Abstract
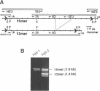
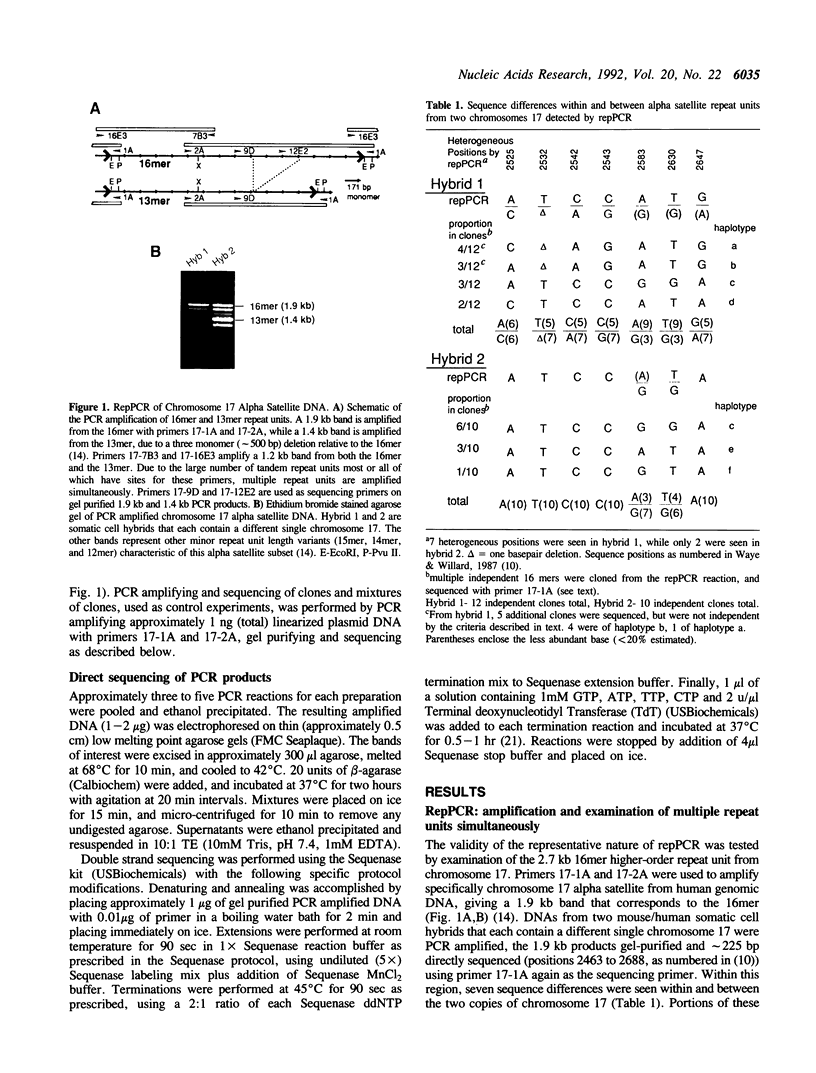
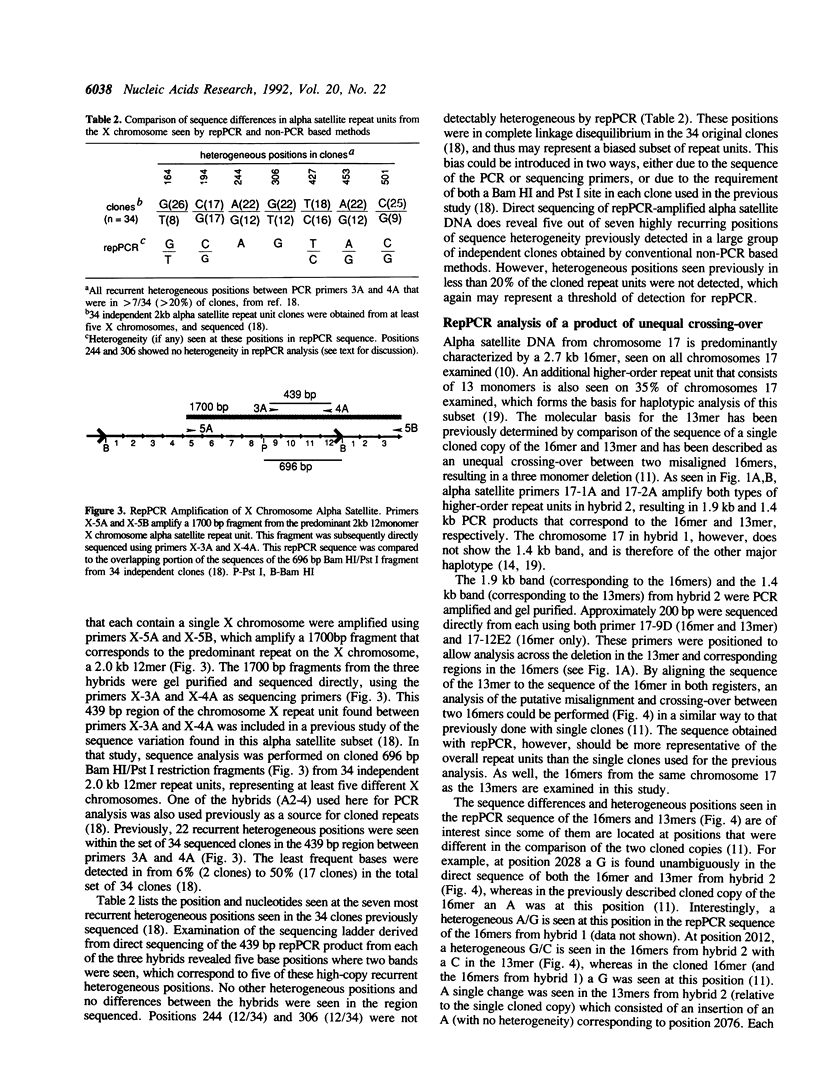
Tandemly repeated DNA can comprise several percent of total genomic DNA in complex organisms and, in some instances, may play a role in chromosome structure or function. Alpha satellite DNA is the major family of tandemly repeated DNA found at the centromeres of all human and primate chromosomes. Each centromere is characterized by a large contiguous array of up to several thousand kb which can contain several thousand highly homogeneous repeat units. By using a novel application of the polymerase chain reaction (repPCR), we are able to amplify a representative sampling of multiple repetitive units simultaneously, allowing rapid analysis of chromosomal subsets. Direct sequence analysis of repPCR amplified alpha satellite from chromosomes 17 and X reveals positions of sequence heterogeneity as two bands at a single nucleotide position on a sequencing ladder. The use of TdT in the sequencing reactions greatly reduces the background associated with polymerase pauses and stops, allowing visualization of heterogeneous bases found in as little as 10% of the repeat units. Confirmation of these heterogeneous positions was obtained by comparison to the sequence of multiple individual cloned copies obtained both by PCR and non-PCR based methods. PCR amplification of alpha satellite can also reveal multiple repeat units which differ in size. Analysis of repPCR products from chromosome 17 and X allows rapid determination of the molecular basis of these repeat unit length variants, which appear to be a result of unequal crossing-over. The application of repPCR to the study of tandemly repeated DNA should allow in-depth analysis of intra- and interchromosomal variation and unequal crossing-over, thus providing insight into the biology and genetics of these large families of DNA.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bevan I. S., Rapley R., Walker M. R. Sequencing of PCR-amplified DNA. PCR Methods Appl. 1992 May;1(4):222–228. doi: 10.1101/gr.1.4.222. [DOI] [PubMed] [Google Scholar]
- Brutlag D. L. Molecular arrangement and evolution of heterochromatic DNA. Annu Rev Genet. 1980;14:121–144. doi: 10.1146/annurev.ge.14.120180.001005. [DOI] [PubMed] [Google Scholar]
- Choo K. H., Brown R., Webb G., Craig I. W., Filby R. G. Genomic organization of human centromeric alpha satellite DNA: characterization of a chromosome 17 alpha satellite sequence. DNA. 1987 Aug;6(4):297–305. doi: 10.1089/dna.1987.6.297. [DOI] [PubMed] [Google Scholar]
- Dunham I., Lengauer C., Cremer T., Featherstone T. Rapid generation of chromosome-specific alphoid DNA probes using the polymerase chain reaction. Hum Genet. 1992 Feb;88(4):457–462. doi: 10.1007/BF00215682. [DOI] [PubMed] [Google Scholar]
- Durfy S. J., Willard H. F. Patterns of intra- and interarray sequence variation in alpha satellite from the human X chromosome: evidence for short-range homogenization of tandemly repeated DNA sequences. Genomics. 1989 Nov;5(4):810–821. doi: 10.1016/0888-7543(89)90123-7. [DOI] [PubMed] [Google Scholar]
- Engelke D. R., Hoener P. A., Collins F. S. Direct sequencing of enzymatically amplified human genomic DNA. Proc Natl Acad Sci U S A. 1988 Jan;85(2):544–548. doi: 10.1073/pnas.85.2.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett T. W., Bartlett G. An effective method for eliminating "artifact banding" when sequencing double-stranded DNA templates. Biotechniques. 1990 Jul;9(1):46–48. [PubMed] [Google Scholar]
- Haaf T., Warburton P. E., Willard H. F. Integration of human alpha-satellite DNA into simian chromosomes: centromere protein binding and disruption of normal chromosome segregation. Cell. 1992 Aug 21;70(4):681–696. doi: 10.1016/0092-8674(92)90436-g. [DOI] [PubMed] [Google Scholar]
- Jørgensen A. L., Bostock C. J., Bak A. L. Chromosome-specific subfamilies within human alphoid repetitive DNA. J Mol Biol. 1986 Jan 20;187(2):185–196. doi: 10.1016/0022-2836(86)90227-5. [DOI] [PubMed] [Google Scholar]
- Lafrenière R. G., Brown C. J., Powers V. E., Carrel L., Davies K. E., Barker D. F., Willard H. F. Physical mapping of 60 DNA markers in the p21.1----q21.3 region of the human X chromosome. Genomics. 1991 Oct;11(2):352–363. doi: 10.1016/0888-7543(91)90143-3. [DOI] [PubMed] [Google Scholar]
- Mahtani M. M., Willard H. F. Pulsed-field gel analysis of alpha-satellite DNA at the human X chromosome centromere: high-frequency polymorphisms and array size estimate. Genomics. 1990 Aug;7(4):607–613. doi: 10.1016/0888-7543(90)90206-a. [DOI] [PubMed] [Google Scholar]
- Masumoto H., Masukata H., Muro Y., Nozaki N., Okazaki T. A human centromere antigen (CENP-B) interacts with a short specific sequence in alphoid DNA, a human centromeric satellite. J Cell Biol. 1989 Nov;109(5):1963–1973. doi: 10.1083/jcb.109.5.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Smith G. P. Evolution of repeated DNA sequences by unequal crossover. Science. 1976 Feb 13;191(4227):528–535. doi: 10.1126/science.1251186. [DOI] [PubMed] [Google Scholar]
- Tyler-Smith C., Brown W. R. Structure of the major block of alphoid satellite DNA on the human Y chromosome. J Mol Biol. 1987 Jun 5;195(3):457–470. doi: 10.1016/0022-2836(87)90175-6. [DOI] [PubMed] [Google Scholar]
- Warburton P. E., Greig G. M., Haaf T., Willard H. F. PCR amplification of chromosome-specific alpha satellite DNA: definition of centromeric STS markers and polymorphic analysis. Genomics. 1991 Oct;11(2):324–333. doi: 10.1016/0888-7543(91)90139-6. [DOI] [PubMed] [Google Scholar]
- Warburton P. E., Willard H. F. Genomic analysis of sequence variation in tandemly repeated DNA. Evidence for localized homogeneous sequence domains within arrays of alpha-satellite DNA. J Mol Biol. 1990 Nov 5;216(1):3–16. doi: 10.1016/s0022-2836(05)80056-7. [DOI] [PubMed] [Google Scholar]
- Waye J. S., Willard H. F. Chromosome-specific alpha satellite DNA: nucleotide sequence analysis of the 2.0 kilobasepair repeat from the human X chromosome. Nucleic Acids Res. 1985 Apr 25;13(8):2731–2743. doi: 10.1093/nar/13.8.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waye J. S., Willard H. F. Molecular analysis of a deletion polymorphism in alpha satellite of human chromosome 17: evidence for homologous unequal crossing-over and subsequent fixation. Nucleic Acids Res. 1986 Sep 11;14(17):6915–6927. doi: 10.1093/nar/14.17.6915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waye J. S., Willard H. F. Structure, organization, and sequence of alpha satellite DNA from human chromosome 17: evidence for evolution by unequal crossing-over and an ancestral pentamer repeat shared with the human X chromosome. Mol Cell Biol. 1986 Sep;6(9):3156–3165. doi: 10.1128/mcb.6.9.3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willard H. F. Centromeres of mammalian chromosomes. Trends Genet. 1990 Dec;6(12):410–416. doi: 10.1016/0168-9525(90)90302-m. [DOI] [PubMed] [Google Scholar]
- Willard H. F. Chromosome-specific organization of human alpha satellite DNA. Am J Hum Genet. 1985 May;37(3):524–532. [PMC free article] [PubMed] [Google Scholar]
- Willard H. F. Evolution of alpha satellite. Curr Opin Genet Dev. 1991 Dec;1(4):509–514. doi: 10.1016/s0959-437x(05)80200-x. [DOI] [PubMed] [Google Scholar]
- Willard H. F., Greig G. M., Powers V. E., Waye J. S. Molecular organization and haplotype analysis of centromeric DNA from human chromosome 17: implications for linkage in neurofibromatosis. Genomics. 1987 Dec;1(4):368–373. doi: 10.1016/0888-7543(87)90041-3. [DOI] [PubMed] [Google Scholar]
- Willard H. F., Smith K. D., Sutherland J. Isolation and characterization of a major tandem repeat family from the human X chromosome. Nucleic Acids Res. 1983 Apr 11;11(7):2017–2033. doi: 10.1093/nar/11.7.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willard H. F., Waye J. S., Skolnick M. H., Schwartz C. E., Powers V. E., England S. B. Detection of restriction fragment length polymorphisms at the centromeres of human chromosomes by using chromosome-specific alpha satellite DNA probes: implications for development of centromere-based genetic linkage maps. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5611–5615. doi: 10.1073/pnas.83.15.5611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt M., Erickson R. P. A rapid method for detection of Y-chromosomal DNA from dried blood specimens by the polymerase chain reaction. Hum Genet. 1989 Jun;82(3):271–274. doi: 10.1007/BF00291168. [DOI] [PubMed] [Google Scholar]
- Yang T. P., Hansen S. K., Oishi K. K., Ryder O. A., Hamkalo B. A. Characterization of a cloned repetitive DNA sequence concentrated on the human X chromosome. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6593–6597. doi: 10.1073/pnas.79.21.6593. [DOI] [PMC free article] [PubMed] [Google Scholar]