Abstract
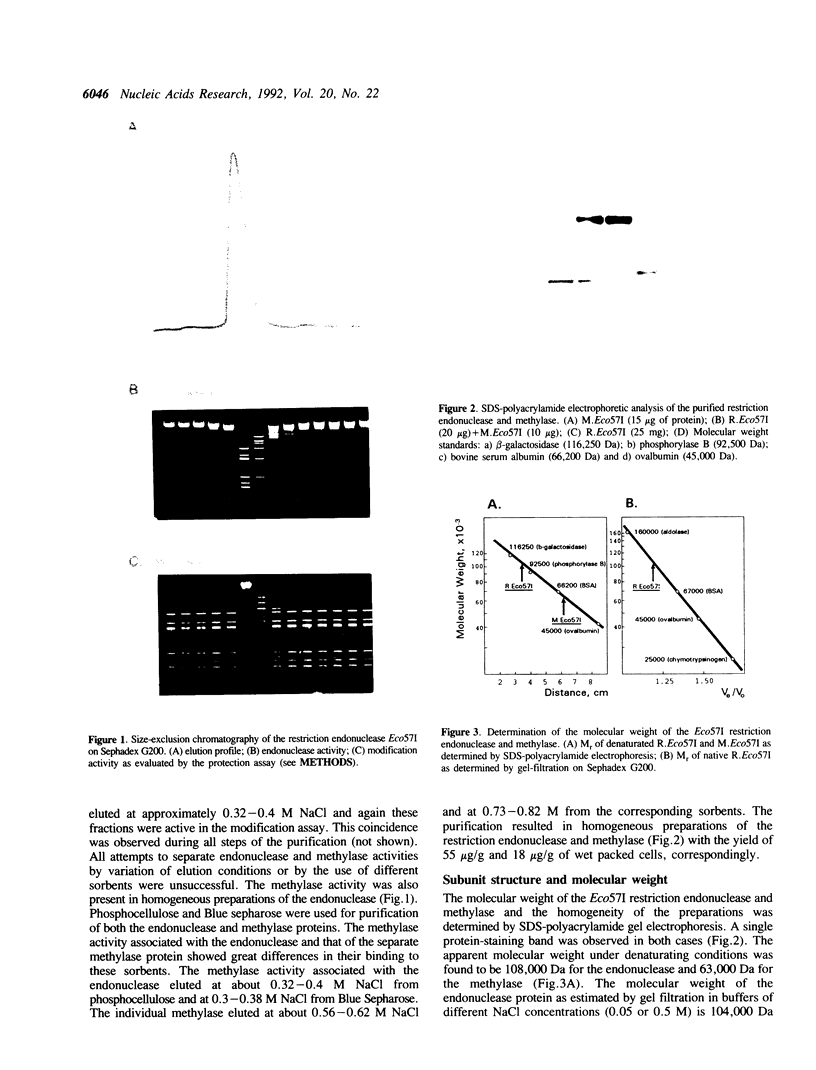
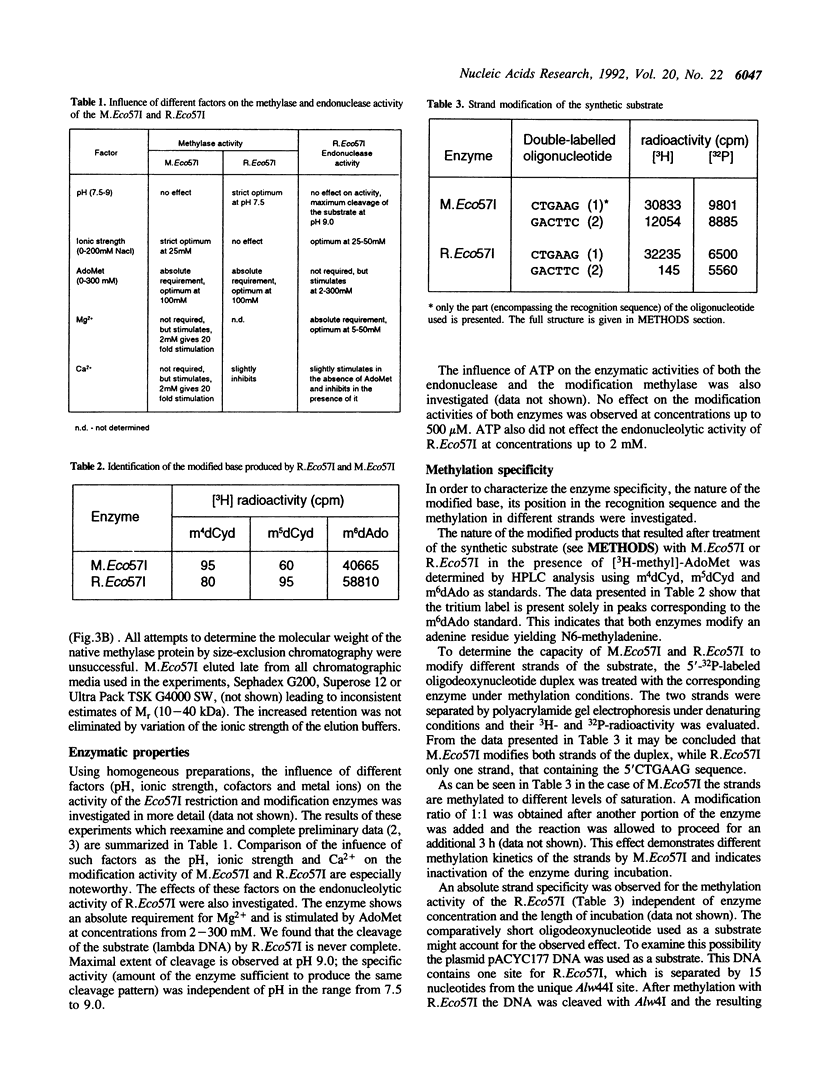
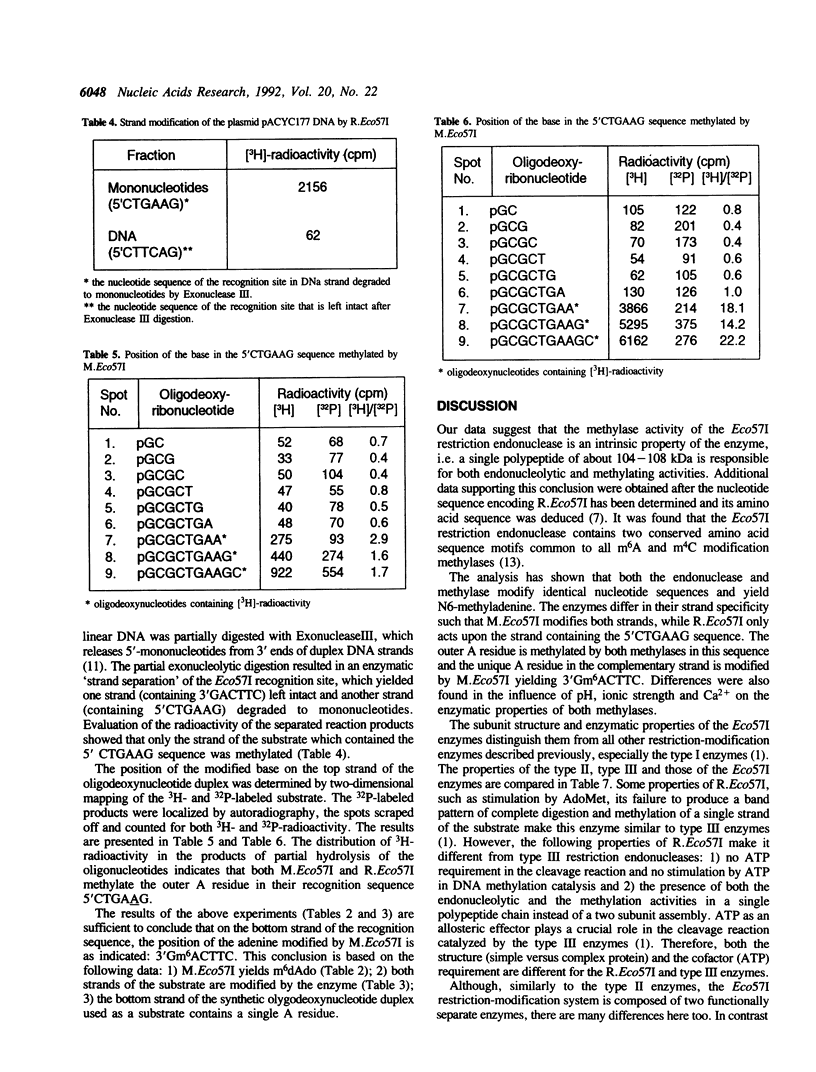
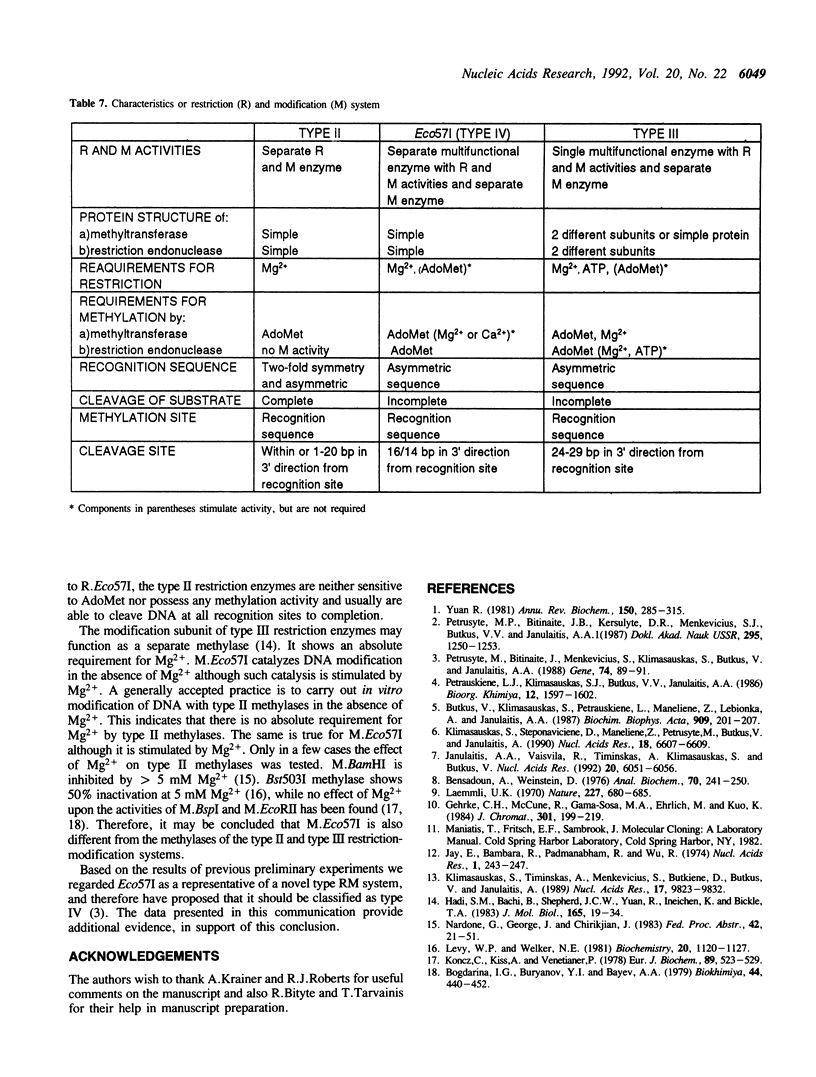
The Eco57I restriction endonuclease and methylase were purified to homogeneity from the E.coli RR1 strain carrying the eco57IRM genes on a recombinant plasmid. The molecular weight of the denaturated methylase is 63 kDa. The restriction endonuclease exists in a monomeric form with an apparent molecular weight of 104-108 kDa. R.Eco57I also possesses methylase activity. The methylation activities of both enzymes modify the outer A residue in the target sequence 5'CTGAAG yielding N6-methyladenine. M.Eco57I modifies both strands of the substrate while R.Eco57I modifies only one. Only the methylase enzyme is stimulated by Ca2+. The restriction endonuclease shows an absolute requirement for Mg2+ and is stimulated by AdoMet. ATP has no influence on either activity of the enzymes. The subunit structure and enzymatic properties of the Eco57I enzymes distinguish them from all other restriction-modification enzymes that have been described previously. Therefore, RM.Eco57I may be regarded as a representative of a novel class of restriction-modification systems, and we propose to classify it as type IV.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bensadoun A., Weinstein D. Assay of proteins in the presence of interfering materials. Anal Biochem. 1976 Jan;70(1):241–250. doi: 10.1016/s0003-2697(76)80064-4. [DOI] [PubMed] [Google Scholar]
- Bogdarina I. G., Bur'ianov Ia I., Baev A. A. Vydelenie i svoistva DNK-tsitozin-metiltransferaz EcoRII i E. coli K12. Biokhimiia. 1979 Mar;44(3):440–452. [PubMed] [Google Scholar]
- Butkus V., Klimasauskas S., Petrauskiene L., Maneliene Z., Lebionka A., Janulaitis A. Interaction of AluI, Cfr6I and PvuII restriction-modification enzymes with substrates containing either N4-methylcytosine or 5-methylcytosine. Biochim Biophys Acta. 1987 Aug 25;909(3):201–207. doi: 10.1016/0167-4781(87)90078-9. [DOI] [PubMed] [Google Scholar]
- Gehrke C. W., McCune R. A., Gama-Sosa M. A., Ehrlich M., Kuo K. C. Quantitative reversed-phase high-performance liquid chromatography of major and modified nucleosides in DNA. J Chromatogr. 1984 Sep 28;301(1):199–219. doi: 10.1016/s0021-9673(01)89189-5. [DOI] [PubMed] [Google Scholar]
- Hadi S. M., Bächi B., Iida S., Bickle T. A. DNA restriction--modification enzymes of phage P1 and plasmid p15B. Subunit functions and structural homologies. J Mol Biol. 1983 Mar 25;165(1):19–34. doi: 10.1016/s0022-2836(83)80240-x. [DOI] [PubMed] [Google Scholar]
- Janulaitis A., Vaisvila R., Timinskas A., Klimasauskas S., Butkus V. Cloning and sequence analysis of the genes coding for Eco57I type IV restriction-modification enzymes. Nucleic Acids Res. 1992 Nov 25;20(22):6051–6056. doi: 10.1093/nar/20.22.6051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimasauskas S., Steponaviciene D., Maneliene Z., Petrusyte M., Butkus V., Janulaitis A. M.Smal is an N4-methylcytosine specific DNA-methylase. Nucleic Acids Res. 1990 Nov 25;18(22):6607–6609. doi: 10.1093/nar/18.22.6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimasauskas S., Timinskas A., Menkevicius S., Butkienè D., Butkus V., Janulaitis A. Sequence motifs characteristic of DNA[cytosine-N4]methyltransferases: similarity to adenine and cytosine-C5 DNA-methylases. Nucleic Acids Res. 1989 Dec 11;17(23):9823–9832. doi: 10.1093/nar/17.23.9823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koncz C., Kiss A., Venetianer P. Biochemical characterization of the restriction-modification system of Bacillus sphaericus. Eur J Biochem. 1978 Sep 1;89(2):523–529. doi: 10.1111/j.1432-1033.1978.tb12557.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levy W. P., Welker N. E. Deoxyribonucleic acid modification methylase from Bacillus stearothermophilus. Biochemistry. 1981 Mar 3;20(5):1120–1127. doi: 10.1021/bi00508a012. [DOI] [PubMed] [Google Scholar]
- Petrusyte M., Bitinaite J., Menkevicius S., Klimasauskas S., Butkus V., Janulaitis A. Restriction endonucleases of a new type. Gene. 1988 Dec 25;74(1):89–91. doi: 10.1016/0378-1119(88)90259-4. [DOI] [PubMed] [Google Scholar]
- Piatrushite M. P., Bitinaite Iu B., Kershulite D. R., Menkiavichius S. Iu, Butkus V. V. Restriktsionnye éndonukleazy novogo tipa. Dokl Akad Nauk SSSR. 1987;295(5):1250–1253. [PubMed] [Google Scholar]
- Stull J. T., Silver P. J., Miller J. R., Blumenthal D. K., Botterman B. R., Klug G. A. Phosphorylation of myosin light chain in skeletal and smooth muscles. Fed Proc. 1983 Jan;42(1):21–26. [PubMed] [Google Scholar]
- Yuan R. Structure and mechanism of multifunctional restriction endonucleases. Annu Rev Biochem. 1981;50:285–319. doi: 10.1146/annurev.bi.50.070181.001441. [DOI] [PubMed] [Google Scholar]





