 0.26374 (10) | 0.25136 (12) | 0.61278 (8) | 0.0638 (4) | |
| Cl3 | −0.49702 (12) | 0.78162 (13) | −0.25924 (10) | 0.0702 (4) | |
| N2 | 0.2182 (3) | 0.4226 (3) | 0.3840 (3) | 0.0455 (7) | |
| H2D | 0.2213 | 0.3602 | 0.4166 | 0.055* | |
| N3 | 0.1121 (3) | 0.5197 (3) | 0.2718 (2) | 0.0391 (6) | |
| N4 | −0.0771 (3) | 0.4117 (3) | 0.1697 (2) | 0.0380 (6) | |
| H4C | −0.1379 | 0.4135 | 0.1234 | 0.046* | |
| N5 | 0.0158 (3) | 0.6172 (3) | 0.1555 (2) | 0.0399 (6) | |
| H5C | 0.0781 | 0.6806 | 0.1710 | 0.048* | |
| N6 | −0.0855 (3) | 0.6209 (3) | 0.0851 (2) | 0.0369 (6) | |
| O1 | 0.3001 (3) | 0.6543 (3) | −0.0088 (2) | 0.0553 (7) | |
| O2 | 0.2245 (3) | 0.8192 (3) | 0.1395 (3) | 0.0617 (8) | |
| O3 | 0.3692 (3) | 0.6777 (3) | 0.1811 (2) | 0.0582 (7) | |
| O4 | 0.4611 (4) | 0.8581 (4) | 0.0865 (4) | 0.1015 (14) | |
| O5 | 0.1324 (4) | 0.2708 (10) | 0.6082 (4) | 0.201 (4) | |
| O6 | 0.2930 (6) | 0.1273 (4) | 0.6352 (4) | 0.123 (2) | |
| O7 | 0.3248 (4) | 0.2711 (4) | 0.5170 (3) | 0.0731 (9) | |
| O8 | 0.3333 (5) | 0.3762 (5) | 0.7247 (4) | 0.1072 (14) | |
| C1 | 0.3790 (6) | 0.9314 (8) | 0.3904 (6) | 0.094 (3) | 0.768 (10) |
| H1B | 0.3551 | 0.9663 | 0.3306 | 0.141* | 0.768 (10) |
| H1C | 0.4612 | 0.8987 | 0.3783 | 0.141* | 0.768 (10) |
| H1D | 0.3842 | 1.0001 | 0.4576 | 0.141* | 0.768 (10) |
| C2 | 0.1534 (15) | 0.8623 (13) | 0.3983 (9) | 0.054 (3) | 0.768 (10) |
| H2A | 0.0894 | 0.7915 | 0.4033 | 0.081* | 0.768 (10) |
| H2B | 0.1373 | 0.8877 | 0.3318 | 0.081* | 0.768 (10) |
| H2C | 0.1493 | 0.9370 | 0.4601 | 0.081* | 0.768 (10) |
| N1 | 0.2784 (4) | 0.8189 (4) | 0.3980 (3) | 0.0711 (11) | 0.768 (10) |
| H1A | 0.2818 | 0.7500 | 0.3379 | 0.085* | 0.768 (10) |
| C3 | 0.3380 (7) | 0.7866 (6) | 0.4925 (5) | 0.071 (2) | 0.768 (10) |
| H3A | 0.3252 | 0.8526 | 0.5596 | 0.085* | 0.768 (10) |
| H3B | 0.4297 | 0.7839 | 0.4841 | 0.085* | 0.768 (10) |
| C4 | 0.2729 (9) | 0.6522 (6) | 0.4957 (5) | 0.079 (3) | 0.768 (10) |
| H4A | 0.1807 | 0.6504 | 0.4836 | 0.095* | 0.768 (10) |
| H4B | 0.2881 | 0.6397 | 0.5683 | 0.095* | 0.768 (10) |
| C5 | 0.3222 (4) | 0.5303 (4) | 0.4057 (4) | 0.0635 (12) | 0.768 (10) |
| H5A | 0.3423 | 0.5563 | 0.3390 | 0.076* | 0.768 (10) |
| H5B | 0.3990 | 0.5033 | 0.4340 | 0.076* | 0.768 (10) |
| C1' | 0.4014 (16) | 0.885 (3) | 0.438 (2) | 0.081 (7) | 0.232 (10) |
| H1'B | 0.4612 | 0.8206 | 0.4339 | 0.121* | 0.232 (10) |
| H1'C | 0.3972 | 0.9346 | 0.5124 | 0.121* | 0.232 (10) |
| H1'D | 0.4297 | 0.9437 | 0.3936 | 0.121* | 0.232 (10) |
| C2' | 0.156 (4) | 0.896 (4) | 0.397 (4) | 0.059 (11) | 0.232 (10) |
| H2'A | 0.1676 | 0.9518 | 0.3478 | 0.089* | 0.232 (10) |
| H2'B | 0.1452 | 0.9492 | 0.4697 | 0.089* | 0.232 (10) |
| H2'C | 0.0805 | 0.8331 | 0.3734 | 0.089* | 0.232 (10) |
| N1' | 0.2784 (4) | 0.8189 (4) | 0.3980 (3) | 0.0711 (11) | 0.232 (10) |
| H1'A | 0.2844 | 0.7628 | 0.3307 | 0.085* | 0.232 (10) |
| C3' | 0.2400 (10) | 0.7379 (10) | 0.4811 (8) | 0.027 (4) | 0.232 (10) |
| H3'A | 0.2259 | 0.7983 | 0.5508 | 0.032* | 0.232 (10) |
| H3'B | 0.1594 | 0.6825 | 0.4552 | 0.032* | 0.232 (10) |
| C4' | 0.3425 (12) | 0.6497 (11) | 0.4986 (10) | 0.033 (4) | 0.232 (10) |
| H4'A | 0.4283 | 0.6954 | 0.4992 | 0.040* | 0.232 (10) |
| H4'B | 0.3320 | 0.6261 | 0.5676 | 0.040* | 0.232 (10) |
| C5' | 0.3222 (4) | 0.5303 (4) | 0.4057 (4) | 0.0635 (12) | 0.232 (10) |
| H5'A | 0.3229 | 0.5634 | 0.3407 | 0.076* | 0.232 (10) |
| H5'B | 0.4003 | 0.4881 | 0.4056 | 0.076* | 0.232 (10) |
| C6 | 0.1190 (3) | 0.4168 (3) | 0.3162 (3) | 0.0377 (7) | |
| C7 | 0.0215 (3) | 0.3013 (3) | 0.2901 (3) | 0.0377 (7) | |
| C8 | 0.0235 (4) | 0.1903 (4) | 0.3319 (3) | 0.0476 (8) | |
| H8 | 0.0881 | 0.1891 | 0.3833 | 0.057* | |
| C9 | −0.0688 (4) | 0.0830 (4) | 0.2980 (3) | 0.0523 (9) | |
| H9 | −0.0663 | 0.0093 | 0.3258 | 0.063* | |
| C10 | −0.1655 (4) | 0.0850 (4) | 0.2224 (3) | 0.0535 (9) | |
| H10 | −0.2279 | 0.0121 | 0.1998 | 0.064* | |
| C11 | −0.1714 (4) | 0.1935 (4) | 0.1798 (3) | 0.0491 (9) | |
| H11 | −0.2375 | 0.1940 | 0.1297 | 0.059* | |
| C12 | −0.0763 (3) | 0.3026 (3) | 0.2132 (3) | 0.0384 (7) | |
| C13 | 0.0161 (3) | 0.5139 (3) | 0.1994 (3) | 0.0353 (7) | |
| C14 | −0.0831 (3) | 0.7243 (3) | 0.0498 (3) | 0.0398 (7) | |
| H14 | −0.0154 | 0.7918 | 0.0722 | 0.048* | |
| C15 | −0.1847 (3) | 0.7380 (3) | −0.0249 (3) | 0.0376 (7) | |
| C16 | −0.2904 (3) | 0.6397 (3) | −0.0569 (3) | 0.0425 (8) | |
| H16 | −0.2963 | 0.5654 | −0.0292 | 0.051* | |
| C17 | −0.3850 (4) | 0.6532 (4) | −0.1289 (3) | 0.0478 (9) | |
| H17 | −0.4547 | 0.5880 | −0.1506 | 0.057* | |
| C18 | −0.3756 (3) | 0.7649 (4) | −0.1690 (3) | 0.0440 (8) | |
| C19 | −0.2739 (4) | 0.8635 (4) | −0.1393 (3) | 0.0464 (8) | |
| H19 | −0.2689 | 0.9374 | −0.1676 | 0.056* | |
| C20 | −0.1798 (3) | 0.8500 (3) | −0.0664 (3) | 0.0437 (8) | |
| H20 | −0.1113 | 0.9168 | −0.0442 | 0.052* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Cl1 | 0.0423 (5) | 0.0379 (5) | 0.0468 (5) | −0.0048 (3) | −0.0039 (3) | 0.0099 (4) |
| Cl2 | 0.0543 (6) | 0.0819 (8) | 0.0474 (6) | −0.0141 (5) | −0.0011 (4) | 0.0116 (5) |
| Cl3 | 0.0688 (7) | 0.0759 (8) | 0.0666 (7) | 0.0096 (6) | −0.0302 (5) | 0.0216 (6) |
| N2 | 0.0436 (16) | 0.0429 (16) | 0.0513 (17) | 0.0030 (12) | −0.0128 (13) | 0.0165 (13) |
| N3 | 0.0363 (14) | 0.0390 (15) | 0.0406 (15) | 0.0044 (11) | −0.0049 (11) | 0.0086 (12) |
| N4 | 0.0353 (14) | 0.0420 (15) | 0.0369 (14) | 0.0026 (11) | −0.0058 (11) | 0.0119 (12) |
| N5 | 0.0338 (14) | 0.0432 (16) | 0.0436 (15) | 0.0020 (11) | −0.0071 (11) | 0.0144 (12) |
| N6 | 0.0329 (13) | 0.0410 (15) | 0.0375 (14) | 0.0041 (11) | −0.0051 (11) | 0.0119 (12) |
| O1 | 0.0659 (17) | 0.0486 (15) | 0.0455 (14) | −0.0049 (13) | −0.0153 (12) | 0.0072 (12) |
| O2 | 0.0433 (15) | 0.0480 (16) | 0.090 (2) | −0.0004 (12) | 0.0120 (14) | 0.0124 (15) |
| O3 | 0.0685 (18) | 0.0532 (16) | 0.0528 (16) | −0.0003 (13) | −0.0138 (13) | 0.0178 (13) |
| O4 | 0.081 (3) | 0.082 (3) | 0.126 (3) | −0.016 (2) | 0.024 (2) | 0.008 (2) |
| O5 | 0.042 (2) | 0.408 (12) | 0.098 (4) | 0.007 (4) | −0.017 (2) | −0.026 (5) |
| O6 | 0.222 (6) | 0.058 (2) | 0.084 (3) | −0.029 (3) | 0.009 (3) | 0.029 (2) |
| O7 | 0.092 (2) | 0.086 (2) | 0.0534 (17) | 0.0278 (19) | 0.0155 (16) | 0.0313 (16) |
| O8 | 0.091 (3) | 0.111 (3) | 0.102 (3) | 0.006 (2) | −0.008 (2) | −0.001 (3) |
| C1 | 0.047 (3) | 0.133 (7) | 0.067 (4) | 0.006 (4) | −0.015 (3) | −0.034 (4) |
| C2 | 0.064 (5) | 0.042 (8) | 0.051 (4) | 0.011 (5) | −0.003 (3) | 0.002 (4) |
| N1 | 0.096 (3) | 0.074 (2) | 0.0416 (18) | 0.044 (2) | −0.0082 (17) | −0.0013 (16) |
| C3 | 0.078 (5) | 0.075 (4) | 0.054 (3) | 0.008 (4) | −0.011 (3) | 0.006 (3) |
| C4 | 0.095 (6) | 0.089 (5) | 0.037 (3) | −0.045 (5) | −0.012 (3) | 0.011 (3) |
| C5 | 0.064 (3) | 0.056 (2) | 0.072 (3) | −0.012 (2) | −0.034 (2) | 0.031 (2) |
| C1' | 0.094 (11) | 0.083 (10) | 0.079 (10) | 0.007 (8) | −0.001 (8) | 0.048 (9) |
| C2' | 0.037 (11) | 0.009 (12) | 0.12 (2) | 0.001 (8) | 0.015 (11) | 0.005 (10) |
| N1' | 0.096 (3) | 0.074 (2) | 0.0416 (18) | 0.044 (2) | −0.0082 (17) | −0.0013 (16) |
| C3' | 0.026 (7) | 0.016 (6) | 0.029 (6) | −0.008 (5) | −0.003 (5) | −0.009 (5) |
| C4' | 0.016 (6) | 0.052 (7) | 0.028 (6) | −0.018 (5) | −0.016 (5) | 0.013 (5) |
| C5' | 0.064 (3) | 0.056 (2) | 0.072 (3) | −0.012 (2) | −0.034 (2) | 0.031 (2) |
| C6 | 0.0398 (17) | 0.0392 (17) | 0.0345 (16) | 0.0077 (13) | −0.0010 (13) | 0.0095 (13) |
| C7 | 0.0377 (17) | 0.0405 (18) | 0.0348 (16) | 0.0041 (13) | −0.0003 (13) | 0.0100 (13) |
| C8 | 0.050 (2) | 0.049 (2) | 0.049 (2) | 0.0068 (16) | −0.0018 (16) | 0.0198 (16) |
| C9 | 0.055 (2) | 0.048 (2) | 0.057 (2) | 0.0001 (17) | 0.0010 (18) | 0.0220 (18) |
| C10 | 0.054 (2) | 0.046 (2) | 0.059 (2) | −0.0074 (17) | −0.0009 (18) | 0.0162 (18) |
| C11 | 0.0455 (19) | 0.054 (2) | 0.047 (2) | −0.0047 (16) | −0.0081 (15) | 0.0169 (17) |
| C12 | 0.0374 (16) | 0.0400 (18) | 0.0375 (17) | 0.0020 (13) | 0.0027 (13) | 0.0102 (14) |
| C13 | 0.0353 (16) | 0.0379 (17) | 0.0333 (15) | 0.0085 (13) | 0.0006 (12) | 0.0088 (13) |
| C14 | 0.0372 (17) | 0.0385 (18) | 0.0428 (18) | 0.0018 (13) | −0.0055 (13) | 0.0102 (14) |
| C15 | 0.0394 (17) | 0.0347 (17) | 0.0379 (16) | 0.0065 (13) | −0.0014 (13) | 0.0073 (13) |
| C16 | 0.0475 (19) | 0.0337 (17) | 0.0449 (18) | 0.0031 (14) | −0.0054 (15) | 0.0091 (14) |
| C17 | 0.049 (2) | 0.0392 (19) | 0.049 (2) | 0.0010 (15) | −0.0108 (16) | 0.0037 (15) |
| C18 | 0.0437 (18) | 0.051 (2) | 0.0357 (17) | 0.0128 (15) | −0.0069 (14) | 0.0059 (15) |
| C19 | 0.052 (2) | 0.0432 (19) | 0.050 (2) | 0.0102 (16) | −0.0016 (16) | 0.0206 (16) |
| C20 | 0.0400 (18) | 0.0367 (18) | 0.056 (2) | 0.0017 (14) | −0.0035 (15) | 0.0155 (15) |
Geometric parameters (Å, º)
| Cl1—O3 | 1.444 (3) | C5—H5A | 0.9700 |
| Cl1—O2 | 1.452 (3) | C5—H5B | 0.9700 |
| Cl1—O1 | 1.454 (3) | C1'—H1'B | 0.9600 |
| Cl1—O4 | 1.775 (4) | C1'—H1'C | 0.9600 |
| Cl2—O5 | 1.412 (5) | C1'—H1'D | 0.9600 |
| Cl2—O7 | 1.430 (3) | C2'—H2'A | 0.9600 |
| Cl2—O6 | 1.464 (5) | C2'—H2'B | 0.9600 |
| Cl2—O8 | 1.747 (5) | C2'—H2'C | 0.9600 |
| Cl3—C18 | 1.741 (4) | C3'—C4' | 1.538 (10) |
| N2—C6 | 1.318 (4) | C3'—H3'A | 0.9700 |
| N2—C5 | 1.450 (5) | C3'—H3'B | 0.9700 |
| N2—H2D | 0.8600 | C4'—H4'A | 0.9700 |
| N3—C13 | 1.330 (4) | C4'—H4'B | 0.9700 |
| N3—C6 | 1.346 (4) | C6—C7 | 1.461 (5) |
| N4—C13 | 1.341 (4) | C7—C12 | 1.398 (5) |
| N4—C12 | 1.392 (4) | C7—C8 | 1.400 (5) |
| N4—H4C | 0.8600 | C8—C9 | 1.374 (5) |
| N5—C13 | 1.338 (4) | C8—H8 | 0.9300 |
| N5—N6 | 1.379 (4) | C9—C10 | 1.382 (6) |
| N5—H5C | 0.8600 | C9—H9 | 0.9300 |
| N6—C14 | 1.272 (4) | C10—C11 | 1.384 (6) |
| C1—N1 | 1.517 (7) | C10—H10 | 0.9300 |
| C1—H1B | 0.9600 | C11—C12 | 1.404 (5) |
| C1—H1C | 0.9600 | C11—H11 | 0.9300 |
| C1—H1D | 0.9600 | C14—C15 | 1.450 (5) |
| C2—N1 | 1.430 (16) | C14—H14 | 0.9300 |
| C2—H2A | 0.9600 | C15—C20 | 1.400 (5) |
| C2—H2B | 0.9600 | C15—C16 | 1.404 (5) |
| C2—H2C | 0.9600 | C16—C17 | 1.372 (5) |
| N1—C3 | 1.465 (6) | C16—H16 | 0.9300 |
| N1—H1A | 0.9100 | C17—C18 | 1.386 (5) |
| C3—C4 | 1.510 (7) | C17—H17 | 0.9300 |
| C3—H3A | 0.9700 | C18—C19 | 1.375 (5) |
| C3—H3B | 0.9700 | C19—C20 | 1.378 (5) |
| C4—C5 | 1.620 (7) | C19—H19 | 0.9300 |
| C4—H4A | 0.9700 | C20—H20 | 0.9300 |
| C4—H4B | 0.9700 | ||
| O3—Cl1—O2 | 110.00 (19) | H5A—C5—H5B | 108.6 |
| O3—Cl1—O1 | 114.28 (17) | H1'B—C1'—H1'C | 109.5 |
| O2—Cl1—O1 | 112.44 (19) | H1'B—C1'—H1'D | 109.5 |
| O3—Cl1—O4 | 107.1 (2) | H1'C—C1'—H1'D | 109.5 |
| O2—Cl1—O4 | 106.45 (18) | H2'A—C2'—H2'B | 109.5 |
| O1—Cl1—O4 | 106.02 (19) | H2'A—C2'—H2'C | 109.5 |
| O5—Cl2—O7 | 110.7 (3) | H2'B—C2'—H2'C | 109.5 |
| O5—Cl2—O6 | 116.7 (5) | C4'—C3'—H3'A | 109.0 |
| O7—Cl2—O6 | 110.9 (3) | C4'—C3'—H3'B | 109.0 |
| O5—Cl2—O8 | 105.3 (3) | H3'A—C3'—H3'B | 107.8 |
| O7—Cl2—O8 | 107.5 (2) | C3'—C4'—H4'A | 110.6 |
| O6—Cl2—O8 | 105.0 (3) | C3'—C4'—H4'B | 110.6 |
| C6—N2—C5 | 122.3 (3) | H4'A—C4'—H4'B | 108.7 |
| C6—N2—H2D | 118.8 | N2—C6—N3 | 117.7 (3) |
| C5—N2—H2D | 118.8 | N2—C6—C7 | 120.5 (3) |
| C13—N3—C6 | 119.0 (3) | N3—C6—C7 | 121.8 (3) |
| C13—N4—C12 | 120.5 (3) | C12—C7—C8 | 119.3 (3) |
| C13—N4—H4C | 119.7 | C12—C7—C6 | 115.8 (3) |
| C12—N4—H4C | 119.7 | C8—C7—C6 | 124.8 (3) |
| C13—N5—N6 | 119.2 (3) | C9—C8—C7 | 120.7 (4) |
| C13—N5—H5C | 120.4 | C9—C8—H8 | 119.6 |
| N6—N5—H5C | 120.4 | C7—C8—H8 | 119.6 |
| C14—N6—N5 | 116.2 (3) | C8—C9—C10 | 119.7 (4) |
| N1—C1—H1B | 109.5 | C8—C9—H9 | 120.1 |
| N1—C1—H1C | 109.5 | C10—C9—H9 | 120.1 |
| H1B—C1—H1C | 109.5 | C9—C10—C11 | 121.2 (4) |
| N1—C1—H1D | 109.5 | C9—C10—H10 | 119.4 |
| H1B—C1—H1D | 109.5 | C11—C10—H10 | 119.4 |
| H1C—C1—H1D | 109.5 | C10—C11—C12 | 119.2 (4) |
| N1—C2—H2A | 109.5 | C10—C11—H11 | 120.4 |
| N1—C2—H2B | 109.5 | C12—C11—H11 | 120.4 |
| H2A—C2—H2B | 109.5 | N4—C12—C7 | 119.5 (3) |
| N1—C2—H2C | 109.5 | N4—C12—C11 | 120.8 (3) |
| H2A—C2—H2C | 109.5 | C7—C12—C11 | 119.8 (3) |
| H2B—C2—H2C | 109.5 | N3—C13—N5 | 116.5 (3) |
| C2—N1—C3 | 123.2 (6) | N3—C13—N4 | 123.4 (3) |
| C2—N1—C1 | 109.2 (6) | N5—C13—N4 | 120.1 (3) |
| C3—N1—C1 | 97.5 (5) | N6—C14—C15 | 120.7 (3) |
| C2—N1—H1A | 108.6 | N6—C14—H14 | 119.6 |
| C3—N1—H1A | 108.6 | C15—C14—H14 | 119.6 |
| C1—N1—H1A | 108.6 | C20—C15—C16 | 118.6 (3) |
| N1—C3—C4 | 106.3 (5) | C20—C15—C14 | 120.6 (3) |
| N1—C3—H3A | 110.5 | C16—C15—C14 | 120.8 (3) |
| C4—C3—H3A | 110.5 | C17—C16—C15 | 120.1 (3) |
| N1—C3—H3B | 110.5 | C17—C16—H16 | 119.9 |
| C4—C3—H3B | 110.5 | C15—C16—H16 | 119.9 |
| H3A—C3—H3B | 108.7 | C16—C17—C18 | 119.4 (3) |
| C3—C4—C5 | 113.7 (5) | C16—C17—H17 | 120.3 |
| C3—C4—H4A | 108.8 | C18—C17—H17 | 120.3 |
| C5—C4—H4A | 108.8 | C19—C18—C17 | 122.2 (3) |
| C3—C4—H4B | 108.8 | C19—C18—Cl3 | 118.9 (3) |
| C5—C4—H4B | 108.8 | C17—C18—Cl3 | 119.0 (3) |
| H4A—C4—H4B | 107.7 | C18—C19—C20 | 118.2 (3) |
| N2—C5—C4 | 106.7 (4) | C18—C19—H19 | 120.9 |
| N2—C5—H5A | 110.4 | C20—C19—H19 | 120.9 |
| C4—C5—H5A | 110.4 | C19—C20—C15 | 121.5 (3) |
| N2—C5—H5B | 110.4 | C19—C20—H20 | 119.3 |
| C4—C5—H5B | 110.4 | C15—C20—H20 | 119.3 |
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N1—H1A···O3 | 0.91 | 2.19 | 2.990 (5) | 147 |
| N5—H5C···O2 | 0.86 | 2.11 | 2.922 (4) | 157 |
| N4—H4C···O1i | 0.86 | 2.14 | 2.964 (4) | 160 |
| N2—H2D···O7 | 0.86 | 2.10 | 2.873 (5) | 149 |
| C19—H19···O2ii | 0.93 | 2.48 | 3.316 (5) | 150 |
| C1—H1D···O6iii | 0.96 | 2.56 | 3.445 (8) | 153 |
| C1—H1C···O6iv | 0.96 | 2.62 | 3.555 (10) | 166 |
Symmetry codes: (i) −x, −y+1, −z; (ii) −x, −y+2, −z; (iii) x, y+1, z; (iv) −x+1, −y+1, −z+1.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: IM2363).
References
- Abouzid, K. & Shouman, S. (2008). Bioorg. Med. Chem. 16, 7543–7551. [DOI] [PubMed]
- An, Z. Y., Yan, Y. Y., Peng, D., Ou, T. M., Tan, J. H., Huang, S. L., An, L. K., Gu, L. Q. & Huang, Z. S. (2010). Eur. J. Med. Chem. 45, 3895–3903. [DOI] [PubMed]
- Ferreira, M. L. de, Souza, M. V. N. de, Howie, R. A., Tiekink, E. R. T., Wardell, J. L. & Wardell, S. M. S. V. (2009). Acta Cryst. E65, o3239–o3240. [DOI] [PMC free article] [PubMed]
- Fun, H.-K., Loh, W.-S. & Nayak, S. P. (2010). Acta Cryst. E66, o2467. [DOI] [PMC free article] [PubMed]
- Horiuchi, T., Nagata, M., Kitagawa, M., Akahane, K. & Uoto, K. (2009). Bioorg. Med. Chem. 17, 119–132. [DOI] [PubMed]
- Loh, W.-S., Fun, H.-K., Kayarmar, R., Viveka, S. & Nagaraja, G. K. (2011). Acta Cryst. E67, o407–o408. [DOI] [PMC free article] [PubMed]
- Sheldrick, G. M. (1996). SADABS University of Göttingen, Germany.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Siemens (1996). SMART and SAINT Siemens Analytical X-ray Instruments Inc., Madison, Wisconsin, USA.
- Souza, M. V. N. de, Howie, R. A., Tiekink, E. R. T., Wardell, J. L. & Wardell, S. M. S. V. (2010). Acta Cryst. E66, o152–o153. [DOI] [PMC free article] [PubMed]
- Zhang, H. W., Solomon, V. R., Hu, C. K., Ulibarri, G. & Lee, H. Y. (2008). Biomed. Pharmacother. 62, 65–69. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S1600536812018272/im2363sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536812018272/im2363Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536812018272/im2363Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report