Abstract
A single 7,8-dihydro-8-oxoguanine (G8-OXO; 8-hydroxyguanine) adduct in the lacZ alpha gene of bacteriophage M13 DNA induces a targeted G-->T transversion after replication in Escherichia coli (Biochemistry, 29, 7024-7031 (1990)). This mutation is thought to be due to the facile formation during DNA synthesis of a G8-OXO.base pair, where G8-OXO is in the syn conformation about the deoxyglycosyl bond. A related modified purine, 7,8-dihydro-8-oxoadenine (A8-OXO; 8-hydroxyadenine), is an abundant product found in irradiated and oxidized DNAs. Similar to G8-OXO, as a mononucleoside A8-OXO assumes the syn conformation. This work has assessed the relative mutagenicities of A8-OXO and G8-OXO in the same experimental system. A deoxypentanucleotide containing A8-OXO [d(GCT-A8-OXOG)] was synthesized. After 5'-phosphorylation with [gamma-32P] ATP, the oligomer was ligated into a duplex M13mp19-derived genome at a unique NheI restriction site. Genomes containing either A8-OXO (at position 6275, [+] strand) or G8-OXO (position 6276) were denatured with heat and introduced into E.coli DL7 cells. Analysis of phage DNA from mutant plaques obtained by plating immediately after transformation (infective centers assay) revealed that G8-OXO induced G-->T transversions at an apparent frequency of approximately 0.3%. The frequency and spectrum of mutations observed in DNA sequences derived from 172 mutant plaques arising from the A8-OXO-modified DNA were almost indistiguishable from those generated from transfection of an adenine-containing control genome. We conclude that A8-OXO is at least an order of magnitude less mutagenic than G8-OXO in E.coli cells with normal DNA repair capabilities.
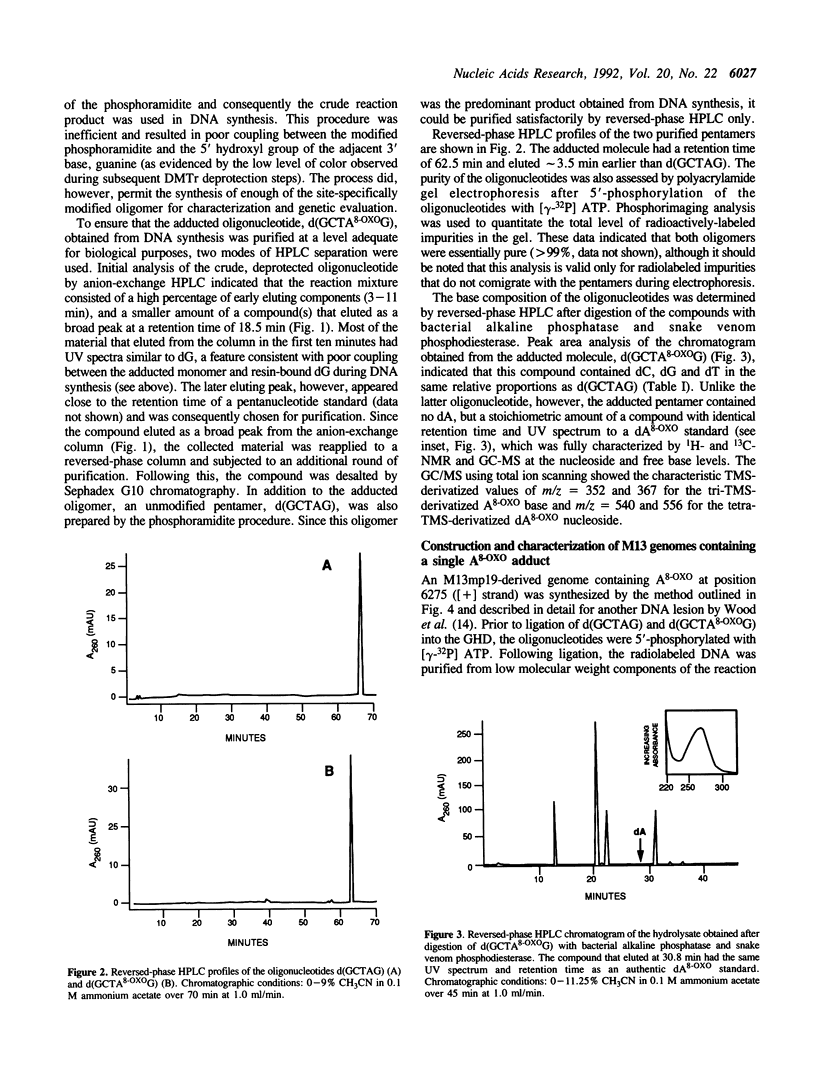
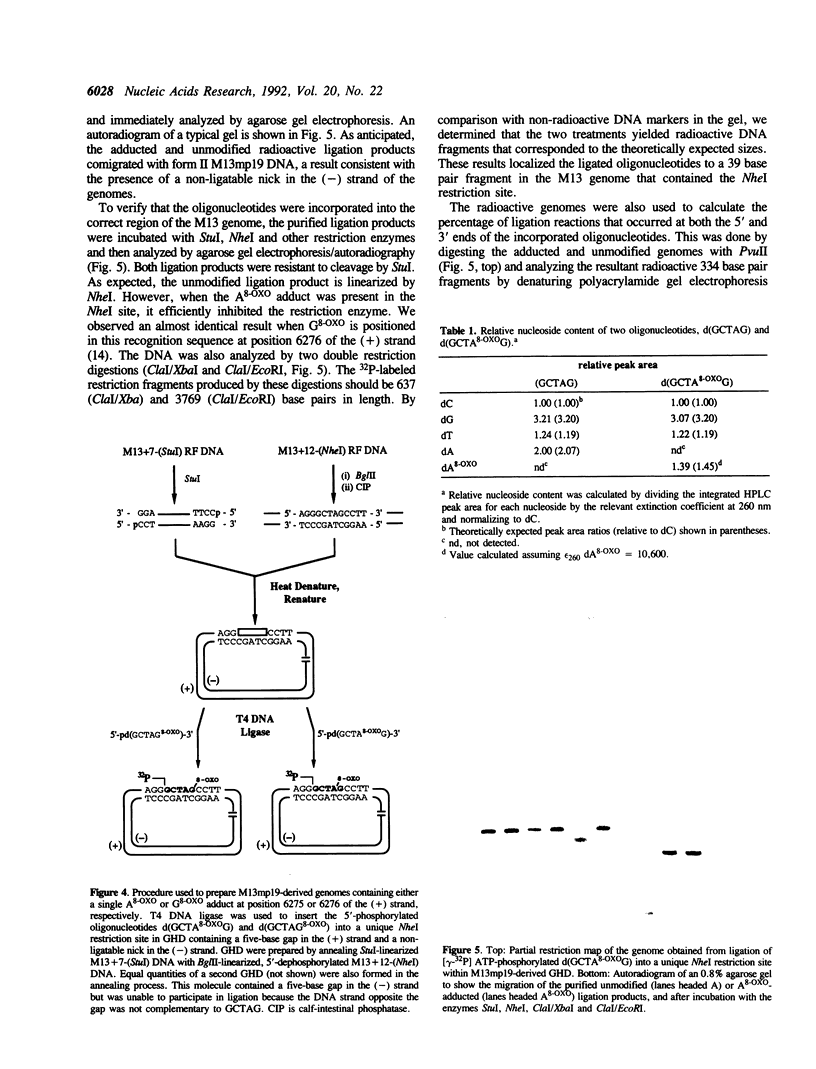
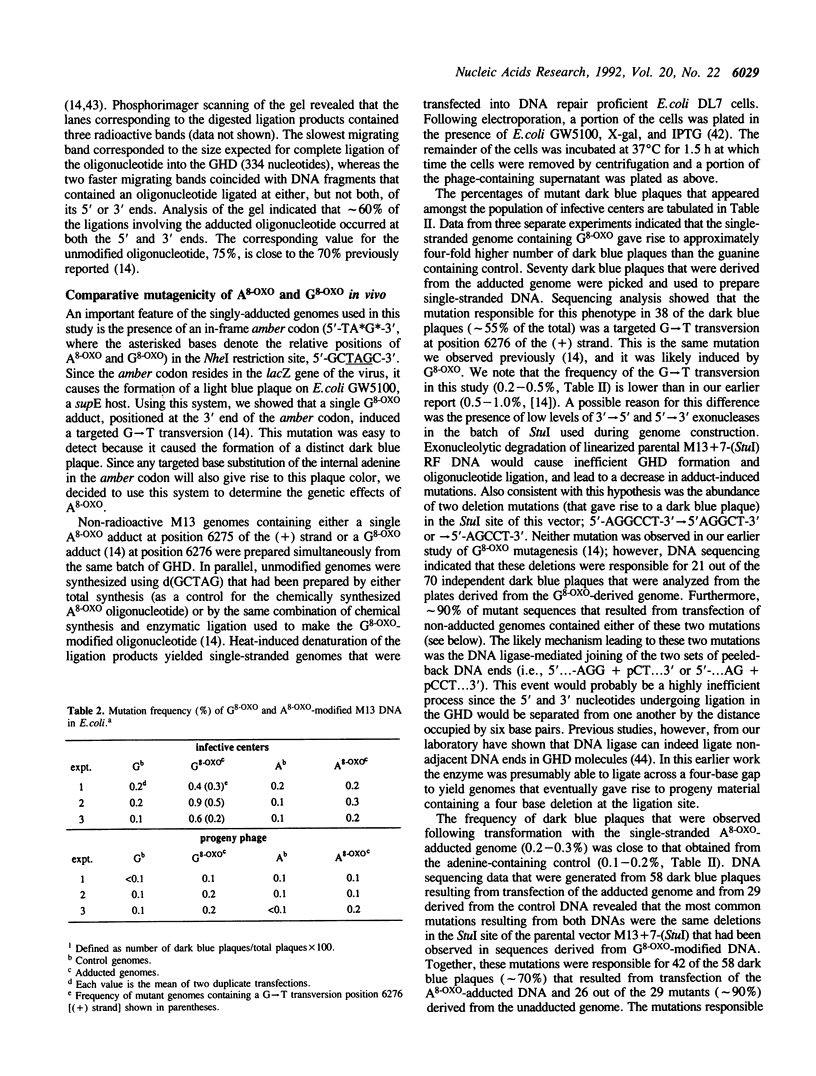
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames B. N. Dietary carcinogens and anticarcinogens. Oxygen radicals and degenerative diseases. Science. 1983 Sep 23;221(4617):1256–1264. doi: 10.1126/science.6351251. [DOI] [PubMed] [Google Scholar]
- Ames B. N. Endogenous oxidative DNA damage, aging, and cancer. Free Radic Res Commun. 1989;7(3-6):121–128. doi: 10.3109/10715768909087933. [DOI] [PubMed] [Google Scholar]
- Breimer L. H. Molecular mechanisms of oxygen radical carcinogenesis and mutagenesis: the role of DNA base damage. Mol Carcinog. 1990;3(4):188–197. doi: 10.1002/mc.2940030405. [DOI] [PubMed] [Google Scholar]
- Cheng K. C., Cahill D. S., Kasai H., Nishimura S., Loeb L. A. 8-Hydroxyguanine, an abundant form of oxidative DNA damage, causes G----T and A----C substitutions. J Biol Chem. 1992 Jan 5;267(1):166–172. [PubMed] [Google Scholar]
- Cho B. P., Evans F. E. Structure of oxidatively damaged nucleic acid adducts. 3. Tautomerism, ionization and protonation of 8-hydroxyadenosine studied by 15N NMR spectroscopy. Nucleic Acids Res. 1991 Mar 11;19(5):1041–1047. doi: 10.1093/nar/19.5.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho B. P., Kadlubar F. F., Culp S. J., Evans F. E. 15N nuclear magnetic resonance studies on the tautomerism of 8-hydroxy-2'-deoxyguanosine, 8-hydroxyguanosine, and other C8-substituted guanine nucleosides. Chem Res Toxicol. 1990 Sep-Oct;3(5):445–452. doi: 10.1021/tx00017a010. [DOI] [PubMed] [Google Scholar]
- Chung M. H., Kasai H., Jones D. S., Inoue H., Ishikawa H., Ohtsuka E., Nishimura S. An endonuclease activity of Escherichia coli that specifically removes 8-hydroxyguanine residues from DNA. Mutat Res. 1991 Jan;254(1):1–12. doi: 10.1016/0921-8777(91)90035-n. [DOI] [PubMed] [Google Scholar]
- Cross C. E., Halliwell B., Borish E. T., Pryor W. A., Ames B. N., Saul R. L., McCord J. M., Harman D. Oxygen radicals and human disease. Ann Intern Med. 1987 Oct;107(4):526–545. doi: 10.7326/0003-4819-107-4-526. [DOI] [PubMed] [Google Scholar]
- Culp S. J., Cho B. P., Kadlubar F. F., Evans F. E. Structural and conformational analyses of 8-hydroxy-2'-deoxyguanosine. Chem Res Toxicol. 1989 Nov-Dec;2(6):416–422. doi: 10.1021/tx00012a010. [DOI] [PubMed] [Google Scholar]
- Dizdaroglu M. Formation of an 8-hydroxyguanine moiety in deoxyribonucleic acid on gamma-irradiation in aqueous solution. Biochemistry. 1985 Jul 30;24(16):4476–4481. doi: 10.1021/bi00337a032. [DOI] [PubMed] [Google Scholar]
- Floyd R. A. The role of 8-hydroxyguanine in carcinogenesis. Carcinogenesis. 1990 Sep;11(9):1447–1450. doi: 10.1093/carcin/11.9.1447. [DOI] [PubMed] [Google Scholar]
- Frei B., Stocker R., Ames B. N. Antioxidant defenses and lipid peroxidation in human blood plasma. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9748–9752. doi: 10.1073/pnas.85.24.9748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuciarelli A. F., Wegher B. J., Gajewski E., Dizdaroglu M., Blakely W. F. Quantitative measurement of radiation-induced base products in DNA using gas chromatography-mass spectrometry. Radiat Res. 1989 Aug;119(2):219–231. [PubMed] [Google Scholar]
- Gajewski E., Rao G., Nackerdien Z., Dizdaroglu M. Modification of DNA bases in mammalian chromatin by radiation-generated free radicals. Biochemistry. 1990 Aug 28;29(34):7876–7882. doi: 10.1021/bi00486a014. [DOI] [PubMed] [Google Scholar]
- Glickman B. W., Rietveld K., Aaron C. S. gamma-Ray induced mutational spectrum in the lacI gene of Escherichia coli: comparison of induced and spontaneous spectra at the molecular level. Mutat Res. 1980 Jan;69(1):1–12. doi: 10.1016/0027-5107(80)90171-2. [DOI] [PubMed] [Google Scholar]
- Grosovsky A. J., de Boer J. G., de Jong P. J., Drobetsky E. A., Glickman B. W. Base substitutions, frameshifts, and small deletions constitute ionizing radiation-induced point mutations in mammalian cells. Proc Natl Acad Sci U S A. 1988 Jan;85(1):185–188. doi: 10.1073/pnas.85.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guschlbauer W., Duplaa A. M., Guy A., Téoule R., Fazakerley G. V. Structure and in vitro replication of DNA templates containing 7,8-dihydro-8-oxoadenine. Nucleic Acids Res. 1991 Apr 25;19(8):1753–1758. doi: 10.1093/nar/19.8.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Role of free radicals and catalytic metal ions in human disease: an overview. Methods Enzymol. 1990;186:1–85. doi: 10.1016/0076-6879(90)86093-b. [DOI] [PubMed] [Google Scholar]
- Ikehara M., Kaneko M. Studies of nucleosides and nucleotides. XLI. Purine cyclonucleosides. 8. Selective sulfonylation of 8-bromoadenosine derivatives and an alternate synthesis of 8,2'-and 8,3'-S-cyclonucleosides. Tetrahedron. 1970 Sep;26(18):4251–4259. doi: 10.1016/s0040-4020(01)93068-6. [DOI] [PubMed] [Google Scholar]
- Kasai H., Tanooka H., Nishimura S. Formation of 8-hydroxyguanine residues in DNA by X-irradiation. Gan. 1984 Dec;75(12):1037–1039. [PubMed] [Google Scholar]
- Kouchakdjian M., Bodepudi V., Shibutani S., Eisenberg M., Johnson F., Grollman A. P., Patel D. J. NMR structural studies of the ionizing radiation adduct 7-hydro-8-oxodeoxyguanosine (8-oxo-7H-dG) opposite deoxyadenosine in a DNA duplex. 8-Oxo-7H-dG(syn).dA(anti) alignment at lesion site. Biochemistry. 1991 Feb 5;30(5):1403–1412. doi: 10.1021/bi00219a034. [DOI] [PubMed] [Google Scholar]
- Kuchino Y., Mori F., Kasai H., Inoue H., Iwai S., Miura K., Ohtsuka E., Nishimura S. Misreading of DNA templates containing 8-hydroxydeoxyguanosine at the modified base and at adjacent residues. Nature. 1987 May 7;327(6117):77–79. doi: 10.1038/327077a0. [DOI] [PubMed] [Google Scholar]
- Lasko D. D., Basu A. K., Kadlubar F. F., Evans F. E., Lay J. O., Jr, Essigmann J. M. A probe for the mutagenic activity of the carcinogen 4-aminobiphenyl: synthesis and characterization of an M13mp10 genome containing the major carcinogen-DNA adduct at a unique site. Biochemistry. 1987 Jun 2;26(11):3072–3081. doi: 10.1021/bi00385a019. [DOI] [PubMed] [Google Scholar]
- Lasko D. D., Harvey S. C., Malaikal S. B., Kadlubar F. F., Essigmann J. M. Specificity of mutagenesis by 4-aminobiphenyl. A possible role for N-(deoxyadenosin-8-yl)-4-aminobiphenyl as a premutational lesion. J Biol Chem. 1988 Oct 25;263(30):15429–15435. [PubMed] [Google Scholar]
- Loechler E. L., Green C. L., Essigmann J. M. In vivo mutagenesis by O6-methylguanine built into a unique site in a viral genome. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6271–6275. doi: 10.1073/pnas.81.20.6271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malins D. C., Haimanot R. 4,6-Diamino-5-formamidopyrimidine, 8-hydroxyguanine and 8-hydroxyadenine in DNA from neoplastic liver of English sole exposed to carcinogens. Biochem Biophys Res Commun. 1990 Dec 14;173(2):614–619. doi: 10.1016/s0006-291x(05)80079-8. [DOI] [PubMed] [Google Scholar]
- McBride T. J., Preston B. D., Loeb L. A. Mutagenic spectrum resulting from DNA damage by oxygen radicals. Biochemistry. 1991 Jan 8;30(1):207–213. doi: 10.1021/bi00215a030. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Michaels M. L., Pham L., Cruz C., Miller J. H. MutM, a protein that prevents G.C----T.A transversions, is formamidopyrimidine-DNA glycosylase. Nucleic Acids Res. 1991 Jul 11;19(13):3629–3632. doi: 10.1093/nar/19.13.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles C., Meuth M. DNA sequence determination of gamma-radiation-induced mutations of the hamster aprt locus. Mutat Res. 1989 Oct;227(2):97–102. doi: 10.1016/0165-7992(89)90004-3. [DOI] [PubMed] [Google Scholar]
- Moriya M., Ou C., Bodepudi V., Johnson F., Takeshita M., Grollman A. P. Site-specific mutagenesis using a gapped duplex vector: a study of translesion synthesis past 8-oxodeoxyguanosine in E. coli. Mutat Res. 1991 May;254(3):281–288. doi: 10.1016/0921-8777(91)90067-y. [DOI] [PubMed] [Google Scholar]
- Naser L. J., Pinto A. L., Lippard S. J., Essigmann J. M. Chemical and biological studies of the major DNA adduct of cis-diamminedichloroplatinum(II), cis-[Pt(NH3)2(d(GpG]], built into a specific site in a viral genome. Biochemistry. 1988 Jun 14;27(12):4357–4367. doi: 10.1021/bi00412a024. [DOI] [PubMed] [Google Scholar]
- Oda Y., Uesugi S., Ikehara M., Nishimura S., Kawase Y., Ishikawa H., Inoue H., Ohtsuka E. NMR studies of a DNA containing 8-hydroxydeoxyguanosine. Nucleic Acids Res. 1991 Apr 11;19(7):1407–1412. doi: 10.1093/nar/19.7.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibutani S., Takeshita M., Grollman A. P. Insertion of specific bases during DNA synthesis past the oxidation-damaged base 8-oxodG. Nature. 1991 Jan 31;349(6308):431–434. doi: 10.1038/349431a0. [DOI] [PubMed] [Google Scholar]
- Stillwell W. G., Xu H. X., Adkins J. A., Wishnok J. S., Tannenbaum S. R. Analysis of methylated and oxidized purines in urine by capillary gas chromatography-mass spectrometry. Chem Res Toxicol. 1989 Mar-Apr;2(2):94–99. doi: 10.1021/tx00008a004. [DOI] [PubMed] [Google Scholar]
- Tchou J., Kasai H., Shibutani S., Chung M. H., Laval J., Grollman A. P., Nishimura S. 8-oxoguanine (8-hydroxyguanine) DNA glycosylase and its substrate specificity. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4690–4694. doi: 10.1073/pnas.88.11.4690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teebor G. W., Boorstein R. J., Cadet J. The repairability of oxidative free radical mediated damage to DNA: a review. Int J Radiat Biol. 1988 Aug;54(2):131–150. doi: 10.1080/09553008814551591. [DOI] [PubMed] [Google Scholar]
- Téoule R. Radiation-induced DNA damage and its repair. Int J Radiat Biol Relat Stud Phys Chem Med. 1987 Apr;51(4):573–589. doi: 10.1080/09553008414552111. [DOI] [PubMed] [Google Scholar]
- Weinberg R. A. Oncogenes, antioncogenes, and the molecular bases of multistep carcinogenesis. Cancer Res. 1989 Jul 15;49(14):3713–3721. [PubMed] [Google Scholar]
- Wood M. L., Dizdaroglu M., Gajewski E., Essigmann J. M. Mechanistic studies of ionizing radiation and oxidative mutagenesis: genetic effects of a single 8-hydroxyguanine (7-hydro-8-oxoguanine) residue inserted at a unique site in a viral genome. Biochemistry. 1990 Jul 31;29(30):7024–7032. doi: 10.1021/bi00482a011. [DOI] [PubMed] [Google Scholar]




