Abstract
White-nose syndrome (WNS) is an emerging disease of hibernating bats associated with cutaneous infection by the fungus Geomyces destructans (Gd), and responsible for devastating declines of bat populations in eastern North America. Affected bats appear emaciated and one hypothesis is that they spend too much time out of torpor during hibernation, depleting vital fat reserves required to survive the winter. The fungus has also been found at low levels on bats throughout Europe but without mass mortality. This finding suggests that Gd is either native to both continents but has been rendered more pathogenic in North America by mutation or environmental change, or that it recently arrived in North America as an invader from Europe. Thus, a causal link between Gd and mortality has not been established and the reason for its high pathogenicity in North America is unknown. Here we show that experimental inoculation with either North American or European isolates of Gd causes WNS and mortality in the North American bat, Myotis lucifugus. In contrast to control bats, individuals inoculated with either isolate of Gd developed cutaneous infections diagnostic of WNS, exhibited a progressive increase in the frequency of arousals from torpor during hibernation, and were emaciated after 3–4 mo. Our results demonstrate that altered torpor-arousal cycles underlie mortality from WNS and provide direct evidence that Gd is a novel pathogen to North America from Europe.
Keywords: fungal pathogen, infectious disease, invasive species, Chiroptera, wildlife conservation
White-nose syndrome (WNS) is a rapidly spreading wildlife disease caused by the cold-tolerant fungus Geomyces destructans (Gd) (1). WNS has killed millions of bats across 16 US states and four Canadian provinces since its emergence in New York State in 2006 (2). So far, nine bat species from three genera, all of which hibernate in caves or mines, have been found to carry Gd, and mortality of infected bats has been observed in six of these species (2). The population dynamics of most affected species are not well understood but the effects of current declines are likely to be drastic; for example, the little brown bat (Myotis lucifugus) was the most widespread and common bat species in North America before WNS, but is now predicted to face local extinction in WNS-affected areas within two decades (3). During hibernation, the skin of WNS-affected bats is colonized by Gd, which invades cutaneous tissues of the muzzle, ears, and wings (4, 5). Major inflammation is usually not observed in infected tissues (6), possibly because immune responses in hibernating animals are suppressed (7). Mortality occurs in the second half of the hibernation season and affected bats are typically emaciated. Recently Lorch et al. (1) showed that experimental inoculation of M. lucifugus with Gd caused the characteristic wing lesions associated with WNS, and confirmed that Gd can be spread by direct contact between bats. However, no study has established a causal mechanism linking Gd with bat mortality.
One possible explanation for mortality from WNS is that Gd causes a disruption of energy balance during hibernation. Hibernating mammals spend the majority of their time in torpor, a state of controlled reduction in body temperature (Tb) and metabolic rate, which is interrupted by brief periodic arousals to normothermic Tb (8). Although these arousals last less than 24 h in most species, the high metabolic cost of thermoregulation during normothermia at a low ambient temperature (Ta) means they account for the vast majority of over-winter energy expenditure (8, 9). Food is unavailable for most temperate-zone bats during winter, so they must survive on stored fat (9). Therefore, one hypothesis to explain WNS-related mortality is that Gd causes bats to increase the duration and/or frequency of periodic arousals, resulting in premature depletion of fat and consequently starvation (10). Preliminary support for this hypothesis was found based on an energetic model (11) but, to date, there is no experimental evidence that bats infected with Gd spend more time out of torpor than uninfected controls.
In addition to the mechanism underlying mortality, the origin of WNS is still unknown. There are two competing explanations for the origin of any emerging infectious disease (12). Such a disease may result from a pathogen that has been present historically but is rendered more pathogenic by a genetic mutation or environmental change (i.e., the endemic pathogen hypothesis). Alternatively, a pathogen may arrive in a new geographic area and encounter a naive host population (the “novel” or invasive pathogen hypothesis) (12). It is now established that Gd occurs at low levels on bats throughout Europe, where it has been isolated from eight Myotis spp., but with no evidence of mass mortality (13, 14). Given that Gd went undiscovered in Europe until WNS was observed in North America, one possibility is that Gd has occurred historically at low levels on bats from both continents but went unnoticed until mass mortality of bats in North America led to intensive sampling for a potential pathogen. This theory is cause for concern because European bats could be at risk from the accidental introduction of North American Gd to bat hibernacula in Europe. Alternatively, Gd may have arrived in North America as a recent invader from Europe, perhaps introduced by tourists visiting caves. Wibbelt et al. (14) hypothesized that under this novel pathogen scenario European bats may have coevolved with Gd over many years, and differences in its apparent pathogenicity for North American versus European bats could reflect differences in the physiology or behavior of the bats or differences in their environments, rather than intercontinental differences in Gd. Confirming one or the other of these hypotheses is essential because different disease-management strategies are warranted for invasive versus endemic pathogens (12).
We conducted an inoculation experiment with M. lucifugus to evaluate three hypotheses important for our understanding of WNS. First, we tested a key prediction of the novel pathogen hypothesis, which predicts that Gd isolated from Europe should cause the same clinical signs in a North American bat species as Gd isolated from North America. Therefore, we inoculated individual M. lucifugus with either a North American isolate of Gd (NAGd) or a European isolate (EUGd) and assessed clinical signs following several months of infection. Second, we tested whether inoculation with Gd, alone, is sufficient to cause mortality, a fundamental question about WNS that has still not been addressed. Third, by monitoring skin temperatures of bats following inoculation, we assessed the hypothesis that infection with Gd causes bats to increase the frequency and/or duration of periodic arousals during hibernation, leading to premature fat depletion (10, 11). Importantly, we kept animals in environmental conditions closely matched to those of M. lucifugus hibernacula (9), particularly in terms of high relative humidity (RH).
Results
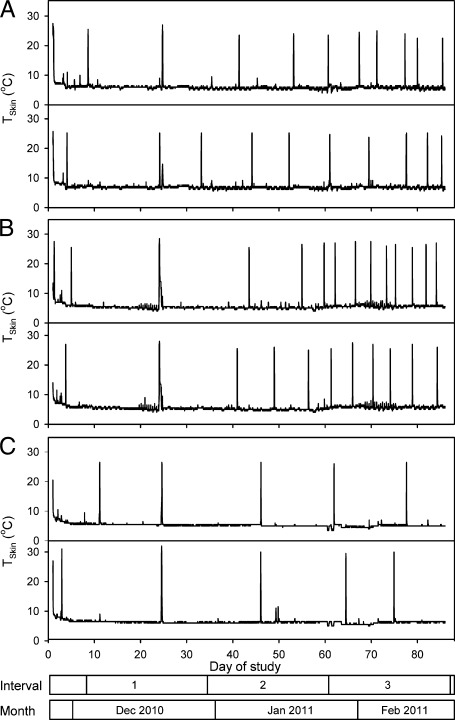
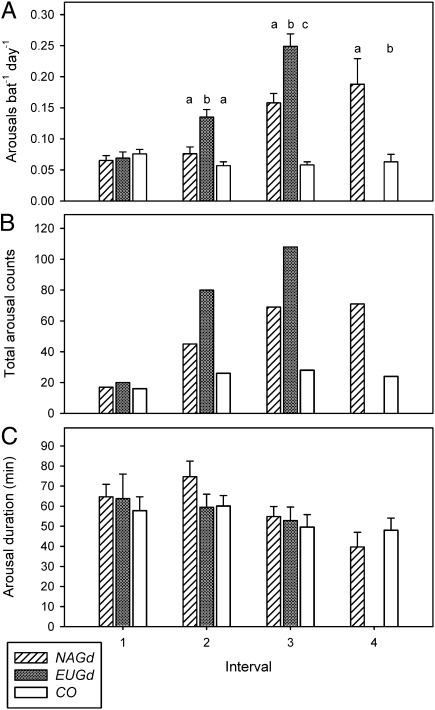
All bats entered multiday torpor bouts (i.e., began hibernating) within the first week of the study (Fig. 1). Average torpor bout duration over the entire study period was 9.0 ± 1.0 d for NAGd bats (individual range 1.2–32.4 d), 6.1 ± 0.6 d for EUGd bats (1.0–21.8 d), and 16.0 ± 0.9 d for sham-infected control group (CO) bats (2.0–33.4 d). Both NAGd and EUGd caused a progressive increase in the frequency of periodic arousals over the course of the experiment. There was no significant difference among groups during Interval 1, but treatment groups aroused significantly more often throughout the rest of hibernation (Table 1). In fact, during Interval 3, arousal frequency of NAGd bats was three times—and EUGd bats four times—that of CO bats (Fig. 2 A and B). The effect of time on arousal frequency was significant for each group (Table 2) with a significant increase over time for both NAGd and EUGd bats, and a decrease for CO bats (Fig. 2A). In contrast, the duration of periodic arousals was not affected by inoculation (Fig. 2C and Table 1), nor by time for any group (Table 2).
Fig. 1.
Representative traces of skin temperature (Tskin) for six M. lucifugus, two each from the following groups: (A) inoculated with NAGd; (B) inoculated with EUGd; (C) sham-inoculated control. The x axis shows the day of study, where day 1 is November 27, 2010; the bars at the bottom indicate the division of the study period into 26-d intervals, and months.
Table 1.
Sample sizes and ANOVA results for arousal frequency and arousal duration
| Interval | NAGd (n) | EUGd (n) | CO (n) | df1 | df2 | F | P | |
| Arousal frequency | 1 | 10 | 11 | 18 | 2 | 36 | 0.52 | 0.601 |
| 2 | 10 | 11 | 18 | 2 | 36 | 20.79 | <0.001 | |
| 3 | 14 | 11 | 18 | 2 | 40 | 52.82 | <0.001 | |
| 4 | 4 | — | 5 | 1 | 7 | 10.79 | 0.013 | |
| Arousal duration | 1 | 8 | 6 | 15 | 2 | 26 | 0.24 | 0.789 |
| 2 | 10 | 13 | 18 | 2 | 38 | 1.57 | 0.221 | |
| 3 | 14 | 12 | 18 | 2 | 41 | 0.21 | 0.811 | |
| 4 | 4 | — | 5 | 1 | 7 | 0.76 | 0.411 |
Sample sizes and ANOVA results for arousal frequency (arousal bat−1⋅d−1) and arousal duration (length of time above skin temperature threshold) for the four time intervals for M. lucifugus inoculated with NAGd and EUGd compared with sham-inoculated controls (CO). The results of the pair-wise post hoc comparisons (SNK) are indicated in Fig. 2. Significant results are in bold.
Fig. 2.
Changes in torpor patterns in M. lucifugus following inoculation with NAGd, EUGd, or CO. Frequency of arousals based on skin temperature (A), total count of arousals based on video observations (B), and mean arousal duration (C). Within intervals, different letters above bars indicate significant differences between groups (SNK post hoc tests following significant ANOVA in Table 1).
Table 2.
Repeated-measures ANOVA results for within-group effects of time on arousal frequency and arousal duration
| Group | n | df1 | df2 | F | P | |
| Arousal frequency | NAGd | 10 | 2 | 8 | 16.10 | 0.002 |
| EUGd | 10 | 2 | 8 | 34.59 | <0.001 | |
| CO | 18 | 2 | 16 | 8.28 | 0.003 | |
| Arousal duration | NAGd | 8 | 2 | 6 | 1.43 | 0.310 |
| EUGd | 6 | 2 | 4 | 1.59 | 0.311 | |
| CO | 15 | 2 | 13 | 1.61 | 0.238 |
Repeated-measures ANOVA results for within-group effects of time on arousal frequency (arousal bat−1⋅d−1) and arousal duration (length of time above skin temperature threshold) for M. lucifugus inoculated with NAGd and EUGd compared with sham inoculated controls (CO). Significant results are in bold.
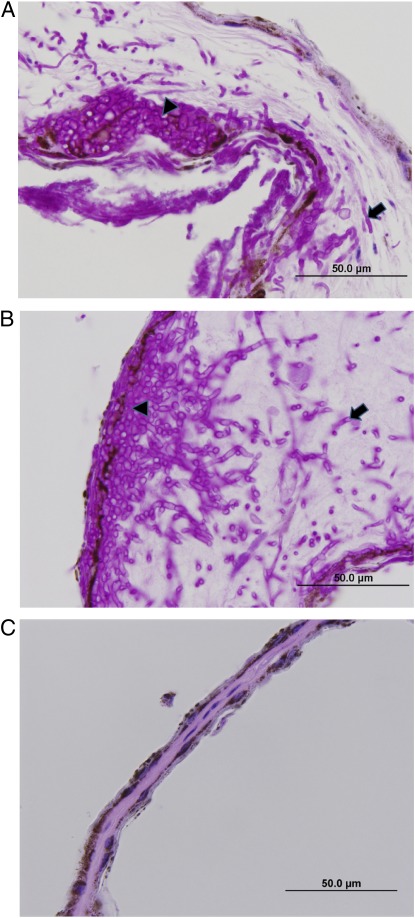
Both isolates of Gd caused all known clinical signs of WNS (5, 6), including loss of elasticity, irregular pigmentation and stickiness of wing tissue, and white surface growth. Histopathology confirmed infection in all NAGd and EUGd bats as fungal hyphae penetrated the epidermis and damaged underlying tissues, consistent with previous studies (5, 6) (Fig. 3 A and B). Gd was also cultured from sections of wing tissue for bats from both treatment groups but no CO bats showed any evidence of infection by Gd (Fig. 3C).
Fig. 3.
Light micrographs of wing membrane in transverse section for three representative M. lucifugus (A) 88 d after inoculation with NAGd (arrowhead shows cup-shaped accumulation of fungal hyphae within the epidermis, growing into the underlying subcutis; arrow shows hyphae deep within the subcutis; brown granular pigment is melanin within the epidermis); (B) 77 d after inoculation with EUGd (arrowhead shows thick mat of fungal hyphae in the epidermis growing into the underlying dermis and subcutis; arrow shows fungal hyphae deep within the wing membrane); or (C) sham-inoculated controls after 119 d.
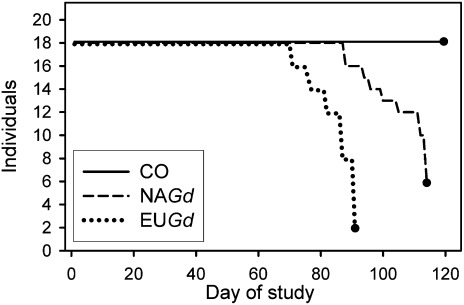
There was a highly significant effect of inoculation on survival for both NAGd (x2 = 17.1, P < 0.001) and EUGd bats (x2 = 26.4, P < 0.001) compared with controls, and NAGd bats survived significantly longer than EUGd bats (Fig. 4) (x2 = 20.3, P < 0.001). Mortality was first observed for EUGd bats on day 71, and surviving bats from the group were euthanized on day 91 after 16 bats reached moribund status. Mortality first occurred for NAGd bats on day 88 and surviving bats from this group were terminated on day 114 after 12 bats reached moribund status. Two EUGd and four NAGd bats were unable to arouse from torpor when removed from the chamber at the end of the experiment and were therefore also considered moribund. Based on necropsies, bats from both treatment groups had virtually no fat reserves remaining. On day 119, when we terminated the CO group, all bats were alive, capable of endogenous arousal from torpor, and still had subcutaneous fat reserves.
Fig. 4.
Survival of individual M. lucifugus over the course of the study for group NAGd (dashed line), EUGd (dotted line), and CO (solid line). The closed circle at the end of each line indicates the day when the group was terminated, day 1 is November 27, 2010.
Discussion
The susceptibility of a North American bat species to both EUGd and NAGd strongly supports the novel pathogen hypothesis that accidental introduction of Gd from Europe is responsible for the WNS-related mass mortality of bats in North America. Our data suggest that the absence of mortality observed among European bats infected with Gd reflects different physiological and behavioral responses of European versus North American bats rather than a heightened pathogenicity of NAGd (14). This finding also supports the hypothesis of Wibbelt et al. (14) that Gd may have impacted European bat populations in the past and that bats in Europe have coevolved resistance to (e.g., via immune system responses), or tolerance of (e.g., via behavioral adaptations), infection with Gd. These findings have significant implications for management and future research. Endemic pathogens are best addressed via management of factors that enhance virulence of the pathogen (e.g., environmental or biotic cofactors), and novel pathogens are best dealt with by managing the agents that spread the disease (12). Managing agents of spread for WNS will be impractical, if not impossible, because the putative agents (i.e., the bats) are highly cryptic, widely dispersed for much of the year, and wide-ranging. However, our results support the high priority of research aimed at understanding temporal and spatial aspects of Gd transmission in the wild, as this work could aid in the development of management strategies focused on critical locations or times of year when Gd is likely to be transmitted. Encouragingly, our findings suggest that European bats face little risk from the possible reintroduction of Gd from North America to Europe, although it would be useful to repeat our experiment with a European bat species.
Interestingly, we found that EUGd affected M. lucifugus more quickly than NAGd (Figs. 1 and 4). Rapid evolution of the host–pathogen interaction between Gd and bats could help explain this pattern (12, 15). For example, if European bats exhibit resistance to infection, Gd in Europe may face intense selection pressure for increased production of potential virulence factors and more rapid growth to facilitate its propagation and transmission. However, if the production of virulence factors and rapid growth are costly for Gd, selective trade-offs could quickly favor a less pathogenic, slower growing variant of the fungus as it infected a naive host population in North America. Moreover, dramatic population declines of North American bats in the early years of the epizootic could have reduced the potential for transmission among bats, enhancing selection for reduced pathogenicity in North America. Despite this potentially encouraging finding, clearly the version of Gd now present in North America is highly pathogenic to a number of bat species. Thus, more laboratory and field experiments are necessary to better understand interactions between bats and Gd, particularly studies aimed at better understanding transmission of the fungus in the wild.
Our study also confirms that Gd causes mortality of hibernating bats and provides direct evidence for the hypothesis that an increase in arousal frequency during hibernation is the mechanism underlying mortality. The three- to fourfold increase in arousal frequency we observed for infected bats is similar to the pattern predicted by Boyles and Willis (11) based on an energetic model. The additional arousals would prematurely deplete the stored energy of a small hibernator like M. lucifugus which, in its northern distribution, must survive >190 d exclusively on fat reserves (9, 16). Periodic arousals account for only 1.2% of the hibernation time budget, yet the thermoregulatory cost of each arousal amounts to about 5% of the winter energy budget (9). Hence, each additional arousal shortens the time a bat is able to hibernate by about 9 d. WNS-affected bats are often observed flying outside hibernacula during the daytime in winter (4), possibly searching for food and, like the Gd-inoculated bats in our study, WNS-affected carcasses collected from hibernacula after mass mortality events were emaciated (4). Hence, we conclude that infection with Gd causes an increase in arousal frequency, leading to emaciation because fat reserves are used prematurely.
One explanation for infected bats spending more time out of torpor during hibernation is that, after rewarming, bats intensify grooming because of skin irritation (17). Another possibility is that infected bats elevate Tb to mount an immune response (18). Both of these hypotheses predict that infected bats should prolong the duration of each periodic arousal, for which we found no evidence. A third hypothesis is that infection influences physiological processes that trigger arousal from torpor. One of the leading explanations for periodic arousals is that evaporative water loss during hibernation leads to dehydration over time, even at high RH, which eventually triggers rewarming (19). Cryan et al. (20) suggested that wing damage caused by Gd infection could elevate cutaneous water loss, reducing the time bats are able to spend in torpor before dehydration triggers arousal, and this hypothesis has circumstantial support (21). We found a progressive increase in arousal frequency, presumably as fungal proliferation increased, with no change in arousal duration. This pattern is counter to the immune response or skin irritation hypotheses but consistent with dehydration. The increased arousal frequency we observed, combined with past work demonstrating the importance of high RH for successful hibernation in M. lucifugus (9), implicates susceptibility to dehydration as an explanation for the high rates of mortality from WNS in this species. Dehydration, prompting arousal from torpor to search for water, could also explain the winter flights of affected bats outside hibernacula (20). Interestingly, a previous inoculation experiment performed under much drier conditions (∼82% RH at 6.5 °C) than those experienced by little brown bats in the wild, or the bats in our study, observed 20% mortality of control bats after 3 mo with no significant difference in survival between inoculated bats and controls (1). This difference between studies could reflect the influence of humidity and dehydration on survival of uninfected bats, or a reduction in the proliferative abilities of Gd under drier conditions. Future detailed studies examining hygric aspects of bat hibernation, as well as the effects of humidity on the growth and pathogenicity of Gd, may help clarify the influence of environmental conditions and water loss on the progression of WNS.
Our study confirms that Gd is the cause of mortality from WNS and strongly implicates premature fat depletion because of increased arousal frequency as the ultimate cause of death. The study also lends strong support to the novel pathogen hypothesis that Gd is an invasive species from Europe. Our findings have implications for future studies on the ecophysiology and susceptibility of WNS-affected bats, as well as the pathogenicity and transmission of Gd.
Materials and Methods
Bats.
Fifty-four male M. lucifugus (8.6 ± 0.1 g) were collected from a WNS-negative cave in Manitoba, Canada, in November 2010 and transported to the University of Saskatchewan. Bats were randomly divided into three groups of 18 individuals each: (i) inoculated with NAGd; (ii) inoculated with EUGd; and (iii) a sham-inoculated control group, CO. Each group was housed in a mesh enclosure contained within a separate environmental chamber at Ta = 7.0 and >97% RH. Bats were not fed but were provided with ad libitum water. In March 2011, surviving bats were removed from the environmental chambers, anesthetized, and humanely euthanized. Methods were approved by the University Committee on Animal Care and Supply of the University of Saskatchewan (Protocol #20100120) under Manitoba Wildlife Scientific Permit WB11145.
Inoculation.
For NAGd and EUGd bats, 20 μL of inoculum containing ∼500,000 Gd conidia suspended in PBS-Tween-20 solution was pipetted onto the dorsal surface of each wing. NAGd was designated type isolate 20631–21 (American Type Culture Collection, ATCC MYA-4855) (5) isolated from a M. lucifugus collected in New York on February 2, 2008. The EUGd isolate (MmyotGER2) was obtained from a greater mouse-eared bat (Myotis myotis) collected in Thuringa, Germany, on March 7, 2009 (14). CO bats were sham-inoculated with 20 μL of PBS-Tween-20 solution lacking fungal conidia.
Skin Temperature.
All bats were equipped with one of two types of device to record skin temperature (Tskin): either temperature-sensitive radio transmitters (LB-2NT; Holohil Systems) or data loggers (DS1922L-F5 Thermochron iButton, Maxim; and iBBat, Alpha Mach). Tskin was recorded every 15 min.
Behavior.
Infrared cameras inside each environment chamber allowed us to monitor behavior and count the total number of arousals from torpor for each group within each interval (Fig. 2B). These data were clearly consistent with Tskin (compare Fig. 2 A and B).
Histopathology.
We examined multiple sections from the left wing, as well as nose and ear, following Meteyer et al. (6). Tissues were fixed in formalin immediately after bats were euthanized and later stained for histopathological examination using the periodic-acid Schiff method. All NAGd and EUGd bats exhibited the epidermal lesions typical of WNS (5, 6).
Analyses.
The study period was divided into four intervals of 26.3 d each. We tabulated the number of arousals from torpor for each individual to generate mean values for each treatment group. Full-factorial ANOVA was used to analyze differences in torpor bout duration and arousal duration among groups within each interval. Student-Newman-Keuls (SNK) post hoc tests were used for pair-wise comparisons following a significant ANOVA result. To examine the effect of time on torpor patterns (i.e., arousal frequency and arousal duration) we used repeated-measures ANOVA testing for differences among the first three intervals within each group. A Breslow–Gehan survival analysis was used to test for differences in the time to mortality/moribund status for the three groups with a Bonferroni correction to account for multiple comparisons between each pair of groups. All analyses were conducted using statistiXL v7.0 and Systat v11.0.
Supplementary Material
Acknowledgments
We thank M. Burmester, P. Mason, and M. Weiss for animal care support; C. Rainbow, C. Wilson, and M. Zimmer for pathology assistance; P. Withers for assistance with statistical analyses; M. Kilpatrick and W. Frick for helpful discussions; and M. Brigham, B. Fenton, and an anonymous reviewer for excellent comments on drafts of the manuscript. Funding was provided by a US Fish and Wildlife Service grant (to C.K.R.W., D.S.B., and P.M.C.); grants from the Natural Sciences and Engineering Research Council, the Canada Foundation for Innovation and Manitoba Research, and Innovation Fund (to C.K.R.W.); and a Government of Canada Post-Doctoral Research Fellowship and a fellowship within the Postdoc Programme of the DAAD, German Academic Exchange Service (to L.W.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1200374109/-/DCSupplemental.
See Commentary on page 6794.
References
- 1.Lorch JM, et al. Experimental infection of bats with Geomyces destructans causes white-nose syndrome. Nature. 2011;480:376–378. doi: 10.1038/nature10590. [DOI] [PubMed] [Google Scholar]
- 2.Turner GG, Reeder DM, Coleman JTH. A five-year assessment of mortality and geographic spread of white-nose syndrome in North American bats and a look to the future. Bat Research News. 2011;52(3):13–27. [Google Scholar]
- 3.Frick WF, et al. An emerging disease causes regional population collapse of a common North American bat species. Science. 2010;329:679–682. doi: 10.1126/science.1188594. [DOI] [PubMed] [Google Scholar]
- 4.Blehert DS, et al. Bat white-nose syndrome: An emerging fungal pathogen? Science. 2009;323:227. doi: 10.1126/science.1163874. [DOI] [PubMed] [Google Scholar]
- 5.Gargas A, Trest MT, Christensen M, Volk TJ, Blehert DS. Geomyces destructans sp. nov. associated with bat white-nose syndrome. Mycotaxon. 2009;108(8):147–154. [Google Scholar]
- 6.Meteyer CU, et al. Histopathologic criteria to confirm white-nose syndrome in bats. J Vet Diagn Invest. 2009;21:411–414. doi: 10.1177/104063870902100401. [DOI] [PubMed] [Google Scholar]
- 7.Burton RS, Reichman OJ. Does immune challenge affect torpor duration? Funct Ecol. 1999;13:232–237. [Google Scholar]
- 8.Geiser F. Metabolic rate and body temperature reduction during hibernation and daily torpor. Annu Rev Physiol. 2004;66:239–274. doi: 10.1146/annurev.physiol.66.032102.115105. [DOI] [PubMed] [Google Scholar]
- 9.Thomas DW, Dorais M, Bergeron J-M. Winter energy budgets and cost of arousals for hibernating little brown bats Myotis lucifugus. J Mammal. 1990;71:475–479. [Google Scholar]
- 10.WNS Science Strategy Group 2008. Questions, observations, hypotheses, predictions, and research needs for addressing effects of white-nose syndrome (WNS) in hibernating bats. http://batcon.org/pdfs/WNSMtgRptFinal2.pdf. Accessed January 8, 2012.
- 11.Boyles JG, Willis CKR. Could localized warm areas inside cold caves reduce mortality of hibernating bats affected by white-nose syndrome? Front Ecol Environ. 2009;18(2):92–98. [Google Scholar]
- 12.Rachowicz LJ, et al. The novel and endemic pathogen hypotheses: Competing explanations for the origin of emerging infectious diseases of wildlife. Conserv Biol. 2005;19:1441–1448. [Google Scholar]
- 13.Puechmaille SJ, et al. Pan-European distribution of white-nose syndrome fungus (Geomyces destructans) not associated with mass mortality. PLoS ONE. 2011;6:e19167. doi: 10.1371/journal.pone.0019167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wibbelt G, et al. White-nose syndrome fungus (Geomyces destructans) in bats, Europe. Emerg Infect Dis. 2010;16:1237–1243. doi: 10.3201/eid1608.100002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Altizer S, Harvell D, Friedle E. Rapid evolutionary dynamics and disease threats to biodiversity. Trends Ecol Evol. 2003;18:589–596. [Google Scholar]
- 16.Jonasson KA, Willis CKR. Changes in body condition of hibernating bats support the thrifty female hypothesis and predict consequences for populations with white-nose syndrome. PLoS ONE. 2011;6:e21061. doi: 10.1371/journal.pone.0021061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giorgi MS, Arlettaz R, Christe P, Vogel P. The energetic grooming costs imposed by a parasitic mite (Spinturnix myoti) upon its bat host (Myotis myotis) Proc Biol Sci. 2001;268:2071–2075. doi: 10.1098/rspb.2001.1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prendergast BJ, Freeman DA, Zucker I, Nelson RJ. Periodic arousal from hibernation is necessary for initiation of immune responses in ground squirrels. Am J Physiol Regul Integr Comp Physiol. 2002;282:R1054–R1062. doi: 10.1152/ajpregu.00562.2001. [DOI] [PubMed] [Google Scholar]
- 19.Thomas DW, Geiser F. Periodic arousals in hibernating mammals: Is evaporative water loss involved? Funct Ecol. 1997;11:585–591. [Google Scholar]
- 20.Cryan PM, Meteyer CU, Boyles JG, Blehert DS. Wing pathology of white-nose syndrome in bats suggests life-threatening disruption of physiology. BMC Biol. 2010;8:135. doi: 10.1186/1741-7007-8-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Willis CKR, Menzies AK, Boyles JG, Wojciechowski MS. Evaporative water loss is a plausible explanation for mortality of bats from white-nose syndrome. Integr Comp Biol. 2011;51:364–373. doi: 10.1093/icb/icr076. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.