Abstract
Hepatitis C virus (HCV) infection is a leading cause of chronic liver diseases worldwide, but treatment options are limited. Basic HCV research required for vaccine and drug development has been hampered by inability to culture patient isolates, and to date only the JFH1 (genotype 2a) recombinant replicates spontaneously in hepatoma cells and releases infectious virus. A JFH1 chimera with the 5′ end through NS2 from another genotype 2a strain, J6, had enhanced infectivity. However, the full-length J6 clone (J6CF), which we previously found to be fully functional in vivo, was replication incompetent in vitro. Through a systematic approach of culturing J6 with minimal JFH1 sequences, we identified three mutations in NS3, NS4A, and NS5B that permitted full-length J6 propagation and adaptation with infectivity titers comparable to JFH1-based systems. The most efficient recombinant, J6cc, had six adaptive mutations and did not accumulate additional changes following viral passage. We demonstrated that HCV NS3/NS4A protease-, NS5A- and NS5B polymerase-directed drugs respectively inhibited full-length J6 infection dose dependently. Importantly, the three J6-derived mutations enabled culture adaptation of the genetically divergent isolate J8 (genotype 2b), which differed from the J6 nucleotide sequence by 24%. The most efficient recombinant, J8cc, had nine adaptive mutations and was genetically stable after viral passage. The availability of these robust JFH1-independent genotype 2a and 2b culture systems represents an important advance, and the approach used might permit culture development of other isolates, with implications for improved individualized treatments of HCV patients and for development of broadly efficient vaccines.
Keywords: RNA, neutralization, inhibitor, interferon, antiviral
Hepatitis C virus (HCV) infection is a major cause of chronic hepatitis, liver cirrhosis, and hepatocellular carcinoma. The outcome of infection is associated with genetic variability of HCV and host factors (1). No vaccine is available, and current IFN-based treatment is suboptimal, with many side effects, with low efficacy against the most prevalent HCV variants (2–4), and with differential influence from host factors (5). Directly acting antivirals (DAA) might improve treatment outcome but also have differential efficacy in treatment of patients with different HCV genotypes (6). The HCV positive sense single-strand RNA genome (∼9.6 kb) contains a single ORF flanked by 5′ and 3′ untranslated regions (UTRs). The ORF encodes virus structural proteins (Core, E1, and E2), p7, and six nonstructural (NS) proteins (7). HCV isolates are classified into seven major genotypes and numerous subtypes differing by 31–33% and 20–25%, respectively (8).
The high heterogeneity of HCV and the lack of representative culture systems have hampered HCV vaccine development, preclinical drug testing, assessment of neutralizing antibodies, and basic HCV research. Although a number of HCV full-length genomes were shown to be infectious in chimpanzees (9–15), to date only the JFH1 strain (genotype 2a) could replicate autonomously in Huh7 human hepatoma cells (16, 17); efficient growth depended on adaptive mutations (18–21). The low probability of isolating a replication-competent HCV genome demands alternative approaches to develop culture systems for HCV isolates. Using the unique replication capacity of JFH1, inter- and intragenotypic recombinants including Core-NS2 (22–29), 5′ UTR-NS2 (30), NS3 protease/NS4A (31, 32), and NS5A (33) of various genotypes have been developed. Besides permitting functional studies of specific regions in a genotype-specific manner, these culture systems have been used for testing HCV inhibitors (31–33), assessment of neutralizing antibodies (23, 24, 27, 34), host microRNA-122 silencing (30), animal model development (35), and HCV entry receptor discovery (36). The JFH1 recombinants with Core-NS2 (25) or 5′ UTR-NS2 (30) from another in vivo infectious genotype 2a clone, J6CF (14), did not require adaptation for efficient growth (22, 30). Both J6 (37) and JFH1 (38) were isolated from Japanese hepatitis C patients. Studies on recombinants containing various JFH1 (17) and J6CF (14) elements demonstrated that the JFH1 NS3 helicase, NS5B polymerase, and 3′ UTR (39), as well as specific amino acids, nucleotides, and structural features in NS5B and the 3′ UTR (40), are important for the replication capacity of JFH1 in Huh7 cells. Substitutions with JFH1-specific NS5B residues and a nucleotide in the 3′ UTR enhanced the J6CF NS5B RNA polymerase activity and the replication of J6CF replicons with JFH1 elements, respectively (40).
Among other culture systems reported (41–45), only H77-S (genotype 1a) carrying mutations in NS3, NS4A, and NS5A, identified in the H77 replicon system, was found to enable studies of the HCV life cycle (44), and its infectivity was improved by an E2 mutation (46). Nevertheless, the extensive heterogeneity of HCV and genotype specificities in virus production (33, 34) and in response to antivirals (7, 31–33) and neutralizing antibodies (23, 24, 27, 34) argue for a critical need for developing full-length culture systems for other HCV isolates. Through a systematic approach, we identified mutations that enabled replication of in vitro-deficient genomes and that permitted establishment of efficient J6 culture systems independent of JFH1 elements. We demonstrated the cross-genotype utility of such mutations by adaptation of a genetically divergent HCV isolate, J8 (genotype 2b). The approach and the identified mutations possibly could be applied to promote the development of full-length culture systems for other HCV patient isolates.
Results
In Vitro Viability of J6 NS3 Helicase, Partial NS5B, and 3′ UTR Demonstrated by Analyses of J6 Recombinants with Minimal JFH1 Sequences and Identification of Virus Production-Enhancing Mutations.
The NS3 helicase, NS5B, and 3′ UTR previously were found to be critical for the unique replication capacity of JFH1 (39), and these genome regions were all included in previously developed JFH1-based HCV genotype recombinants (22–33, 39). Here we demonstrated that the J6 NS3 helicase, NS5B finger, palm, and partial thumb domains and 3′ UTR were functional for virus production in Huh7.5 cells, and we identified virus production-enhancing mutations F1468L (NS3 helicase) and A1676S (NS4A). Moreover, we demonstrated that a short C-terminal region in JFH1 NS5B contained elements critical for virus production.
J6 NS5B finger-to-partial thumb domain (NS5B amino acids 1–464) was functional for virus production.
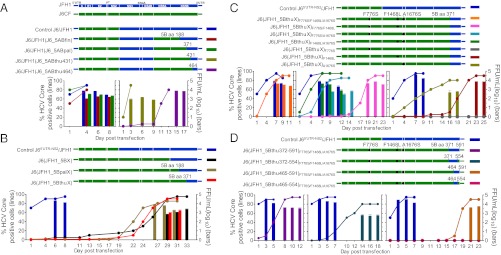
JFH1 with Core-NS2 and NS5A from J6CF (J6/JFH1_J6NS5A) replicated efficiently in Huh7.5 cells (33). Here J6/JFH1_J6NS5A recombinants in which we replaced the JFH1 NS5B finger domain (amino acids 1–188), finger-palm domains (amino acids 1–371), or finger-palm-partial thumb domains (amino acids 1–431 or 1–464) (47) with J6 sequences were found viable in transfected Huh7.5 cells (Fig. 1A and Table S1), indicating that wild-type J6 NS5B (amino acids 1–464) was functional for virus production.
Fig. 1.
Analysis of the in vitro viability of J6 recombinants with minimal JFH1 sequences. (A) J6/JFH1 with J6 NS5A-to-NS5B thumb domain was viable in Huh7.5 cells. (Upper) JFH1 (17), J6CF (14), and J6/JFH1 (25) recombinants. The NS5B finger (amino acids 1–188), finger-palm (amino acids 1–371), and finger-thumb (amino acids 1–431 and amino acids 1–464) domains of J6/JFH1 were replaced with J6 sequences. (Lower) RNA transcripts of J6/JFH1-based recombinants were transfected into Huh7.5 cells, HCV core antigen was detected by immunostaining, and the percentage of positive cells was estimated (left y-axis). HCV infectivity titers in supernatant at peak infection (≥80% HCV-positive cells) were determined by FFU assay (mean of triplicate infections ± SEM; right y-axis). J6/JFH1 was included for comparison. (B) J6 recombinant with JFH1 thumb domain-to-3′ UTR was viable. Huh7.5 cells were transfected with J6CF recombinants with JFH1 NS5B-to-3′ UTR or partial NS5B-to-3′ UTR. J65′ UTR-NS2/JFH1 (30) was used as a positive control. J6(JFH1_5BpalX) was from a separate experiment. Experimental details are as in A. (C) Mutations F776S (p7), F1468L (NS3 helicase), and A1676S (NS4A), identified by analysis of viruses shown in B and Table 1, enhanced J6(JFH1_5BthuX) production in transfected Huh7.5 cells. J65′ UTR-NS2/JFH1 served as a positive control. Experimental details are as in A. (D) NS5B amino acids 465–591 within the thumb domain was the minimal JFH1 element required for adaptation of the J6CF with mutations F776S/F1468L/A1676S. J6 recombinants with F776S/F1468L/A1676S, in which different regions of the NS5B thumb domain were replaced by JFH1 sequences, were tested in Huh7.5 cells. J65′ UTR-NS2/JFH1 served as a positive control. Experimental details are as in A. J6CFF776S/F1468L/A1676S had no evidence of HCV infection during 41 d posttransfection.
Identification of virus production-enhancing mutations F1468L and A1676S through adaptation of J6 recombinant with JFH1 NS5B thumb domain and 3′ UTR.
Given the results for J6/JFH1 with J6 NS5A-NS5B and the viability of J65′ UTR-NS2/JFH1 (30) and J6/JFH1 with J6 NS3/NS4A protease (31), we tested the viability of J6CF recombinants with the following JFH1 sequences: (i) complete NS5B and 3′ UTR [J6(JFH1_5BX), X indicating inclusion of entire 3′ UTR with 3′ X-region]; (ii) NS5B palm and thumb domains (amino acids 189–591) and 3′ UTR [J6(JFH1_5BpalX)]; and (iii) NS5B thumb domain (amino acids 372–591) and 3′ UTR [J6(JFH1_5BthuX)]. In transfected Huh7.5 cells, these recombinants had delayed spread with peak infection (≥80% of cells infected) at days 29, 27, and 29, respectively, and reached peak infectivity titers of 103.4, 103.9, and 103.3 focus-forming units (FFU)/mL (Fig. 1B). Recovered J6(JFH1_5BthuX) viruses encoded F776S (p7), F1468L (NS3 helicase), and F1676S (NS4A) changes [nucleotide and amino acid positions based on J6CF (14), GenBank accession no. AF177036]; F776S and A1676S also were found in other genomes (Table 1 and Table S1). We next engineered these three mutations singly or in double and triple combinations into J6(JFH1_5BthuX) and tested viability in Huh7.5 cells (Fig. 1C). J6(JFH1_5BthuX) with F1468L/A1676S and F776S/F1468L/A1676S spread to ≥80% of cells within 7 d, reaching peak HCV infectivity titers of 103.6 FFU/mL (Fig. 1C); in first-passage viruses, engineered mutations were maintained (Table S2). Thus, these mutations could promote replication and virus production of J6(JFH1_5BthuX) effectively, showing that the J6 helicase and partial NS5B were functional for virus production in cultured cells.
Table 1.
Sequence analysis of J6 recombinants with JFH1 complete or partial NS5B-to-3′ UTR
| HCV | Experiment (day) | p7 | NS2 | NS2 | NS3 | NS3 | NS4A | NS4A | NS4A | NS4B | NS5A |
| Nucleotide position | |||||||||||
| Recombinant-specific | 2667 | 2835 | 2981 | 4727 | 4742 | 5331 | 5332 | 5366 | 6132 | 7655 | |
| H77 ref. (AF009606) | 2656 | 2824 | 2970 | 4716 | 4731 | 5320 | 5321 | 5355 | 6121 | 7590 | |
| Construct nucleotide | T | T | G | A | T | G | G | G | A | G | |
| J6 recombinant virus | |||||||||||
| J6(JFH1_5BX), exp. 1* | First passage (14) | T/C | A/G | G/C | G/T | A/G | |||||
| J6(JFH1_5BX), exp. 2* | First passage (8) | T/C | G/c | G/t | |||||||
| J6(JFH1_5BpalX) | Transfection (31) | A/g | G/T | G/T | T/g | ||||||
| J6(JFH1_5BthuX)* | First passage (8) | C | T/C | G/T | |||||||
| Amino acid position | |||||||||||
| Recombinant-specific | 776 | 832 | 881 | 1463 | 1468 | 1664 | 1664 | 1676 | 1931 | 2439 | |
| H77 ref. (AF009606) | 772 | 828 | 877 | 1459 | 1464 | 1660 | 1660 | 1672 | 1927 | 2417 | |
| Amino acid change | F–S | L–P | V–I | T–A | F–L | W–S | W–C | A–S | N–S | V–F | |
Transfection-derived J6 recombinants (Fig. 1B) were passaged to naive Huh7.5 cells, and culture supernatant collected at peak infection (≥80% cells infected) was subjected to RNA extraction and RT-PCR for HCV ORF sequence analysis. Nucleotide and amino acid positions of the specific recombinant with coding mutations are listed; the corresponding position of H77 reference sequence (AF009606) is given. Two capital letters separated by a slash indicate a nucleotide quasispecies (50/50); a capital letter separated from a lowercase letter by a slash indicates a dominant/minor sequencing read.
*The sequence of the 5′ UTR of recovered virus was determined by the 5′ RACE procedure using HCV RNA extracted from infection supernatant. The G inserted immediately before the 5′-terminal nucleotide A for enhancing in vitro transcription was deleted, consistent with our previous observations in JFH1-based culture systems (30, 67). No changes were observed in the 5′ UTR.
C-terminal JFH1 NS5B amino acids 465–591 contained elements essential for J6 virus production.
Because J6(JFH1_5BthuX)F776S/F1468L/A1676S spread efficiently in Huh7.5 cells without requiring coding changes (Fig. 1C and Table S2) and J6 NS5B amino acids 1–464 were functional for virus production (Fig. 1A), we aimed at minimizing its JFH1 sequence. We thus replaced the following regions with J6CF sequences: (i) 3′ UTR; (ii) NS5B amino acids 555–591 and 3′ UTR; (iii) NS5B amino acids 372–464 and 3′ UTR; or (iv) NS5B amino acids 372–464, amino acids 555–591, and 3′ UTR, and tested the viability in Huh7.5 cells (Fig. 1D). The last recombinant was nonviable, without HCV-positive cells through 26 d. The other recombinants showed delayed spread with peak HCV infectivity titers of 102.8 to 103.7 FFU/mL. In recovered viruses, large deletions were detected in the 3′ UTR polyU/UC tract (Table S3). Overall, these data showed that the C-terminal JFH1 NS5B amino acids 465–591 contained elements of importance for rescuing J6 replication but that the J6 3′ UTR was functional in vitro.
Development of Full-Length J6 (Genotype 2a) Infectious Culture System.
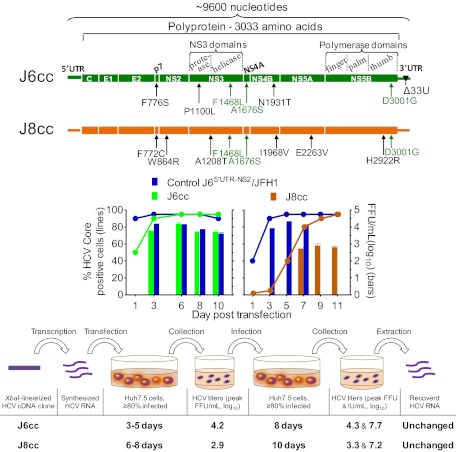
The J6CF genome was found previously to be infectious for chimpanzees (14) but did not replicate in Huh7.5 cells (10). We succeeded in identifying mutations that permitted culture of this important reference strain without JFH1 elements and with HCV infectivity titers comparable to JFH1-based systems. The ORF and polyprotein sequence of JFH1 and J6 differ by 11% and 9%, respectively. We showed that DAA, including NS5B inhibitors, inhibited J6 full-length virus in a dose-dependent manner.
J6 full-length genomes with specific mutations were adapted to growth in Huh7.5 cells.
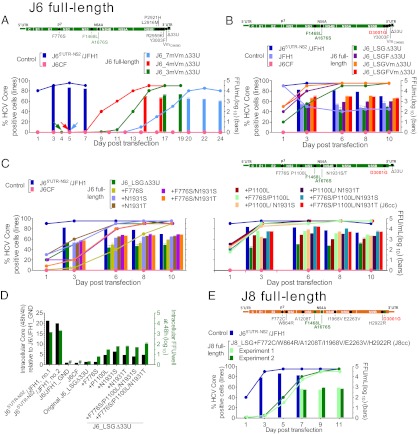
Within NS5B amino acids 465–591 of HCV genotype 1–6 strains, M474, H479, and F561 (corresponding to the J6 polyprotein amino acids L2916, P2921, and Y3003, respectively) were found exclusively in JFH1, whereas L474, P479, and Y561 were found primarily in other isolates. Recently, JFH1 NS5B amino acids K517 (J6 amino acid R2959) and F561, as well as nucleotide G at position 9458 in the 3′ UTR variable region (VR) and the length of the poly(U/UC) tract were demonstrated to be important for HCV RNA replication (40). We demonstrated the importance of F776S/F1468L/A1676S for virus production (Fig. 1C) and recovered shortened polyU/UC tracts from viable J6 recombinants with minimal JFH1 sequences (Table S3). We thus mutated the corresponding J6CF VR nucleotide (Vm, C9458G) and shortened the J6CF polyU/UC tract to match the length in JFH1 (Δ33U). These modifications (VmΔ33U) were in combination with mutations F776S/F1468L/A1676S/L2916M/P2921H/R2959K/Y3003F (designated “7m”), F776S/F1468L/A1676S/Y3003F (“4m”), F1468L/A1676S/Y3003F (“3m”), or Y3003F introduced into J6CF (Fig. 2A). In addition, a J6CF genome with only the four NS5B mutations L2916M/P2921H/R2959K/Y3003F (J6_4m5B) or with Δ33U (J6_Δ33U) was constructed. In transfected Huh7.5 cells, no HCV-positive cells were detected for J6_4m5B, J6_Y3003FVmΔ33U, or J6_Δ33U cultures, but J6_7mVmΔ33U, J6_4mVmΔ33U, and J6_3mVmΔ33U cultures became HCV positive and spread to ≥80% of cells at day 20, 13, and 15, respectively (Fig. 2A). These infections produced HCV peak infectivity titers of 103.3, 103.5, and 103.6 FFU/mL, respectively. In recovered viruses, the engineered mutations were maintained. Interestingly, D3001G (J6 NS5B amino acid D559G) was the only additional mutation in J6_7mVmΔ33U and J6_3mVmΔ33U (Table 2). Thus, the combination of F1468L/A1676S/Y3003F with a modified 3′ UTR (Vm C9458G and Δ33U) could initiate the replication of the J6 full-length genome in Huh7.5 cells and promote adaptation by long-term culture.
Fig. 2.
Development of J6 and J8 full-length culture systems. RNA transcripts of HCV genomes with indicated mutations (genome illustrations in upper panels) were transfected into Huh7.5 cells (graphs in lower panels), and the estimated percentage of HCV-positive cells (left y-axis) and peak HCV infectivity titers (right y-axis) were determined. J65′ UTR-NS2/JFH1 (30) served as a positive control. (A) Long-term culture adaptation of J6CF (14) with mutations. Arrows indicate the day HCV-positive cells emerged. 7m, F776S/F1468L/A1676S/L2916M/P2921H/R2959K/Y3003F; 4m, F776S/F1468L/A1676S/Y3003F; 3m, F1468L/A1676S/Y3003F; Vm, C9458G (40); and Δ33U, 33 U deletion in the polyU/UC tract. Data are from three different experiments with a representative J65′ UTR-NS2/JFH1 shown. In addition, J6_Y3003FVmΔ33U and J6_Δ33U remained HCV negative through 40 and 33 d, respectively. (B) Combination of F1468L/A1676S/D3001G (LSG) and Δ33U enabled full-length J6 to produce infectious particles efficiently. Mutations F1468L (NS3 helicase) and A1676S (NS4A) were identified by analysis of viruses shown in Fig. 1B and Table 1 (green type in A, B, C, and E), and D3001G (NS5B) was identified by analysis of viruses shown in A and Table 2 (red type in B, C, and E). “F” indicates Y3003F. Wild-type JFH1 (17) and J6CF were included for comparison. (C) Mutations F776S, P1100L, and N1931S/T, identified by analysis of J6_LSGΔ33U viruses shown in B and Table 3, adapted J6_LSGΔ33U reaching HCV infectivity titers >104 FFU/mL. *, titer <1.7 log10 FFU/mL. (D) Mutations adapting J6 enhanced replication and assembly of intracellular infectious HCV particles. RNA transcripts were transfected into HCV entry-deficient S29 cells (18). The HCV core level at 48 h (relative to 4 h) was normalized to that of replication-negative J6/JFH1_GND (arbitrary value as 1). #, No FFU detected. (E) Efficient full-length J8 culture system based on F1468L/A1676S/D3001G mutations and further adaptation. Transfection of J8_LSG with additional mutations F772C/W864R/A1208T/I1968V/E2263V/H2922R, identified by analysis of J8_LSG-derived viruses (Table 4 and Table S4), yielded rapid spread and significant infectivity titers. Infectivity titers increased after passage to naive Huh7.5 cells, and recovered viruses did not have other mutations (Table 4).
Table 2.
Sequence analysis of J6 full-length genome with mutations recovered from long-term cultures
| HCV | Experiment (day) | E1 | p7 | NS3 | NS4A | NS5A | NS5B | NS5B | NS5B | NS5B | NS5B | VR |
| Nucleotide position | ||||||||||||
| Recombinant-specific | 1325 | 2667 | 4742 | 5366 | 7656 | 9086 | 9102 | 9216 | 9342 | 9348 | 9458 | |
| H77 ref. (AF009606) | 1326 | 2656 | 4731 | 5355 | 7591 | 9021 | 9037 | 9151 | 9277 | 9283 | 9397 | |
| J6CF nucleotide | A | T | T | G | T | C | C | G | A | A | C | |
| J6CF with mutations | ||||||||||||
| J6_7mVmΔ33U* | Transfection (27) | C | C | T | A | A | A | G/a | T | G | ||
| J6_4mVmΔ33U | Fourth passage (5)† | T/a | C | C | T | C/T | G | T | G | |||
| J6_3mVmΔ33U | First passage (18) | C | T | G | T | G | ||||||
| Amino acid position | ||||||||||||
| Recombinant-specific | 329 | 776 | 1468 | 1676 | 2439 | 2916 | 2921 | 2959 | 3001 | 3003 | ||
| H77 ref. (AF009606) | 329 | 772 | 1464 | 1672 | 2417 | 2894 | 2899 | 2937 | 2979 | 2981 | ||
| Amino acid change | T–S | F–S | F–L | A–S | V–A | L–M | P–H | R–K | D–G | Y–F | ||
Transfection- or first passage-recovered viable J6 recombinants (transfection in Fig. 2A) with different engineered mutations were subjected to ORF (coding changes are shown) and 3′ UTR sequence analysis. See legend of Table 1 for nucleotide annotations. Shading indicates engineered mutations. Mutations introduced into J6CF: 7m, F776S/F1468L/A1676S/L2916M/P2921H/R2959K/Y3003F; 4m, F776S/F1468L/A1676S/Y3003F; 3m, F1468L/A1676S/Y3003F; Vm, nucleotide change C9458G in 3′ UTR variable region (VR); Δ33U, 33 U in polyU tract deleted. The 3′ UTR of recovered virus was determined by 5′ RACE on an HCV-negative-strand RNA. No consensus changes were observed in the variable and 3′-X regions. The length of the polyU/UC tract was found to be variable among analyzed clones: Recombinants with 7m, 4m, and 3m mutations were, on average, six nucleotides (seven clones with three U insertions to 15 U deletions), eight nucleotides (six clones with seven U insertions to 19 U deletions) shorter, and four nucleotides (seven clones with 19 U insertions to six U deletions) longer than the original polyU/UC tract, respectively.
*Six U were deleted in the polyU/UC tract of the final construct plasmid.
†The first-, second-, and third-passage viruses were not sequenced.
Robust full-length J6 culture systems based on F1468L, A1676S, and D3001G mutations.
Because D3001G was identified in all three mutated J6 full-length viruses (Table 2) and in a J6 recombinant with JFH1 elements (Table S1), and F1468L/A1676S facilitated replication of J6(JFH1_5BthuX) (Fig. 1B), we introduced F1468L/A1676S/D3001G into J6_Δ33U to obtain J6_LSGΔ33U. Further, Y3003F and Vm C9458G were engineered, singly or in combination, into J6_LSGΔ33U to generate J6_LSGFΔ33U, J6_LSGVmΔ33U, and J6_LSGFVmΔ33U (Fig. 2B). All four recombinants showed efficient HCV replication at day 1, with 20–50% antigen-positive cells, and spread to >80% of culture cells within 6 d with peak infectivity titers of 102.7 to 103.5 FFU/mL. Controls J65′ UTR-NS2/JFH1 and wild-type JFH1 had peak HCV infectivity titers of 104.0 and 102.8 FFU/mL, respectively. No HCV infection was detected for J6CF throughout. In first-passage viruses, the engineered mutations were maintained, but ORF changes were identified in J6_LSGΔ33U (Table 3) and J6_LSGFΔ33U (Table 3, legend). Because residues F1468, A1676, and D3001 are identical for J6 and JFH1, we had successfully adapted the J6 full-length genome to replicate efficiently in Huh7.5 cells independent of JFH1 elements.
Table 3.
Analyses of the recovered J6_LSG∆33U full-length viruses
| HCV | Peak titer (mL, log10) |
Passage (day) | E2 | p7 | NS2 | NS2 | NS3 | NS3 | NS3 | NS3 | NS4A | NS4B | NS5A | NS5A | NS5B | |
| FFU | RNA | |||||||||||||||
| Nucleotide position | ||||||||||||||||
| Recombinant-specific | 1826 | 2667 | 2873 | 3119 | 3639 | 3953 | 3962 | 4742 | 5366 | 6132 | 6857 | 7375 | 9342 | |||
| H77 ref. (AF009606) | 1821 | 2656 | 2862 | 3108 | 3628 | 3942 | 3951 | 4731 | 5355 | 6121 | 6846 | 7376 | 9277 | |||
| J6CF nucleotide | T | T | C | A | C | C | G | T | G | A | G | A | A | |||
| J6CF with mutations | ||||||||||||||||
| J6_LSGΔ33U* | 3.8 | 6.9 | First (16) | C/T | C | T | A/g/c | G | ||||||||
| 4.2 | 7.8 | Second (9)† | C/t | T/c | C | T | A/g/c | G | ||||||||
| 4.3 | 8.1 | Fourth (7) | C/t | T/c | T/c | C | T | A/g/c | G | |||||||
| 3.9 | 7.5 | Fifth (12) ‡ | C/t | C/t | C/t | C | T | A/G/C | G | |||||||
| 4.8 | 7.6 | Sixth (13) | C | A/G | C/t | C | T | C/a | A/C | G | ||||||
| J6_LSGΔ33U mutations | ||||||||||||||||
| +F776S | 4.1 | 7.5 | First (12) | T/C§ | C | T | C | G | ||||||||
| +P1100L | 3.7 | 7.4 | First (12) | T/C | T | C | T | G | ||||||||
| +N1931S | 3.7 | 7.1 | First (12) | C/t | C | T | G | G | ||||||||
| +N1931T | 3.5 | 6.4 | First (12) | C | T | C | G | |||||||||
| +F776S/P1100L | 4.2 | 7.7 | First (12) | C | T | C | T | G | ||||||||
| +F776S/N1931S | 4.0 | 7.6 | First (12) | C | C | T | G | G | ||||||||
| +F776S/N1931T | 4.2 | 7.3 | First (12) | T/C | C | T | T | A | C | T | C | G | ||||
| +P1100L/N1931S | 4.5 | 7.7 | First (12) | T/C | T | C | T | G | G | |||||||
| +P1100L/N1931T | 4.4 | 7.7 | First (12) | T/C | T | C | T | C | G | |||||||
| +F776S/P1100L/N1931S | 4.3 | 8.1 | First (12) †‡ | C | T | C | T | G | A | G | ||||||
| +F776S/P1100L/N1931Ta | 4.3 | 7.7 | First (12) †‡ | C | T | C | T | C | G | |||||||
| Amino acid position | ||||||||||||||||
| Recombinant-specific | 496 | 776 | 845 | 927 | 1100 | 1205 | 1208 | 1468 | 1676 | 1931 | 2173 | 2345 | 3001 | |||
| H77 ref. (AF009606) | 494 | 772 | 841 | 923 | 1096 | 1201 | 1204 | 1464 | 1672 | 1927 | 2169 | 2345 | 2979 | |||
| Amino acid change | C–R | F–S | R–W | T–A | P–L | L–F | V–I | F–L | A–S | N–S/T | D–N | Q–H | D–G | |||
For each passaged J6_LSG∆33U (original transfection shown in Fig. 2B) and its derived viruses with additional mutations (transfection in Fig. 2C), a representative peak infectivity titer (FFU/mL) with associated HCV RNA titer (IU/mL) is shown. Viruses from the indicated passage day were sequenced for ORF. Coding changes are shown. Shading indicates engineered mutations. See legend of Table 1 for nucleotide annotations. a, This virus was named “J6cc” (for “J6 cell culture-derived”).
*In a separate transfection experiment, first passage J6_LSGΔ33U (103.8 FFU/mL) acquired mutations T2667C/t (amino acid F776S), T2876T/G (F846V), A3548A/G (T1070A), A6132A/g/c (N1931S/T).
†The 5′ UTR was determined by 5′ RACE; no change was identified in the 5′ UTR. However, the G inserted immediately before the 5′-terminal A for enhancing in vitro transcription was deleted, consistent with our previous observations in JFH1-based systems (30, 67).
‡The 3′ UTR was determined by 5′ RACE on HCV-negative-strand RNA; no consensus changes were found in variable and 3′-X regions. However, the polyU/UC tract was variable in length among sequenced clones, being on average four nucleotides (eight clones with one U insertion to six U deletions) and 10 nucleotides (eight clones with 3–23 U deletions) shorter than the original polyU/UC for F776S/P1100L/N1931S and F776S/P1100L/N1931T mutants, respectively. In addition, genome sequence analysis of first-passage J6_LSGFΔ33U, J6_LSGVmΔ33U, and J6_LSGFVmΔ33U (transfection in Fig. 2B) revealed that the engineered mutations were maintained; no additional coding mutations were found.
§Engineered C was partially reverted.
Additional mutations F776S, P1100L, and N1931S/T enhanced virus production of J6_LSGΔ33U.
Transfection-recovered J6_LSGΔ33U reached infectivity titers of only 102.5 to 103.0 FFU/mL (range of 10 transfection experiments). However, passage-recovered polyclonal J6_LSGΔ33U viruses reached infectivity titers above 104.0 FFU/mL (Table 3), with a peak titer determined from the sixth passage (104.8 FFU/mL), higher than J65′ UTR-NS2/JFH1 (104.2 to104.5 FFU/mL). Sequence analysis of passaged J6_LSGΔ33U viruses identified additional mutations, of which F776S, P1100L, and N1931S/T appeared in most viruses (Table 3). We thus engineered these three mutations, singly or in double and triple combinations, into J6_LSGΔ33U (Fig. 2C). In transfection, the two J6_LSGΔ33U recombinants with all three mutations reached viral infectivity titers of 104.2 FFU/mL, equivalent to J65′ UTR-NS2/JFH1, and the virus with F776S/P1100L/N1931T did not acquire additional ORF mutations after passage to naive Huh7.5 cells (Table 3). Further analysis of the 5′ UTR and the 3′ UTR of both recombinants revealed that no mutations were required in these regions. We here named the most efficient recombinant, J6_LSGΔ33UF776S/P1100L/N1931T, “J6cc” (for “J6 cell culture-derived”). Thus, we established a robust J6 full-length culture system with infectivity titers comparable to JFH1-based systems.
Mutations adapting J6 enhanced replication and assembly of intracellular infectious HCV particles.
To address which steps of the viral life cycle were affected by mutations conferring efficient growth of J6, we first measured intracellular HCV core levels after transfection of S29 cells, a cell line deficient for the putative HCV receptor CD81 (18). J6_LSGΔ33U had only marginal enhancement of replication compared with J6CF. Introduction of single mutations P1100L, N1931S, or N1931T or of triple combinations (F776S/P1100L/N1931S or T) further increased replication (Fig. 2D). Next, we investigated the effect of these mutations on the production of intracellular infectious HCV. J6_LSGΔ33U was assembly competent, but introduction of single mutations or combinations increased the production of intracellular infectious virus particles (Fig. 2D). Thus, these mutations apparently enhanced the replication as well as assembly of intracellular infectious HCV particles.
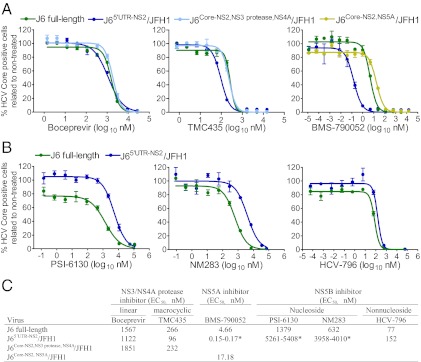
Full-length J6 virus was inhibited by HCV protease, NS5A, and NS5B inhibitors in a dose-dependent manner.
We previously tested protease and NS5A inhibitors against J6/JFH1 and J6/JFH1 with J6 NS3/NS4A protease or NS5A (31, 33). Here, we demonstrated that the full-length J6 culture viruses were inhibited by linear and macrocyclic NS3 protease inhibitors [boceprevir (3, 48) and TMC435 (49), respectively], by the NS5A inhibitor daclatasvir (BMS-790052) (50, 51) (Fig. 3A), and by nucleoside [PSI-6130 (52) and NM283 (53)] and nonnucleoside [HCV-796 (54)] NS5B inhibitors (Fig. 3B) in a dose-dependent manner. Compared with J65′ UTR-NS2/JFH1, both the J6 virus and J6/JFH1 with J6-specific NS3/NS4A protease were less sensitive to TMC435 with an EC50 two- to threefold higher than found for J65′ UTR-NS2/JFH1 (30); no apparent difference was observed for boceprevir (Fig. 3C). Both J6 full-length virus and J6/JFH1 with J6-specific NS5A were highly resistant to BMS790052 compared with J65′ UTR-NS2/JFH1 (Fig. 3 A and C), in agreement with our previous observations with the J6 NS5A recombinant (33).
Fig. 3.
Dose-dependent efficacy of NS3/NS4A protease and NS5A and NS5B polymerase inhibitors against J6 full-length virus and JFH1-based recombinants with J6-specific elements. Naive Huh7.5 cells were infected with indicated viruses, and antiviral treatments were carried out at 24 and 48 h postinfection. The HCV-positive cells were counted 72 h postinfection as previously described (31). Fourth-passage J6 full-length J6_LSGΔ33U virus (Table 3) was used for treatment. Treatment experiments with recombinants J65′ UTR-NS2/JFH1 (30), J6Core-NS2,NS3 protease,NS4A/JFH1 (31), and J6Core-NS2,NS5A/JFH1 (33) were included for comparison. (A) Treatment with protease inhibitors (boceprevir and TMC435) and the NS5A inhibitor daclatasvir (BMS-790052). (B) Treatment with NS5B inhibitors. (C) EC50 of the inhibitors used against the indicated viruses. *, Range of two experiments; experiments with the lowest EC50 shown in A or B. In A and B, values are means of triplicate determinations.
The J6 full-length culture system allowed us to test the effect of NS5B inhibitors against J6 NS5B in the context of the complete viral life cycle in vitro. Compared with J65′ UTR-NS2/JFH1 (30), the J6 virus was more sensitive to PSI-6130, NM283, and HCV-796 treatment, with around four-, six-, and twofold lower EC50, respectively (Fig. 3 B and C). Thus, this study demonstrated strain-specific efficacy of NS5B polymerase inhibitors in vitro.
Development of Full-Length J8 (Genotype 2b) Infectious Culture System.
There is an urgent need to identify mutations that will permit replication of clinical HCV isolates. Here we demonstrated that mutations important for adaptation of J6 could promote adaptation of J8, which is a prototype genotype 2b isolate (55, 56).
Key mutations adapting full-length J6 initiated replication of HCV strain J8 (genotype 2b) in Huh7.5 cells.
Because F1468L, A1676S, and D3001G resulted in adaptation of J6, we tested the effect of these mutations on an HCV isolate of another subtype of genotype 2. We thus generated a consensus full-length cDNA clone of the genotype 2b strain J8 (J8CF) using the sequence determined from virus recovered from a chimpanzee plasma-challenge pool (56). The ORF and polyprotein sequence of J6 and J8 differed by 24% and 16%, respectively. In three Huh7.5 cell transfections, the wild-type J8 did not show evidence of HCV replication through 3–4 wk, but J8 with three J6-derived mutations F1468L/A1676S/D3001G (named “J8_LSG”) showed HCV-positive cells at day 3 posttransfection. Among three J8_LSG cultures that initially started with <1% HCV antigen-positive cells, two cultures (J8_LSGa and J8_LSGb) were followed long-term and spread to ≥80% at days 60 and 77, respectively, reaching infectivity titers of 103.3 and 102.8 FFU/mL. In addition, we tested J8_LSG with Y3003F (J8_LSGF) and detected HCV-positive cells at day 3 posttransfection; the infection spread to ≥80% cells at day 81. After passage to naive Huh7.5 cells, we recovered viruses with infectivity titers of 103.2 to 103.6 FFU/mL (first passage) and 103.8 to 104.2 FFU/mL (second passage). A number of mutations were identified in transfection- and passage-recovered viruses (Table 4 and Tables S4 and S5). Thus, three J6-derived mutations could initiate J8 replication and promote further adaptation in vitro.
Table 4.
Analyses of recovered J8 full-length viruses
| HCV | Peak titer (mL, log10) |
Experiment (day) * | p7 | NS2 | NS2 | NS3 | NS3 | NS3 | NS3 | NS4A | NS4B | NS5A | NS5B | NS5B | NS5B | NS5B | |
| FFU | RNA (IU) | ||||||||||||||||
| Nucleotide position | |||||||||||||||||
| Recombinant-specific | 2656 | 2931 | 3115 | 3963 | 4209 | 4413 | 4743 | 5367 | 6243 | 7129 | 7657 | 9031 | 9106 | 9343 | |||
| H77 ref. (AF009606) | 2644 | 2919 | 3103 | 3951 | 4297 | 4401 | 4731 | 5355 | 6231 | 7129 | 7591 | 8965 | 9040 | 9277 | |||
| J8CF nucleotide | T | T | T | G | A | A | T | G | A | A | T | A | A | A | |||
| J8CF with mutations | |||||||||||||||||
| J8_LSGa† | 3.3 | — | Transfection (63/65) | G | C | A | G | C | T | G | T | G | G | ||||
| 3.6 | 6.9 | First passage (14) | T/G | C | A | A/g | C | T | G | T | G | G | G | ||||
| J8_LSGF772C/I1968V/H2922R | 3.6 | 7.0 | First passage (25) | G | G | C | T | G | G | G | |||||||
| +W864R | 3.6 | 7.2 | First passage (13) | G | C | A/G | C | T | G | G | G | ||||||
| +A1208T | 3.1 | 6.1 | First passage (16) | G | C | A | a/G | C | T | G | C | G | G | ||||
| +W864R/A1208T | 3.4 | 7.0 | First passage (12) | G | C | A | C | T | G | G | G | ||||||
| +W864R/E2263V | 3.3 | 7.2 | first passage (13) | G | C | A/G | C | T | G | T | G | G | |||||
| +A1208T/E2263V | 3.2 | 7.1 | First passage (13) | G | A | C | T | G | T | G | G | ||||||
| +W864R/A1208T/E2263V‡ | 3.3 | 7.2 | First passage (12) | G | C | A | C | T | G | T | G | G | |||||
| Amino acid position | |||||||||||||||||
| Recombinant-specific | 772 | 864 | 925 | 1208 | 1290 | 1358 | 1468 | 1676 | 1968 | 2263 | 2439 | 2897 | 2922 | 3001 | |||
| H77 ref. (AF009606) | 768 | 860 | 921 | 1204 | 1319 | 1354 | 1464 | 1672 | 1964 | 2263 | 2417 | 2875 | 2900 | 2979 | |||
| Amino acid change | F–C | W–R | V–A | A–T | T–A | T–A | F–L | A–S | I–V | E–V | V-A | N–S | H–R | D–G | |||
For each passaged J8 virus a representative peak infectivity titer (FFU/mL) with associated HCV RNA titer (IU/mL) is shown. Viruses of transfection- and passage-derived J8_LSGa (J8 with F1468L/A1676S/D3001G, one of two transfections) and first-passage J8_LSG with additional mutations were sequenced for ORF. Nucleotide and amino acid positions of the specific recombinant with complete coding changes are listed; noncoding and quasispecies mutations are shown in Table S4. Shading indicates engineered mutations: J6-derived mutations are indicated by dark gray shading and J8-derived mutations by light gray shading. See Table 1 legend for nucleotide annotations. –, RNA titer not determined.
*Viruses from indicated experiment were sequenced.
†The 5′ UTR was determined, and the 5′-terminal G was changed to A, consistent with our previous observations in JFH1-based systems (30, 67). The 3′ UTR of third-passage viruses was determined; no consensus changes were observed in variable and 3′-X regions. However, the polyU/UC tract had 4–23 (on average 14 ) U deletions among four sequenced clones. Second-passage J8_LSGa sequence is shown in Table S4.
‡This virus was named “J8cc” (for “J8 cell culture-derived”); in another experiment, no mutation was found. In addition, sequence analysis of transfection- and first passage-derived J8_LSGb showed that this virus also acquired mutations T2655T/G (amino acid F772V), A6243A/G (I1968V), and A9106G (H2922R), representing three of the six coding mutations found in J8_LSGa transfection and first-passage viruses.
Adaptation of full-length J8 to efficient growth in Huh7.5 cells by additional mutations.
Six coding mutations, F772C (p7), W864R (NS2), A1208T (NS3), I1968V (NS4B), E2263V (NS5A), and H2922R (NS5B), were identified in J8_LSGa transfection-, first-, and second-passage viruses (Table 4 and Table S4). H2922R and I1968V (as quasispecies) also were present in J8_LSGb (Table 4, legend) and J8_LSGF (Table S5), indicating their importance for viability. A partial change at F772, in this case to valine, also was observed in J8_LSGb (Table 4, legend). We thus tested viability in transfected Huh7.5 cells of J8_LSG recombinants with H2922R alone or in combination with each of the other five mutations and F772C/H2922R in combination with each of the other four mutations. All 10 mutants showed HCV-positive cells at day 1 posttransfection; however, only J8_LSGI1968V/H2922R and J8_LSGF772C/I1968V/H2922R spread within 2 wk, and only J8_LSGF772C/I1968V/H2922R spread to the entire culture. Sequence analysis of first passage J8_LSGF772C/I1968V/H2922R with infectivity titers of 103.1 to 103.6 FFU/mL identified a number of mutations (Table 4 and Table S4), indicating the need for further adaptation. Thus, we added W864R, A1208T, and E2263V, singly or in double or triple combinations, into J8_LSGF772C/I1968V/H2922R. The resulting mutants showed HCV-positive cells or ∼1% infection at day 1 posttransfection. All tested recombinants spread to ∼80% of culture cells within 6–10 d. The highest infectivity titers were found for the J8_LSGF772C/I1968V/H2922R recombinant with W864R/A1208T/E2263V (102.7 to 102.9 FFU/mL) (Fig. 2E). After passage to Huh7.5 cells, several such J8 recombinants had infectivity titers of 103.2 to 103.4 FFU/mL and did not acquire additional mutations (Table 4 and Table S4). We named the J8_LSGF772C/W864R/A1208T/I1968V/E2263V/H2922R recombinant, which was the most efficient among the ones tested, “J8cc” (for “J8 cell culture-derived”). Thus, we had developed J8 full-length genomes with in vitro efficient virus production without requirement for additional mutations.
Discussion
A major challenge for HCV research is the development of culture systems that support replication of clinical HCV isolates. In 2005, Wakita et al. (17) demonstrated that isolate JFH1 (genotype 2a) could replicate and produce infectious virus particles in Huh7.5 cells; its efficient growth required adaptive mutations (18–21). Since then, only a single other genome, H77C (genotype 1a) with replication-adaptive mutations, has been found to produce significant titers in culture (44, 46). We originally showed that H77C is infectious for chimpanzees (9, 57). We similarly demonstrated that J6CF (genotype 2a) is infectious in vivo (14), but despite numerous studies by other investigators this full-length clone has not been cultivated in vitro (25, 26, 39, 40, 58, 59). In the present study we developed a full-length culture system for J6 and demonstrated how other HCV isolates potentially could be adapted to grow in Huh7.5 cells. By studying J6CF with minimal JFH1 elements or NS5B residues, we identified three key mutations, F1468L in NS3 helicase, A1676S in NS4A, and D3001G in NS5B, which permitted culture replication and further adaptation of full-length J6. We generated genetically stable J6 genomes producing viral infectivity titers comparable to JFH1-based systems. HCV drugs inhibited J6 virus in a dose-dependent manner, providing proof of principle for studies of antivirals targeting any region of the viral genome in the context of the complete viral life cycle in cell culture. Importantly, the three key mutations showed cross-genotype adaptation effect by initiating the replication of a genetically divergent strain, J8 (genotype 2b), and we succeeded in generating several J8 genomes that efficiently produced infectious viruses in vitro without acquiring additional mutations after viral passage. These two culture systems (J6cc and J8cc) represent a significant advance in HCV research and provide valuable tools for studies of two epidemiologically important HCV genotypes. Importantly, the identified mutations and the approach we used to establish these culture systems potentially could be applied to further development of culture systems for other HCV patient isolates.
It took 16 y from the discovery of HCV until the first cell culture system was developed for a single HCV isolate and in a single hepatoma-derived cell line, and growing other full-length HCV isolates has proved an enormous challenge. Naturally existing HCV patient isolates apparently are not replication competent in cell culture. Our study demonstrates a common evolutionary approach to overcome this host restriction and thus has general interest for the studies of other viruses or organisms that have been impossible to culture or for which it has been possible to culture only a limited number of strains, such as other hepatitis viruses. Although identifying the required adaptive mutations was a complex process, in the end a limited number of mutations were required to overcome a complete host restriction. These unique mutations had the capacity to influence not only replication but also several other steps in the viral life cycle. Thus, this knowledge could be of common interest for understanding virus–host interactions. Specific knowledge is available for the roles of some of these amino acid positions, but the roles of others remain to be studied.
Overall, we identified mutations in p7 (F776S), NS3 (P1100L and F1468L), NS4A (A1676S), NS4B (N1931S/T), and NS5B (D3001G) (Fig. 2C and Table 3) supporting efficient growth of full-length J6; these genome regions play important roles in HCV RNA replication and other steps of the HCV life cycle (7). F776 in p7 (corresponding to p7 amino acid 26) is conserved among genotypes 1, 2, and 3, whereas P1100, F1468, A1676, N1931, and D3001 are highly conserved among all HCV genotypes (HCV database at Los Alamos National Lab), indicating their importance for the HCV life cycle. Of these mutations, no direct mutational analysis data were reported for P1100 in the NS3 protease (NS3 amino acid 70), A1676 in the transmembrane α-helix of NS4A (NS4A amino acid 15) (60), or D3001G in the C terminus of NS5B (NS5B amino acid 559). F776 was mapped within the first transmembrane domain of p7 (61), and F776S increased the infectivity of J6/JFH1 with J6 NS5A (33), but had no apparent effect on J6/JFH1 (62). F1468, corresponding to NS3 amino acid 438, is in a Phe-loop motif (DFSLDPTF) within the NS3 helicase that connects two antiparallel sheets between superfamily 2 helicase motifs 5 and 6 (63). Alanine substitution of F1468 resulted in the inability of the NS3 helicase to unwind RNA (63) and in the inability of the Con1 (1b) replicon to form colonies (64), suggesting its importance for HCV RNA replication. N1931 is located between helices 1 and 2 of the NS4B C-terminus (NS4B amino acid 216) and is critical for HCV RNA replication. Alanine substitution of this residue increased virus production of JFH1 and decreased virus production of J6/JFH1 recombinant Jc1 (65), indicating its importance for regulating HCV production, even though the mechanism remains unknown. We demonstrated that these mutations permitted establishment of an efficient J6 culture system by enhancing RNA replication as well as assembly and release of infectious virus particles (Fig. 2 B–E). Importantly, we showed that three of these J6-derived mutations (F1468L, A1676S, and D3001G) could promote a replication-incompetent and genetically divergent full-length J8 genome to replicate in Huh7.5 cells, thus allowing further adaptation to efficient growth in Huh7.5 cells with infectivity titers of 103.6 FFU/mL (Table 4). Even though the J8 isolate belongs to genotype 2b, it varies significantly from J6, with 24% difference at the ORF nucleotide level. The J8 NS5B polymerase sequence differs from J6 and JFH1 by ∼13%. It should be noted that the identified mutations are different from those in the H77 adapted genome (44, 46), whose ability to adapt other HCV isolates was not reported. Thus, the J6-derived adaptive mutations identified here potentially could initiate the replication of other HCV genotype isolates. Moreover, the J8-derived mutations tested in this study also were conserved in genotypes 1, 2, 3, and 7 for F772, in genotypes 1, 2, 3, 5, 6, and 7 for W864, in genotypes 1, 4, 5, and 6 for A1208, in genotypes 2, 3, 4, and 5 for I1968, in genotypes 1, 2b, and 7 for E2263, and in genotypes 2 and 4 for H2922 (HCV database at Los Alamos National Lab). Thus, in future studies the ability of these mutations to aid the development of other full-length HCV culture systems could be investigated.
Numerous HCV patient isolates have been identified, and a number of full-length HCV clones have proved infectious in vivo (9–15); however, only JFH1 could replicate autonomously in cultured cells (17). These results highlight the low probability of isolating an in vitro replication-competent HCV genome and thus argue for an urgent need for a systematic approach that permits the development of culture systems for HCV isolates. The natural existence of HCV quasispecies validates the development of a culture system by introducing replication- and/or virus production-enhancing mutations into a consensus genome sequence. Nevertheless, identification of such mutations has been difficult, because the replication-enhancing mutations identified in the Con1 (1b) replicon system were found to impair in vitro virus assembly (41) and in vivo infectivity (12). We took advantage of the replication capacity of the JFH1 NS5B polymerase and successfully identified mutations from culture, permitting adaptation of full-length J6 and J8. Because culture-derived mutations may be most efficient in aiding replication of full-length HCV isolates, it might be advantageous to use the high replication capacity of the JFH1 NS5B polymerase to adapt isolate-specific 5′ UTR-NS5A or 5′ UTR-partial NS5B recombinants to a highly permissive cell line, e.g., Huh7.5 cells; this approach may identify mutations permitting the replication of a full-length HCV genome as shown here. Because the J6-derived mutations increased RNA replication, virus assembly, and release (Fig. 2 B–D) and had a general adaptive effect for J8 (Fig. 2E), these mutations potentially also could facilitate the adaptation process of other genotype recombinants with JFH1 NS5B- or partial NS5B-3′ UTR regions. Therefore, the mutations identified in this study, in combination with the approach leading to their identification, may be able to break the barrier of culturing HCV in vitro and thus pave the way toward the development of full-length culture systems for clinical HCV isolates.
With J6 and J8 full-length culture systems, all HCV inhibitors now can be tested, in either single or combination treatments, at an isolate-specific level. As an example of the utility of these culture systems, we demonstrated that the newly developed J6 full-length virus and J6/JFH1 with J6 or with JFH1 NS3/NS4A protease and NS5A responded differentially to protease inhibitors (31) and NS5A inhibitor daclatasvir (BMS-790052) (33) (Fig. 3), although J6 and JFH1 belong to the same subtype. Importantly, we compared the efficacy of NS5B inhibitors against J6 full-length or recombinants with JFH1 NS5B polymerase in culture; previously this comparison was not possible because of the lack of a robust culture system with J6 NS5B. Interestingly, differential responses to three NS5B inhibitors were evident (Fig. 3 B and C), even though J6 and JFH1 differ by only 5% in the NS5B amino acid sequence. These differences indicate that the efficacy of these NS5B inhibitors or other antivirals may be more variable among HCV variants with a higher degree of heterogeneity. However, the possibility can not be excluded that cell culture-adaptive mutations in the recombinants used for in vitro testing might have affected sensitivity to the tested antivirals. Even though the observations made in cell culture require verification in the clinical setting, additional full-length HCV culture systems are needed to allow screening for antivirals with universal effects on various genotypes or of new drugs targeting any regions of the HCV protein or RNA. Development of full-length J6cc and J8cc culture systems and possibly additional systems meets these needs and will be a major asset to the hepatitis C field. These culture systems will contribute directly to and also will provide an alternative model for the development of HCV full-length culture systems for HCV patient isolates, with application for HCV vaccine and drug development and for better individualized treatment of HCV-infected patients.
Materials and Methods
Plasmids.
PCR-fused or synthesized (GenScript) chimeric NS5B or NS5B-to-3′ UTR with J6 and JFH1 sequences were cloned into J6/JFH1-J6NS5A (33) or J6CF (14) (Fig. 1 A–D). Mutations in full-length J6 and J8 genomes were generated by PCR or synthesized (GenScript). For J6 recombinants, G was inserted between the T7 promoter and the HCV 5′ UTR to enhance in vitro transcription (14). All recombinant constructs were sequenced covering the T7 promoter and the entire HCV genome.
Construction of Full-Length J8 Genome.
The 5′ UTR-NS2 sequence of J8 had been determined previously (23, 30) using an experimentally infected chimpanzee plasma pool (56, 66). With virus recovered from this plasma pool, the NS3 to NS5B sequence was determined by sequencing three overlapping RT-PCR amplicons covering nucleotides 2522–9474 [according to J8 sequence (GenBank accession no. D10988) (55)]. A consensus sequence of five to seven clones was synthesized (GenScript) and cloned into pJ85′ UTR-NS2/JFH1 (30) to generate pJ8/J8. The 3′ UTR VR and polyU/UC tract were amplified by nested PCR (10), cloned, and sequenced. A representative sequence was determined from 14 clones with different polyU/UC lengths. The 3′ UTR X-region sequence was taken from JPUT971017 (genotype 2b) (AB030907). The entire 3′ UTR together with the upstream NS5B sequence to Sse232I site was synthesized (GenScript) and cloned into pJ8/J8 by Sse232I-XbaI sites to obtain the full-length J8 plasmid, pJ8CF.
Transfection and infection of Huh7.5 cells.
The human hepatoma cell line Huh7.5 was maintained as described (22, 25). Twenty-four hours before transfection or infection, 4.2 × 105 cells per well were seeded in six-well plates. For transfections, 10 μg of HCV recombinant plasmid was linearized with XbaI, treated with mung bean nuclease, purified, and in vitro transcribed using T7 RNA polymerase (Promega) (100 μL total). The resulting HCV RNA transcripts were mixed with 150 μL Opti-MEM (Invitrogen) and incubated for 10 min at room temperature, mixed with 255 μL transfection complex [5 μL of Lipofectamine 2000 (Invitrogen) in 250 μL of Opti-MEM with 10-min incubation], incubated for 20 min, and added dropwise into the Huh7.5 cell cultures that had been preincubated in 2 mL of Opti-MEM for 20 min. The transfected cultures were left for ∼16 h and then were subcultured every 2–3 d; the supernatant was collected, filtered (pore size 0.45 μm), and stored at −80 °C. To passage virus, Huh7.5 cells grown in six-well plates were incubated with 1 mL transfection-collected culture supernatant for ∼16 h and then were subcultured every 2–3 d.
Determination of virus infection, HCV infectivity titers, and HCV RNA titers.
Monoclonal anti-core antibody B2 (Anogen) was used for immunostaining for HCV core for J6/JFH1 and full-length J6 viruses, and monoclonal anti-NS5A antibody 9E10 (25) was used for J8 viruses, as previously described (22, 33, 67). The percentage of HCV antigen-positive cells was estimated using fluorescence microscopy and was used as an indication of the status of HCV infection in the culture.
HCV infectivity titers were determined by FFU assay as previously described (23, 33, 67). Primary monoclonal anti-core antibody C7-50 (Enzo Life Sciences) was used in 1/500 dilutions for J6 recombinant and full-length viruses [NS3 helicase monoclonal antibody H23 (Abcam) was used for experiments in Fig. 1A]. 9E10 (25) was used in 1/1,000 dilutions for J8 viruses. The number of FFU was counted manually using an inverted light microscope (Olympus CK2) or automatically by an ImmunoSpot Series 5 UV Analyzer with customized software (CTL Europe GmbH) (10, 33). The viral infectivity titers (FFU/mL) are averages from three independent infections.
Supernatant HCV RNA titers were determined using the real-time RT-PCR TaqMan assay as previously described (22).
Sequence analysis of the culture-derived HCV.
ORF sequence analysis of the J6 recombinant viruses with JFH1 sequences was described previously (22, 30, 33, 67). ORF RT-PCR primers for J6 and J8 full-length viruses are given in Table S6. The 5′ UTR and 3′ UTR sequences were determined using 5′ RACE Systems for Rapid Amplification of cDNA Ends (Invitrogen) with dA or dC tailing technology, as described previously (30, 67). HCV RNA extracted from culture supernatant was used for 5′ UTR 5′ RACE (30). HCV-negative-strand RNA extracted from infected cells was used in 5′ RACE to determine the 3′ UTR sequence (30, 67); primers are given in Table S6.
Determination of intracellular HCV core and infectivity titer.
HCV RNA transcripts were transfected into the HCV entry-deficient cell line, S29 (18). As previously described (28), HCV core level at 4 and 48 h posttransfection was determined by ELISA, and the intracellular HCV infectivity titer at 48 h was determined by FFU assay on Huh7.5 cells.
HCV antiviral treatment.
HCV antivirals were purchased from Acme Bioscience and were dissolved in dimethyl sulfoxide. Huh7.5 cells grown in poly-d-lysine–coated 96-well plates (Nunc) were infected with HCV and treated with antivirals 24 and 48 h postinfection as previously described (31). Single HCV core-positive cells (33) were determined by immunostaining with C7-50 (Enzo Life Sciences) 72 h postinfection. No cytotoxic effects were observed in inhibitor treatments, as shown previously for NS3/NS4A protease and NS5A inhibitors (31, 33) and as monitored in this study for NS5B inhibitors using CellTiter 96 AQueous One Solution Cell Proliferation Assay (Promega).
Supplementary Material
Acknowledgments
We thank L. Ghanem, A. L. Sørensen, and B. Landt for technical assistance; S. B. Serre and T. Carlsen for discussions; and J. O. Nielsen and O. Andersen for providing valuable support (all from Copenhagen University Hospital). We thank S. U. Emerson (National Institutes of Health, Bethesda, MD), C. M. Rice (Rockefeller University) and T. Wakita (National Institute of Infectious Diseases, Tokyo, Japan) for providing reagents. This study was supported by research grants from Lundbeck Foundation (to J.G.M., T.H.K.S., and J.B.); The Danish Cancer Society (to J.M.G. and J.B.); The Novo Nordisk Foundation (to J.M.G. and J.B.); The A.P. Møller og Hustru Chastine Mc-Kinney Møllers Fondation (to J.B.); the Danish Council for Independent Research - Medical Sciences (to Y.-P.L., S.R., and J.B.); and in part by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health (to R.H.P. and J.B.). S.R. and T.K.H.S. are the recipients of Individual Postdoctoral Stipends from the Danish Council for Independent Research - Medical Sciences.
Footnotes
The authors declare no conflict of interest.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database [accession nos. JQ745651 (pJ8CF), JQ745650 (pJ6cc), and JQ745652 (pJ8cc)].
See Author Summary on page 6806 (volume 109, number 18).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1203829109/-/DCSupplemental.
References
- 1.Thomas DL, et al. Genetic variation in IL28B and spontaneous clearance of hepatitis C virus. Nature. 2009;461:798–801. doi: 10.1038/nature08463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jacobson IM, et al. ADVANCE Study Team Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011;364:2405–2416. doi: 10.1056/NEJMoa1012912. [DOI] [PubMed] [Google Scholar]
- 3.Poordad F, et al. SPRINT-2 Investigators Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1195–1206. doi: 10.1056/NEJMoa1010494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zeuzem S. Interferon-based therapy for chronic hepatitis C: Current and future perspectives. Nat Clin Pract Gastroenterol Hepatol. 2008;5:610–622. doi: 10.1038/ncpgasthep1274. [DOI] [PubMed] [Google Scholar]
- 5.Ge D, et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature. 2009;461:399–401. doi: 10.1038/nature08309. [DOI] [PubMed] [Google Scholar]
- 6.Foster GR, et al. Telaprevir alone or with peginterferon and ribavirin reduces HCV RNA in patients with chronic genotype 2 but not genotype 3 infections. Gastroenterology. 2011;141:881–889. doi: 10.1053/j.gastro.2011.05.046. [DOI] [PubMed] [Google Scholar]
- 7.Moradpour D, Penin F, Rice CM. Replication of hepatitis C virus. Nat Rev Microbiol. 2007;5:453–463. doi: 10.1038/nrmicro1645. [DOI] [PubMed] [Google Scholar]
- 8.Simmonds P, et al. Consensus proposals for a unified system of nomenclature of hepatitis C virus genotypes. Hepatology. 2005;42:962–973. doi: 10.1002/hep.20819. [DOI] [PubMed] [Google Scholar]
- 9.Yanagi M, Purcell RH, Emerson SU, Bukh J. Transcripts from a single full-length cDNA clone of hepatitis C virus are infectious when directly transfected into the liver of a chimpanzee. Proc Natl Acad Sci USA. 1997;94:8738–8743. doi: 10.1073/pnas.94.16.8738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gottwein JM, et al. Novel infectious cDNA clones of hepatitis C virus genotype 3a (strain S52) and 4a (strain ED43): Genetic analyses and in vivo pathogenesis studies. J Virol. 2010;84:5277–5293. doi: 10.1128/JVI.02667-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gottwein JM, Bukh J. Cutting the gordian knot-development and biological relevance of hepatitis C virus cell culture systems. Adv Virus Res. 2008;71:51–133. doi: 10.1016/S0065-3527(08)00002-X. [DOI] [PubMed] [Google Scholar]
- 12.Bukh J, et al. Mutations that permit efficient replication of hepatitis C virus RNA in Huh-7 cells prevent productive replication in chimpanzees. Proc Natl Acad Sci USA. 2002;99:14416–14421. doi: 10.1073/pnas.212532699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kolykhalov AA, et al. Transmission of hepatitis C by intrahepatic inoculation with transcribed RNA. Science. 1997;277:570–574. doi: 10.1126/science.277.5325.570. [DOI] [PubMed] [Google Scholar]
- 14.Yanagi M, Purcell RH, Emerson SU, Bukh J. Hepatitis C virus: An infectious molecular clone of a second major genotype (2a) and lack of viability of intertypic 1a and 2a chimeras. Virology. 1999;262:250–263. doi: 10.1006/viro.1999.9889. [DOI] [PubMed] [Google Scholar]
- 15.Yanagi M, et al. Transcripts of a chimeric cDNA clone of hepatitis C virus genotype 1b are infectious in vivo. Virology. 1998;244:161–172. doi: 10.1006/viro.1998.9092. [DOI] [PubMed] [Google Scholar]
- 16.Zhong J, et al. Robust hepatitis C virus infection in vitro. Proc Natl Acad Sci USA. 2005;102:9294–9299. doi: 10.1073/pnas.0503596102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wakita T, et al. Production of infectious hepatitis C virus in tissue culture from a cloned viral genome. Nat Med. 2005;11:791–796. doi: 10.1038/nm1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Russell RS, et al. Advantages of a single-cycle production assay to study cell culture-adaptive mutations of hepatitis C virus. Proc Natl Acad Sci USA. 2008;105:4370–4375. doi: 10.1073/pnas.0800422105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Delgrange D, et al. Robust production of infectious viral particles in Huh-7 cells by introducing mutations in hepatitis C virus structural proteins. J Gen Virol. 2007;88:2495–2503. doi: 10.1099/vir.0.82872-0. [DOI] [PubMed] [Google Scholar]
- 20.Kaul A, Woerz I, Meuleman P, Leroux-Roels G, Bartenschlager R. Cell culture adaptation of hepatitis C virus and in vivo viability of an adapted variant. J Virol. 2007;81:13168–13179. doi: 10.1128/JVI.01362-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhong J, et al. Persistent hepatitis C virus infection in vitro: Coevolution of virus and host. J Virol. 2006;80:11082–11093. doi: 10.1128/JVI.01307-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gottwein JM, et al. Robust hepatitis C genotype 3a cell culture releasing adapted intergenotypic 3a/2a (S52/JFH1) viruses. Gastroenterology. 2007;133:1614–1626. doi: 10.1053/j.gastro.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 23.Gottwein JM, et al. Development and characterization of hepatitis C virus genotype 1-7 cell culture systems: Role of CD81 and scavenger receptor class B type I and effect of antiviral drugs. Hepatology. 2009;49:364–377. doi: 10.1002/hep.22673. [DOI] [PubMed] [Google Scholar]
- 24.Jensen TB, et al. Highly efficient JFH1-based cell-culture system for hepatitis C virus genotype 5a: Failure of homologous neutralizing-antibody treatment to control infection. J Infect Dis. 2008;198:1756–1765. doi: 10.1086/593021. [DOI] [PubMed] [Google Scholar]
- 25.Lindenbach BD, et al. Complete replication of hepatitis C virus in cell culture. Science. 2005;309:623–626. doi: 10.1126/science.1114016. [DOI] [PubMed] [Google Scholar]
- 26.Pietschmann T, et al. Construction and characterization of infectious intragenotypic and intergenotypic hepatitis C virus chimeras. Proc Natl Acad Sci USA. 2006;103:7408–7413. doi: 10.1073/pnas.0504877103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scheel TK, et al. Development of JFH1-based cell culture systems for hepatitis C virus genotype 4a and evidence for cross-genotype neutralization. Proc Natl Acad Sci USA. 2008;105:997–1002. doi: 10.1073/pnas.0711044105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scheel TK, et al. Efficient culture adaptation of hepatitis C virus recombinants with genotype-specific core-NS2 by using previously identified mutations. J Virol. 2011;85:2891–2906. doi: 10.1128/JVI.01605-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yi M, Ma Y, Yates J, Lemon SM. Compensatory mutations in E1, p7, NS2, and NS3 enhance yields of cell culture-infectious intergenotypic chimeric hepatitis C virus. J Virol. 2007;81:629–638. doi: 10.1128/JVI.01890-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li YP, Gottwein JM, Scheel TK, Jensen TB, Bukh J. MicroRNA-122 antagonism against hepatitis C virus genotypes 1-6 and reduced efficacy by host RNA insertion or mutations in the HCV 5′ UTR. Proc Natl Acad Sci USA. 2011;108:4991–4996. doi: 10.1073/pnas.1016606108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gottwein JM, Scheel TK, Jensen TB, Ghanem L, Bukh J. Differential efficacy of protease inhibitors against HCV genotypes 2a, 3a, 5a, and 6a NS3/4A protease recombinant viruses. Gastroenterology. 2011;141:1067–1079. doi: 10.1053/j.gastro.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 32.Imhof I, Simmonds P. Development of an intergenotypic hepatitis C virus (HCV) cell culture method to assess antiviral susceptibilities and resistance development of HCV NS3 protease genes from HCV genotypes 1 to 6. J Virol. 2010;84:4597–4610. doi: 10.1128/JVI.02698-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scheel TK, Gottwein JM, Mikkelsen LS, Jensen TB, Bukh J. Recombinant HCV variants with NS5A from genotypes 1-7 have different sensitivities to an NS5A inhibitor but not interferon-α. Gastroenterology. 2011;140:1032–1042. doi: 10.1053/j.gastro.2010.11.036. [DOI] [PubMed] [Google Scholar]
- 34.Prentoe J, et al. Hypervariable region 1 differentially impacts viability of hepatitis C genotype 1-6 strains and impairs virus neutralization. J Virol. 2011;85:2224–2234. doi: 10.1128/JVI.01594-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dorner M, et al. A genetically humanized mouse model for hepatitis C virus infection. Nature. 2011;474:208–211. doi: 10.1038/nature10168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sainz B, Jr, et al. Identification of the Niemann-Pick C1-like 1 cholesterol absorption receptor as a new hepatitis C virus entry factor. Nat Med. 2012;18:281–285. doi: 10.1038/nm.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Okamoto H, et al. Nucleotide sequence of the genomic RNA of hepatitis C virus isolated from a human carrier: Comparison with reported isolates for conserved and divergent regions. J Gen Virol. 1991;72:2697–2704. doi: 10.1099/0022-1317-72-11-2697. [DOI] [PubMed] [Google Scholar]
- 38.Kato T, et al. Sequence analysis of hepatitis C virus isolated from a fulminant hepatitis patient. J Med Virol. 2001;64:334–339. doi: 10.1002/jmv.1055. [DOI] [PubMed] [Google Scholar]
- 39.Murayama A, et al. The NS3 helicase and NS5B-to-3'X regions are important for efficient hepatitis C virus strain JFH-1 replication in Huh7 cells. J Virol. 2007;81:8030–8040. doi: 10.1128/JVI.02088-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murayama A, et al. RNA polymerase activity and specific RNA structure are required for efficient HCV replication in cultured cells. PLoS Pathog. 2010;6:e1000885. doi: 10.1371/journal.ppat.1000885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pietschmann T, et al. Production of infectious genotype 1b virus particles in cell culture and impairment by replication enhancing mutations. PLoS Pathog. 2009;5:e1000475. doi: 10.1371/journal.ppat.1000475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shimizu YK, Iwamoto A, Hijikata M, Purcell RH, Yoshikura H. Evidence for in vitro replication of hepatitis C virus genome in a human T-cell line. Proc Natl Acad Sci USA. 1992;89:5477–5481. doi: 10.1073/pnas.89.12.5477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sung VM, et al. Establishment of B-cell lymphoma cell lines persistently infected with hepatitis C virus in vivo and in vitro: The apoptotic effects of virus infection. J Virol. 2003;77:2134–2146. doi: 10.1128/JVI.77.3.2134-2146.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yi M, Villanueva RA, Thomas DL, Wakita T, Lemon SM. Production of infectious genotype 1a hepatitis C virus (Hutchinson strain) in cultured human hepatoma cells. Proc Natl Acad Sci USA. 2006;103:2310–2315. doi: 10.1073/pnas.0510727103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Silberstein E, et al. Persistent growth of a human plasma-derived hepatitis C virus genotype 1b isolate in cell culture. PLoS Pathog. 2010;6:e1000910. doi: 10.1371/journal.ppat.1000910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shimakami T, et al. Protease inhibitor-resistant hepatitis C virus mutants with reduced fitness from impaired production of infectious virus. Gastroenterology. 2011;140:667–675. doi: 10.1053/j.gastro.2010.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lesburg CA, et al. Crystal structure of the RNA-dependent RNA polymerase from hepatitis C virus reveals a fully encircled active site. Nat Struct Biol. 1999;6:937–943. doi: 10.1038/13305. [DOI] [PubMed] [Google Scholar]
- 48.Venkatraman S, et al. Discovery of (1R,5S)-N-[3-amino-1-(cyclobutylmethyl)-2,3-dioxopropyl]- 3-[2(S)-[[[(1,1-dimethylethyl)amino]carbonyl]amino]-3,3-dimethyl-1-oxobutyl]- 6,6-dimethyl-3-azabicyclo[3.1.0]hexan-2(S)-carboxamide (SCH 503034), a selective, potent, orally bioavailable hepatitis C virus NS3 protease inhibitor: A potential therapeutic agent for the treatment of hepatitis C infection. J Med Chem. 2006;49:6074–6086. doi: 10.1021/jm060325b. [DOI] [PubMed] [Google Scholar]
- 49.Raboisson P, et al. Structure-activity relationship study on a novel series of cyclopentane-containing macrocyclic inhibitors of the hepatitis C virus NS3/4A protease leading to the discovery of TMC435350. Bioorg Med Chem Lett. 2008;18:4853–4858. doi: 10.1016/j.bmcl.2008.07.088. [DOI] [PubMed] [Google Scholar]
- 50.Gao M, et al. Chemical genetics strategy identifies an HCV NS5A inhibitor with a potent clinical effect. Nature. 2010;465:96–100. doi: 10.1038/nature08960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lok AS, et al. Preliminary study of two antiviral agents for hepatitis C genotype 1. N Engl J Med. 2012;366:216–224. doi: 10.1056/NEJMoa1104430. [DOI] [PubMed] [Google Scholar]
- 52.Clark JL, et al. Design, synthesis, and antiviral activity of 2′-deoxy-2′-fluoro-2′-C-methylcytidine, a potent inhibitor of hepatitis C virus replication. J Med Chem. 2005;48:5504–5508. doi: 10.1021/jm0502788. [DOI] [PubMed] [Google Scholar]
- 53.Pierra C, et al. Synthesis and pharmacokinetics of valopicitabine (NM283), an efficient prodrug of the potent anti-HCV agent 2′-C-methylcytidine. J Med Chem. 2006;49:6614–6620. doi: 10.1021/jm0603623. [DOI] [PubMed] [Google Scholar]
- 54.Howe AY, et al. Molecular mechanism of hepatitis C virus replicon variants with reduced susceptibility to a benzofuran inhibitor, HCV-796. Antimicrob Agents Chemother. 2008;52:3327–3338. doi: 10.1128/AAC.00238-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Okamoto H, et al. Full-length sequence of a hepatitis C virus genome having poor homology to reported isolates: Comparative study of four distinct genotypes. Virology. 1992;188:331–341. doi: 10.1016/0042-6822(92)90762-e. [DOI] [PubMed] [Google Scholar]
- 56.Bukh J, et al. Challenge pools of hepatitis C virus genotypes 1-6 prototype strains: Replication fitness and pathogenicity in chimpanzees and human liver-chimeric mouse models. J Infect Dis. 2010;201:1381–1389. doi: 10.1086/651579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bukh J, et al. Previously infected chimpanzees are not consistently protected against reinfection or persistent infection after reexposure to the identical hepatitis C virus strain. J Virol. 2008;82:8183–8195. doi: 10.1128/JVI.00142-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schmitt M, et al. A comprehensive structure-function comparison of hepatitis C virus strain JFH1 and J6 polymerases reveals a key residue stimulating replication in cell culture across genotypes. J Virol. 2011;85:2565–2581. doi: 10.1128/JVI.02177-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Simister P, et al. Structural and functional analysis of hepatitis C virus strain JFH1 polymerase. J Virol. 2009;83:11926–11939. doi: 10.1128/JVI.01008-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brass V, et al. Structural determinants for membrane association and dynamic organization of the hepatitis C virus NS3-4A complex. Proc Natl Acad Sci USA. 2008;105:14545–14550. doi: 10.1073/pnas.0807298105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Montserret R, et al. NMR structure and ion channel activity of the p7 protein from hepatitis C virus. J Biol Chem. 2010;285:31446–31461. doi: 10.1074/jbc.M110.122895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Murray CL, Jones CT, Tassello J, Rice CM. Alanine scanning of the hepatitis C virus core protein reveals numerous residues essential for production of infectious virus. J Virol. 2007;81:10220–10231. doi: 10.1128/JVI.00793-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lam AM, Keeney D, Frick DN. Two novel conserved motifs in the hepatitis C virus NS3 protein critical for helicase action. J Biol Chem. 2003;278:44514–44524. doi: 10.1074/jbc.M306444200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lam AM, Frick DN. Hepatitis C virus subgenomic replicon requires an active NS3 RNA helicase. J Virol. 2006;80:404–411. doi: 10.1128/JVI.80.1.404-411.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jones DM, Patel AH, Targett-Adams P, McLauchlan J. The hepatitis C virus NS4B protein can trans-complement viral RNA replication and modulates production of infectious virus. J Virol. 2009;83:2163–2177. doi: 10.1128/JVI.01885-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Engle RE, Russell RS, Purcell RH, Bukh J. Development of a TaqMan assay for the six major genotypes of hepatitis C virus: Comparison with commercial assays. J Med Virol. 2008;80:72–79. doi: 10.1002/jmv.21043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li YP, Ramirez S, Gottwein JM, Bukh J. Non-genotype-specific role of the hepatitis C virus 5′ untranslated region in virus production and in inhibition by interferon. Virology. 2011;421:222–234. doi: 10.1016/j.virol.2011.10.002. [DOI] [PubMed] [Google Scholar]